Abstract
Protein Z-dependent protease inhibitor (ZPI) is a 72-kd member of the serpin superfamily of proteinase inhibitors that produces rapid inhibition of factor Xa in the presence of protein Z (PZ), procoagulant phospholipids, and Ca++ (t1/2 less than 10 seconds). The rate of factor Xa inhibition by ZPI is reduced more than 1000-fold in the absence of PZ. The factor Xa–ZPI complex is not stable to sodium dodecyl sulfate–polyacrylamide gel electrophoresis, but is detectable by alkaline–polyacrylamide gel electrophoresis. The combination of PZ and ZPI dramatically delays the initiation and reduces the ultimate rate of thrombin generation in mixtures containing prothrombin, factor V, phospholipids, and Ca++. In similar mixtures containing factor Va, however, PZ and ZPI do not inhibit thrombin generation. Thus, the major effect of PZ and ZPI is to dampen the coagulation response prior to the formation of the prothrombinase complex. Besides factor Xa, ZPI also inhibits factor XIa in the absence of PZ, phospholipids, and Ca++. Heparin (0.2 U/mL) enhances the rate (t1/2 = 25 seconds vs 50 seconds) and the extent (99% vs 93% at 30 minutes) of factor XIa inhibition by ZPI. During its inhibitory interaction with factor Xa and factor XIa, ZPI is proteolytically cleaved with the release of a 4.2-kd peptide. The N-terminal amino acid sequence of this peptide (SMPPVIKVDRPF) establishes Y387 as the P1 residue at the reactive center of ZPI. ZPI activity is consumed during the in vitro coagulation of plasma through a proteolytic process that involves the actions of factor Xa with PZ and factor XIa.
Introduction
Vitamin K is required for the post-translational formation of a unique amino acid, gamma-carboxyglutamic acid (Gla), which is present in a number of plasma proteins that are involved in hemostasis.1,2 Gla-mediated Ca++ binding in these proteins is necessary for their association with phospholipid surfaces and is critical for their hemostatic function.3Protein Z (PZ) is a 62-kd vitamin K–dependent plasma protein whose structure is very similar to coagulation factors VII, IX, and X and protein C. In contrast to these serine protease zymogens, however, in PZ the region around the typical activation site is absent, and the histidine and serine residues of the canonical catalytic triad are missing.4,5 The plasma half-life of PZ is approximately 2.5 days, and plasma PZ levels in blood donors span a broad range, with a mean of 2.9 ± 1.0 μg/mL in EDTA-anticoagulated samples.6 PZ was noted to differ from the other vitamin K–dependent coagulation factors in 2 important respects: (1) markedly slower association and dissociation rates with/from phospholipid vesicles7; and (2) a dramatic reduction in the plasma PZ level with warfarin therapy.6 Although bovine PZ was isolated in 1977 and human PZ was isolated in 1984,8 9 the function of PZ remained uncertain.
In more recent studies, it was found that the procoagulant activity of factor Xa in a one-stage plasma coagulation assay was reduced when factor Xa was first incubated with PZ. This apparent inhibitory effect was time-dependent (maximal by 90 to 120 seconds) and required the presence of Ca++ and procoagulant phospholipids.10 The time dependence was related predominantly to the incubation period of PZ with phospholipid, probably reflecting the relatively slow rate of PZ's association with phospholipid.7 PZ that was proteolytically cleaved at R-43 (single-letter amino acid code), thereby separating its Gla domain from the remainder of the molecule, lacked inhibitory activity.10 These results suggested that an interaction between factor Xa and PZ occurs at the phospholipid surface. Consistent with this notion, the rate of inhibition of factor Xa by antithrombin was slowed by PZ in the presence of phospholipids and Ca++.10 Additional work showed that the inhibitory effect of PZ on factor Xa activity in the one-stage coagulation assay was due at least in part to a previously unidentified plasma proteinase inhibitor that recognizes the factor Xa–PZ complex.
Protein Z–dependent protease inhibitor (ZPI) is an approximately 72-kd member of the serpin superfamily of proteinase inhibitors.11 In the presence of PZ, procoagulant phospholipids, and Ca++, ZPI produces rapid inhibition of factor Xa (t1/2 less than 10 seconds) in a process that appears to involve the formation of a Ca++-dependent tertiary complex containing factor Xa, PZ, and ZPI at the phospholipid surface.10 Northern analysis of tissues suggests that a major site of ZPI synthesis is the liver.11 On the basis of studies of the inhibitory activity of an altered form of recombinant ZPI, Y387 is thought to be the P1 residue at the reactive center of ZPI.11 Initial investigations of the inhibitory spectrum of ZPI failed to identify a proteinase besides factor Xa that was inactivated by ZPI in the presence or absence of PZ.
The presence of PZ delays the initiation and reduces the ultimate extent of thrombin generation in plasma induced to clot by low concentrations of factor IXa.12 PZ null mice have an apparently normal phenotype, but PZ deficiency dramatically increases the severity of the thrombotic phenotype of mice carrying the factor VLeiden genotype.12 These in vitro and in vivo studies suggest that PZ plays an important role in dampening coagulation. Presumably the cofactor effect of PZ for the inactivation of factor Xa by ZPI is an important part of this regulatory action of PZ. Here we report experiments designed to further characterize the properties of ZPI.
Materials and methods
Materials
Pooled normal human plasma (20 donors) and factor-deficient plasmas were from George King Biomedical, Inc (Overland Park, KS). Chromogenic substrates S2366 (pyroGlu-P-R-pNA) and S2302 (H-D-P-F-R-pNA) were from Pharmacia (Franklin, OH); Spectrazyme Xa (MeO-CO-D-cyclohexylglycyl-G-R-pNA) was from American Diagnostica, Inc (Greenwich, CT). Prestained molecular weight standards and Affigel-10 were purchased from Bio-Rad Laboratories (Hercules, CA). Rabbit brain cephalin, bovine serum albumin, Tween 20, and 3,3′,5′,5-tetramethylbenzidine (TMB) liquid substrate were from Sigma Chemical Co (St Louis, MO). Kaolin was from Fisher Scientific, Inc (Fairlawn, NJ).
Proteins
PZ and ZPI were isolated from human plasma.10 An altered form of recombinant ZPI, rZPI(Y387A), in which Y387 was replaced with an alanine, was produced as previously described.11 Factor Xa, factor V, and factor Va were from Haematologic Technologies, Inc (Essex Junction, VT). Factor XIa, high molecular weight kininogen (HK), factor XIIa, and the X coagulant protein (XCP) from Russell's Viper venom were purchased from Enzyme Research Laboratories (South Bend, IN). Plasma kallikrein was from Sigma and thrombin from American Diagnostica. Human brain thromboplastin was prepared as previously described.13Taipan snake venom was purchased from the Miami Serpentarium (Miami, FL).
Antibodies and ZPI immunoassay
Rabbit polyclonal anti-ZPI antibodies have been previously described.10 Rabbit polyclonal antibodies against a peptide representing the N-terminal sequence of the ZPI molecule (residues 1 through 14, LAPSPQSPETRAPQ were produced as previously described.14 Mouse monoclonal anti-ZPI antibodies were produced by means of established and previously reported techniques6 and conjugated with biotin with the use of EZ-Link Sulfo-NHS-LC-Biotin (Pierce; Rockford, IL) as described by the manufacturer. For ZPI immunoassay, 150 μL of rabbit anti-ZPI polyclonal antibodies (20 μg/mL) in 0.1 mol/L NaCl, 0.05 mol/L Hepes, pH 7.4 (HS), was incubated overnight at room temperature in the well of a microtiter plate (Nunc, Fisher Scientific; Hanover Park, IL). After the wells were washed with phosphate-buffered saline containing 0.05% Tween 20 (PBST) (200 μL, 4 ×), samples (150 μL) diluted in PBST were applied to the wells and allowed to incubate 1 hour. After washing the wells with PBST, a solution (150 μL) containing biotin-conjugated anti-ZPI monoclonal antibodies MC4249.2 (2 μg/mL) and MC4249.3 (2 μg/mL) in HS with 1 mg/mL bovine serum albumin (HSA) was added to each well and incubated for 1 hour. The wells were washed with PBST, and 150 μL of a 1:100 dilution of streptavidin–horseradish peroxidase complex (Pierce) in PBST was applied. After 1 hour, the wells were washed with PBST, and 200 μL of TMB liquid substrate was added. After exactly 5 minutes, 100 μL 0.5 mol/L H2SO4 was added to stop the reaction, and the A450 was measured by means of a Vmax microtiter plate reader (Molecular Devices; Sunnyvale, CA). Purified ZPI produced a linear dose response in this assay at concentrations of 0 to 15 ng/mL; pooled plasma was tested at 1:200 and 1:400 dilutions. ZPI concentration of the standard was determined by amino acid composition (Protein Chemistry Laboratory, Washington University, St Louis, MO).
Factor Xa inhibition by ZPI
Mixtures containing factor Xa (5 nmol/L), CaCl2 (4 mmol/L), phospholipids (15 μmol/L) with or without PZ (40 nmol/L) were constructed at room temperature in HSA, and the reaction was initiated by the addition of ZPI (69 nmol/L). At various times thereafter, samples were removed from the mixtures and diluted in HSA, and the remaining factor Xa activity was determined by coagulation assay.10 To evaluate the effect of heparin on factor Xa inhibition by ZPI in the absence of PZ, ZPI (42 nmol/L) was added to mixtures containing factor Xa (1.5 nmol/L), CaCl2(4 mmol/L), phospholipids (15 μmol/L), with or without heparin (0.25 U/mL), at room temperature. After 1 hour, Spectrazyme Xa (200 μmol/L) was added, and remaining factor Xa was determined as amidolytic activity and compared with a factor Xa standard curve. Owing to the rapid inhibition of factor Xa produced by ZPI in the presence of PZ, the effect of heparin on the inhibition of factor Xa by ZPI in the presence of PZ was tested by examining the progress curve (A405) of factor Xa inhibition in the presence of Spectrazyme Xa (250 μmol/L).
Factor Xa–ZPI complex on alkaline–polyacrylamide gel electrophoresis
Mixtures containing phospholipids (15 μmol/L) and CaCl2 (4 mmol/L), with various combinations of factor Xa (10 nmol/L), PZ (32 nmol/L), and ZPI (28 nmol/L), were incubated at room temperature. After 2 minutes, 10 μL samples were separated by 8% alkaline–polyacrylamide gel electrophoresis (alkaline-PAGE) for 90 minutes at 120 V and analyzed by Western blotting with the use of goat anti-factor X antibodies (Enzyme Research Laboratories) and chemiluminescence detection.11,15 16
Inhibition of thrombin generation by ZPI
Mixtures containing PZ (40 nmol/L), ZPI (69 nmol/L), phospholipids (15 μmol/L), CaCl2 (4 mmol/L), and factor V (6 nmol/L) or factor Va (6 nmol/L) in HSA were incubated 2 minutes at room temperature. Prothrombin (1.4 μmol/L) and factor Xa (0.1 nmol/L) were then added in succession to initiate the reaction. Samples were removed at various times and diluted with HSA containing 10 mmol/L EDTA, and the thrombin concentration was determined as amidolytic activity against S2238 (200 μmol/L) in comparison with a standard curve constructed with purified thrombin. In certain experiments, factor Xa was included with other reactants during the initial 2-minute incubation, and the reaction was initiated by the addition of prothrombin.
Reversibility of ZPI-mediated factor Xa inhibition
Mixtures containing factor Xa (20 nmol/L), phospholipids (25 μmol/L), CaCl2 (5 mmol/L), with and without factor Va (30 nmol/L) and with and without PZ (80 nmol/L) and ZPI (56 nmol/L), were constructed in HS. After 3 minutes at room temperature, a one-tenth volume of EDTA (100 mmol/L) or HS was added, and the mixtures were transferred to a 37° water bath. At various times thereafter, samples were removed for Western blot analysis with rabbit anti-ZPI antibodies and factor Xa coagulation assay following dilution.10
ZPI inhibition of factor XIIa, kallikrein, and factor XIa
Proteinases were incubated with or without ZPI (35 nmol/L) at room temperature in the presence or absence of PZ (40 nmol/L), phospholipids (15 μmol/L), and CaCl2 (4 mmol/L). After 15 minutes, remaining proteinase activity was determined by amidolytic assay. The tested proteinases and their respective chromogenic substrates were as follows: factor XIIa (12.5 nmol/L), S2302 (130 μmol/L); kallikrein (8.1 nmol/L), S2302 (400 μmol/L); and factor XIa (2.5 nmol/L), S2366 (1 mmol/L). In experiments to further examine the inhibition of factor XIa by ZPI, mixtures were constructed containing factor XIa (1 nmol/L), with or without HK (0.5 μmol/L) and with or without heparin (0.20 U/mL), and the inhibitory process was initiated with the addition of ZPI (69 nmol/L). At various times thereafter, samples were removed, and residual factor XIa activity was determined by the addition of S2366 (250 μmol/L) and comparison of the amidolytic activity with a factor XIa standard curve.
Isolation and amino acid sequencing of the ZPI proteolytic fragments produced by factors Xa and XIa
A mixture containing factor Xa (3.6 μmol/L), ZPI (5.56 μmol/L), PZ (4.84 μmol/L), CaCl2 (4 mmol/L), and phospholipids (150 μmol/L) and a mixture containing factor XIa (1 μmol/L) and ZPI (5.56 μmol/L) in HS were incubated at 37°C. After 1 hour, 50 μL samples were separated by 10% to 20% gradient sodium dodecyl sulfate (SDS)–PAGE by means of a tricine/glycine buffer system (Bio-Rad Laboratories) and electrotransferred onto a polyvinylidene fluoride membrane (Micron Separations, Inc; Westborough, MA) in 10 mmol/L CAPS, pH 11.0, 10% (vol/vol) methanol. The membrane was stained with 0.025% (wt/vol) Coomassie Brilliant Blue R-250 in 40% methanol for 5 minutes and then destained with 50% methanol for 10 minutes. The membrane was rinsed several times with distilled water and allowed to air dry. The discernible 4.2 kd-protein bands were cut from the membrane, and their N-terminal amino acid sequences determined by the Protein Chemistry Laboratory.
Proteolysis of ZPI during coagulation in plasma
Coagulation was induced in normal and various factor-deficient citrated plasmas (George King Biomedical) by the addition of a one-tenth volume of human brain thromboplastin with CaCl2(250 mmol/L); the addition of a one-tenth volume of freshly prepared 1% (wt/vol) kaolin, phospholipids (200 μmol/L), and CaCl2 (250 mmol/L); or the addition of a one-tenth volume of crude Taipan snake venom (10 μg/mL), phospholipids (200 μmol/L), and CaCl2 (250 mmol/L). In certain experiments, plasmas were treated with kaolin alone. The mixtures were incubated for 30 minutes at 37°C and centrifuged (10 000g, 5 minutes) to separate the fibrin pellet from the serum. In additional experiments, a one-tenth volume of XCP from Russell's Viper venom (1.0 μg/mL), phospholipids (200 μmol/L), and CaCl2 (150 mmol/L) was used to induce coagulation in barium-adsorbed plasma12that had been supplemented with factor X (8 μg/mL) and PZ (10 μg/mL). ZPI in plasma and serum samples (1 mL) was immunoadsorbed by adding 100 μL of a 75% (vol/vol) slurry of rabbit anti-ZPI polyclonal immunoglobulin (Ig)–G linked to Affigel-10 (2.5 mg/mL) and rocking the mixture for 1 hour. The Affigel was isolated by centrifugation (10 000g, 2 minutes) and washed 3 times with 1 mL HS. SDS-PAGE sample buffer (50 μL) was added to the Affigel pellet to extract the ZPI, and the Affigel was removed by centrifugation. Samples containing ZPI (5 μL) were separated by SDS-PAGE and analyzed by Western blotting with the use of mouse monoclonal anti-ZPI IgG (MC4249.2) or rabbit polyclonal anti-ZPI N-terminal peptide antibodies and alkaline phosphatase detection.11 16
Other assays
SDS-PAGE and ZPI functional assay were performed as previously described.10
Results
Inhibition of factor Xa by ZPI
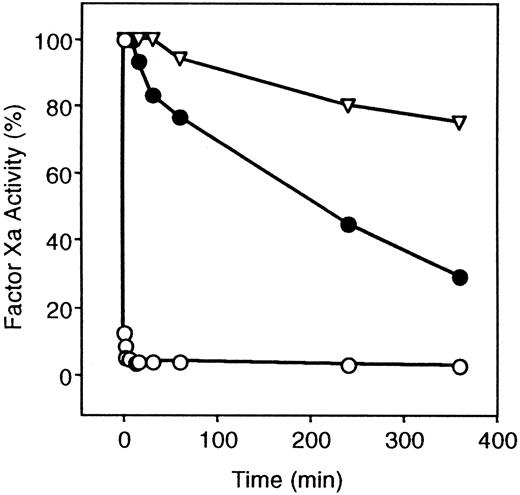
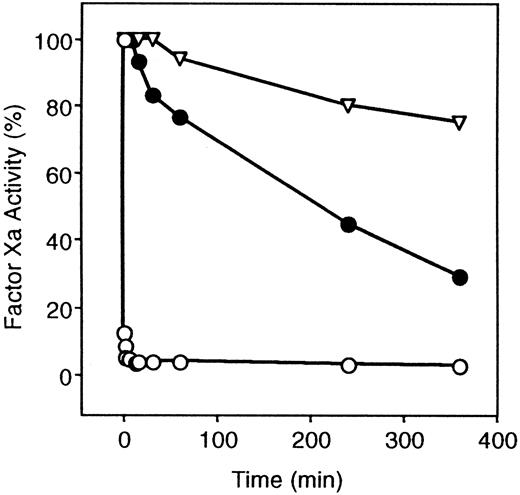
Once a Ca++-dependent complex between PZ and factor Xa has formed at a phospholipid surface, the inhibition of factor Xa by ZPI occurs very rapidly (t1/2 less than 10 seconds). The requirement of PZ for ZPI-mediated factor Xa inhibition, however, is not absolute, and ZPI alone will inhibit factor Xa, although at a much slower rate (t1/2 = 210 minutes) (Figure1). Heparin (0.25 U/mL) did not significantly affect ZPI inhibition of factor Xa in the presence of PZ (not shown) and enhanced ZPI inhibition of factor Xa in the absence of PZ to only a minor extent (factor Xa inhibition at 60 minutes: without heparin, 29 ± 10%; with heparin, 61 ± 8%).
Inhibition of factor Xa by ZPI.
ZPI inhibition of factor Xa is shown in the presence and absence of PZ. Mixtures containing factor Xa (5 nmol/L), phospholipids (15 μmol/L), CaCl2 (4 mmol/L), with or without PZ (40 nmol/L), were constructed in HSA at room temperature, and the reaction was initiated by the addition of ZPI (69 nmol/L). Samples were removed at various times and diluted in HSA, and remaining factor Xa activity was determined by coagulation assay. ▿, without PZ or ZPI; ○, ZPI with PZ; and ●, ZPI without PZ.
Inhibition of factor Xa by ZPI.
ZPI inhibition of factor Xa is shown in the presence and absence of PZ. Mixtures containing factor Xa (5 nmol/L), phospholipids (15 μmol/L), CaCl2 (4 mmol/L), with or without PZ (40 nmol/L), were constructed in HSA at room temperature, and the reaction was initiated by the addition of ZPI (69 nmol/L). Samples were removed at various times and diluted in HSA, and remaining factor Xa activity was determined by coagulation assay. ▿, without PZ or ZPI; ○, ZPI with PZ; and ●, ZPI without PZ.
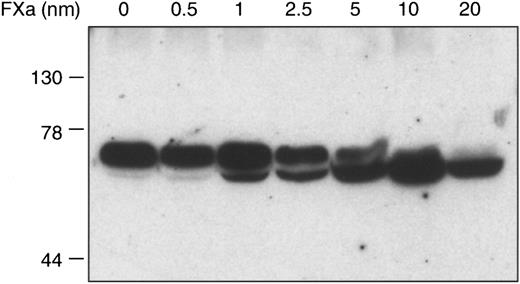
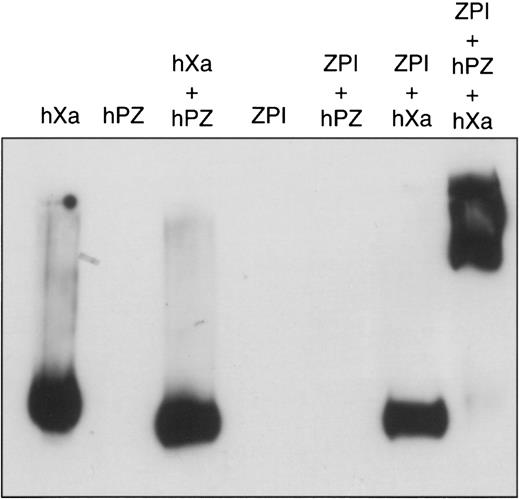
As is typical for members of the serpin superfamily of proteinase inhibitors, apparent proteolytic cleavage of ZPI occurs during its inhibition of factor Xa (Figure 2). In contrast to many other proteinase-serpin interactions, however, the factor Xa–ZPI inhibitory complex is disrupted during SDS-PAGE (Figure2), but can be detected by alkaline-PAGE (Figure3). Under nondenaturing conditions, the PZ–factor Xa–ZPI complex migrates as a smear. The apparently clear area in the center of the smear in Figure 3 represents a staining artifact. This peculiar migration pattern of the complex is most likely due to variable loss of PZ and/or the dissociation of the factor Xa–ZPI complex during the electrophoresis itself. The lack of Ca++ and phospholipid during electrophoresis favors the release of PZ, and the high pH (8.8) of the separation buffer reduces the stability of the factor Xa–ZPI complex (unpublished observations, 1999).
Cleavage of ZPI by factor Xa.
Mixtures containing factor Xa (0 to 20 nmol/L), PZ (80 nmol/L), ZPI (15 nmol/L), phospholipids (75 μmol/L), and CaCl2 (4 mmol/L) in HSA were incubated 15 minutes at room temperature before SDS-PAGE and Western blot analysis with rabbit anti-ZPI antibodies.
Cleavage of ZPI by factor Xa.
Mixtures containing factor Xa (0 to 20 nmol/L), PZ (80 nmol/L), ZPI (15 nmol/L), phospholipids (75 μmol/L), and CaCl2 (4 mmol/L) in HSA were incubated 15 minutes at room temperature before SDS-PAGE and Western blot analysis with rabbit anti-ZPI antibodies.
Factor Xa–ZPI interaction on alkaline gel electrophoresis.
Mixtures containing various combinations of factor Xa (10 nmol/L), PZ (32 nmol/L), ZPI (28 nmol/L), cephalin (15 μmol/L), and CaCl2 (4 mmol/L) were incubated in HSA at room temperature. After 2 minutes, samples were separated by 8% alkaline-PAGE and analyzed by Western blotting with goat anti–factor Xa antibodies.
Factor Xa–ZPI interaction on alkaline gel electrophoresis.
Mixtures containing various combinations of factor Xa (10 nmol/L), PZ (32 nmol/L), ZPI (28 nmol/L), cephalin (15 μmol/L), and CaCl2 (4 mmol/L) were incubated in HSA at room temperature. After 2 minutes, samples were separated by 8% alkaline-PAGE and analyzed by Western blotting with goat anti–factor Xa antibodies.
Previous work has shown that an altered form of recombinant ZPI, rZPI(Y387A) (in which tyrosine 387 is replaced with an alanine), does not inhibit factor Xa.11 This strongly implies that Y387 is the P1 residue at the reactive center of ZPI. To confirm this notion, the peptide (4.2 kd) released from ZPI following its interaction with factor Xa was isolated and sequenced (see “Materials and methods”). As anticipated, the N-terminal amino acid sequence of the peptide, SMPPVIKVDRPF, directly corresponded to the amino acid sequence of the ZPI molecule following Y387.11
ZPI inhibition of thrombin generation
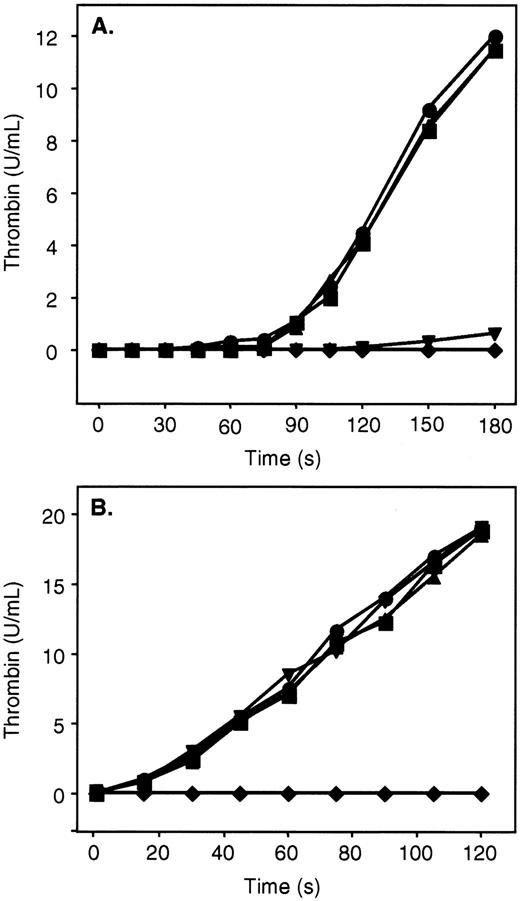
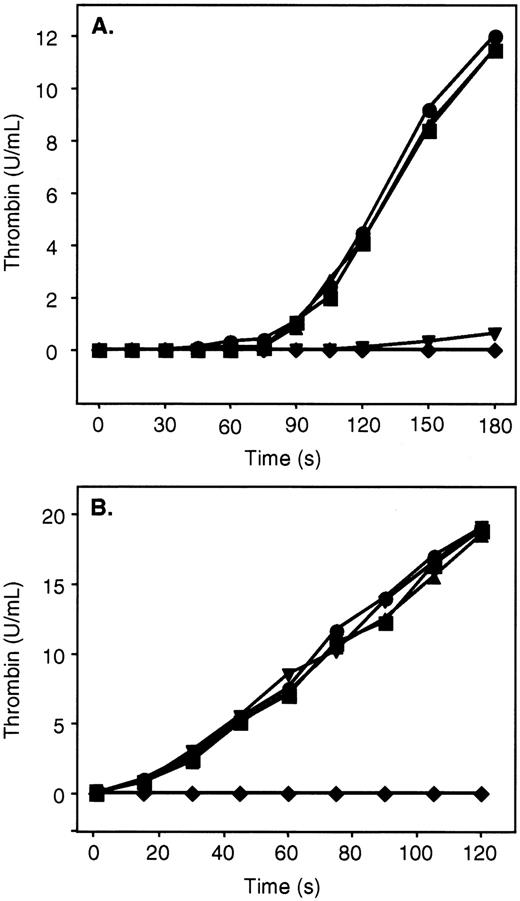
ZPI in the presence of PZ, phospholipids, and Ca++ is a potent inhibitor of factor Xa, and additional studies were undertaken to determine its effect on the prothrombinase complex (Figure4). Preincubation (2 minutes) of PZ and ZPI with factor Xa, factor Va, phospholipids, and Ca++dramatically reduced subsequent thrombin generation following the addition of prothrombin (Figure 4B). PZ and ZPI, however, failed to inhibit thrombin production in a similar mixture when prothrombin was present and factor Xa was added last to initiate the reaction (Figure4B). In contrast, the combination of PZ and ZPI substantially delayed the initiation and reduced the ultimate rate of thrombin generation in the presence of prothrombin when factor V, rather than factor Va, was included in the reaction mixtures (Figure 4A). Presumably, under the latter conditions, PZ and ZPI are able to produce significant factor Xa inhibition prior to the generation of factor Va and the formation of the prothrombinase complex.
Inhibition of thrombin generation by ZPI.
Mixtures containing PZ (40 nmol/L), ZPI (69 nmol/L), phospholipids (15 μmol/L), CaCl2 (4 mmol/L), and factor V or Va (6 nmol/L) in HSA were incubated for 2 minutes at room temperature. Prothrombin (1.4 μmol/L) and factor Xa (0.1 nmol/L) were then added in succession to initiate the reaction. Thrombin activity was determined by amidolytic assay. (A) Mixtures containing factor V. (B) Mixtures containing factor Va. ●, without PZ, without ZPI; ▪, with PZ, without ZPI; ▴, without PZ, with ZPI; ▾, with PZ, with ZPI; ♦, factor Xa preincubated with PZ, ZPI, factor Va, phospholipids, and CaCl2 prior to the addition of prothrombin.
Inhibition of thrombin generation by ZPI.
Mixtures containing PZ (40 nmol/L), ZPI (69 nmol/L), phospholipids (15 μmol/L), CaCl2 (4 mmol/L), and factor V or Va (6 nmol/L) in HSA were incubated for 2 minutes at room temperature. Prothrombin (1.4 μmol/L) and factor Xa (0.1 nmol/L) were then added in succession to initiate the reaction. Thrombin activity was determined by amidolytic assay. (A) Mixtures containing factor V. (B) Mixtures containing factor Va. ●, without PZ, without ZPI; ▪, with PZ, without ZPI; ▴, without PZ, with ZPI; ▾, with PZ, with ZPI; ♦, factor Xa preincubated with PZ, ZPI, factor Va, phospholipids, and CaCl2 prior to the addition of prothrombin.
Reversibility of the factor Xa–ZPI complex
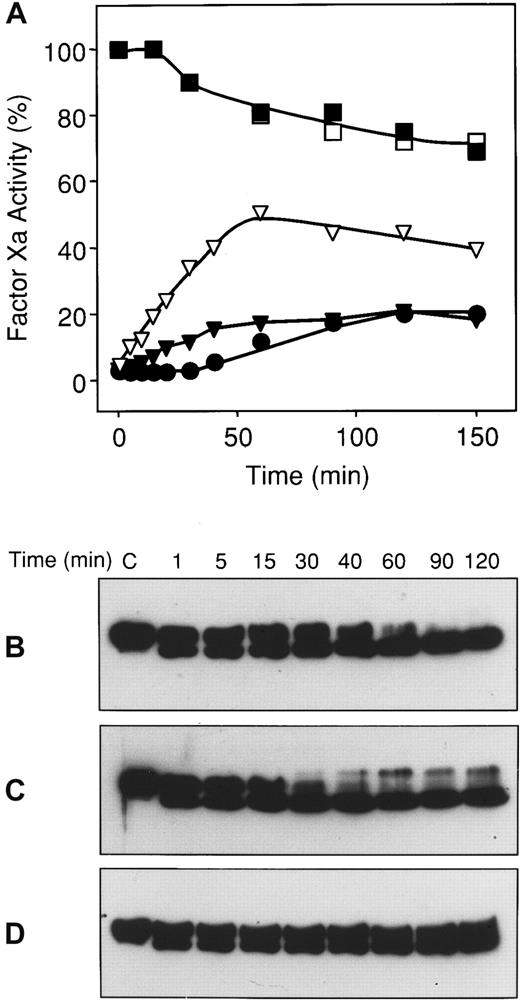
The inability to detect a factor Xa–ZPI complex on SDS-PAGE suggested that the stability of the factor Xa–ZPI complex is considerably less than that of other proteinase-serpin interactions. To examine the dissociation of the factor Xa–ZPI complex, EDTA was added to mixtures incubated at 37° that contained factor Xa which had been inhibited more than 97% by ZPI in the presence of PZ, phospholipids, and Ca++. The chelation of Ca++ prevents factor Xa released from a factor Xa–ZPI complex from forming a subsequent, PZ-dependent interaction with additional ZPI molecules. EDTA treatment induced the progressive, though partial, return of factor Xa activity (Figure 5A). In a similar mixture that was not treated with EDTA, progressive cleavage of ZPI was observed with the ultimate return of some factor Xa activity (Figure 5A-B). The inclusion of factor Va in the mixture produced an earlier return of factor Xa activity and faster ZPI cleavage (Figure 5A,C). EDTA treatment prevented this progressive cleavage of ZPI (Figure5D).
Reversibility of the factor Xa–ZPI interaction.
Mixtures containing factor Xa (20 nmol/L), phospholipids (25 μmol/L), and CaCl2 (4 mmol/L), with and without PZ (80 nmol/L), ZPI (56 nmol/L), and factor Va (20 nmol/L), were incubated in HSA at room temperature. After 3 minutes, a one-tenth volume of EDTA (100 mmol/L) or HS buffer was added to certain reactions, and the mixtures were transferred to a 37°C water bath. At various times thereafter, samples were removed for factor Xa assay and Western blot analysis with rabbit anti-ZPI antibodies. (A) Factor Xa activity. ■, factor Xa alone; ▪, factor Xa and factor Va; ●, factor Xa, PZ, and ZPI; ▾, factor Xa, factor Va, PZ, and ZPI; ▿, factor Xa, PZ, ZPI, with EDTA. (B) Anti-ZPI Western blots of reactions containing factor Xa, PZ, and ZPI. (C) Anti-ZPI Western blots of reactions containing factor Xa, factor Va, PZ, and ZPI. (D) Anti-ZPI Western blots of reactions containing factor Xa, PZ, and ZPI with EDTA.
Reversibility of the factor Xa–ZPI interaction.
Mixtures containing factor Xa (20 nmol/L), phospholipids (25 μmol/L), and CaCl2 (4 mmol/L), with and without PZ (80 nmol/L), ZPI (56 nmol/L), and factor Va (20 nmol/L), were incubated in HSA at room temperature. After 3 minutes, a one-tenth volume of EDTA (100 mmol/L) or HS buffer was added to certain reactions, and the mixtures were transferred to a 37°C water bath. At various times thereafter, samples were removed for factor Xa assay and Western blot analysis with rabbit anti-ZPI antibodies. (A) Factor Xa activity. ■, factor Xa alone; ▪, factor Xa and factor Va; ●, factor Xa, PZ, and ZPI; ▾, factor Xa, factor Va, PZ, and ZPI; ▿, factor Xa, PZ, ZPI, with EDTA. (B) Anti-ZPI Western blots of reactions containing factor Xa, PZ, and ZPI. (C) Anti-ZPI Western blots of reactions containing factor Xa, factor Va, PZ, and ZPI. (D) Anti-ZPI Western blots of reactions containing factor Xa, PZ, and ZPI with EDTA.
ZPI inhibition of factor XIa
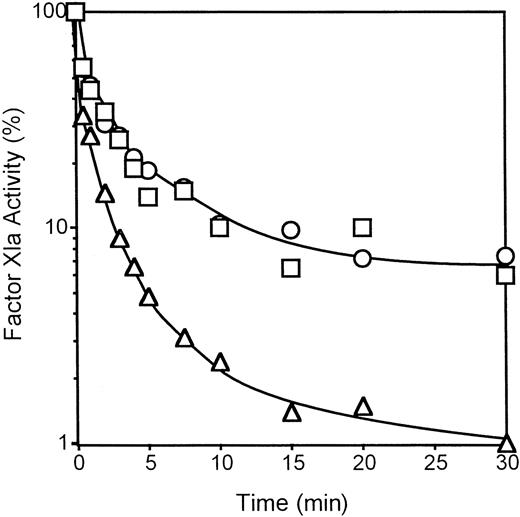
ZPI does not produce significant inhibition of thrombin, meizothrombin, factor VIIa, factor IXa, activated protein C, t-PA, u-PA, plasmin, trypsin, leukocyte elastase, chymotrypsin, or cathepsin G in the presence or absence of PZ, phospholipids, and Ca++.11 Additional studies showed that ZPI does not affect the activities of factor XIIa or kallikrein (not shown), but does inhibit factor XIa. Factor XIa inhibition by ZPI does not require the presence of PZ, phospholipids, or Ca++ and is not affected by the presence of high molecular weight kininogen (Figure 6, and not shown). Heparin (0.20 U/mL) increases the rate (t1/2 25 seconds vs 50 seconds) and the extent (99% vs 93%) of the factor XIa inhibition produced by ZPI (Figure 6).
Inhibition of factor XIa by ZPI.
Mixtures containing factor XIa (1 nmol/L), with or without high molecular weight kininogen (HK, 0.5 μmol/L), and with or without heparin (0.20 U/mL), were constructed in HSA at room temperature, and the reactions were initiated by the addition of ZPI (69 nmol/L). Remaining factor XIa activity was determined by amidolytic assay. ○, factor XIa alone; ■, factor XIa with HK; ▵, factor XIa with heparin.
Inhibition of factor XIa by ZPI.
Mixtures containing factor XIa (1 nmol/L), with or without high molecular weight kininogen (HK, 0.5 μmol/L), and with or without heparin (0.20 U/mL), were constructed in HSA at room temperature, and the reactions were initiated by the addition of ZPI (69 nmol/L). Remaining factor XIa activity was determined by amidolytic assay. ○, factor XIa alone; ■, factor XIa with HK; ▵, factor XIa with heparin.
Like the interaction of ZPI with factor Xa, the ZPI–factor XIa complex is not stable to SDS-PAGE, and the inhibition reaction produces cleavage of ZPI with a reduction in its apparent molecular weight from 72 kd to 68 kd (not shown). To determine the site in the reactive loop of ZPI that is involved in the inhibition of factor XIa, the peptide (4.2 kd) released following the interaction of ZPI with factor XIa was isolated following SDS-PAGE (see “Materials and methods). The N-terminal amino acid sequence of the peptide (SMPPVIKVDRPF) matched that of the peptide produced by the interaction of ZPI with factor Xa (above) and that following Y387 in the ZPI molecule.11 Moreover, the altered form of ZPI, rZPI(Y387A), does not inhibit factor XIa (not shown). Thus, the reactive center of ZPI that is involved in its inactivation of both factors Xa and XIa is Y387-S388 (P1-P1′).
ZPI is consumed during coagulation
The ZPI concentration in pooled normal human plasma was 3.84 μg/mL (53 nmol/L), but the serum produced following the induction of coagulation with kaolin, phospholipids, and CaCl2 contained little ZPI functional activity (less than 0.1 μg/mL). Western blot analysis of the plasma and serum produced by the induction of coagulation by kaolin, phospholipids, and Ca++ or tissue thromboplastin and Ca++ showed that ZPI was proteolytically cleaved during the clotting process with reduction in its apparent molecular weight from 72 kd to 68 kd (Figure7A; lanes 1, 2, 5). On the basis of Western analysis using anti-peptide antibodies directed against the N-terminus of ZPI, this cleavage occurs at the C-terminus of the molecule and appears similar to the one that occurs during ZPI's inhibitory interactions with factor Xa and factor XIa (not shown). In additional experiments, proteolysis of ZPI was detected following the induction of coagulation with kaolin, phospholipids, and Ca++ in plasma deficient in factor VII or prothrombin, and following the induction of coagulation with tissue thromboplastin and Ca++ in plasma deficient in factors XII, XI, IX, VIII, or V or prothrombin (not shown). ZPI degradation was not detected when Taipan snake venom, which produces direct prothrombin activation, was used to initiate coagulation in normal plasma (not shown).
Cleavage of ZPI during coagulation of plasma.
ZPI in plasma and serum samples (see “Materials and methods”) was immunoadsorbed with rabbit polyclonal anti-ZPI antibodies bound to Affigel and detected following SDS-PAGE by Western blotting with monoclonal anti-ZPI antibody (MC4249.2). (A) Lane 1: normal plasma; lane 2: normal plasma treated with kaolin, phospholipids, and Ca++; lane 3: normal plasma treated with kaolin alone; lane 4: factor XI–deficient plasma treated with kaolin alone; lane 5: normal plasma treated with tissue thromboplastin and Ca++; lane 6: factor XI–deficient plasma treated with tissue thromboplastin and Ca++. (B) Lane 1: factor X–deficient plasma; lane 2: factor X–deficient plasma treated with kaolin, phospholipids, and Ca++; lane 3: factor X–deficient plasma treated with tissue thromboplastin and Ca++; lane 4: barium adsorbed plasma (BAP) treated with XCP, phospholipids, and Ca++; lane 5: BAP supplemented with factor X treated with XCP, phospholipids, and Ca++; lane 6: BAP supplemented with factor X and PZ treated with XCP, phospholipids, and Ca++.
Cleavage of ZPI during coagulation of plasma.
ZPI in plasma and serum samples (see “Materials and methods”) was immunoadsorbed with rabbit polyclonal anti-ZPI antibodies bound to Affigel and detected following SDS-PAGE by Western blotting with monoclonal anti-ZPI antibody (MC4249.2). (A) Lane 1: normal plasma; lane 2: normal plasma treated with kaolin, phospholipids, and Ca++; lane 3: normal plasma treated with kaolin alone; lane 4: factor XI–deficient plasma treated with kaolin alone; lane 5: normal plasma treated with tissue thromboplastin and Ca++; lane 6: factor XI–deficient plasma treated with tissue thromboplastin and Ca++. (B) Lane 1: factor X–deficient plasma; lane 2: factor X–deficient plasma treated with kaolin, phospholipids, and Ca++; lane 3: factor X–deficient plasma treated with tissue thromboplastin and Ca++; lane 4: barium adsorbed plasma (BAP) treated with XCP, phospholipids, and Ca++; lane 5: BAP supplemented with factor X treated with XCP, phospholipids, and Ca++; lane 6: BAP supplemented with factor X and PZ treated with XCP, phospholipids, and Ca++.
Treatment of normal plasma, but not factor XI–deficient plasma, with kaolin alone produced partial cleavage of ZPI (Figure 7A; lanes 3-4). Thus, factor XIa appears to be responsible at least in part for the proteolysis of ZPI that occurs when coagulation is induced through contact activation by kaolin. Cleavage of ZPI appears complete in normal plasma induced to clot with kaolin or tissue thromboplastin (Figure 7A; lanes 2, 5), whereas only partial cleavage of ZPI occurs in factor X–deficient plasma in which coagulation is initiated with kaolin, phospholipids, and Ca++, and no degradation of ZPI is seen in factor X–deficient plasma induced to clot with tissue thromboplastin and Ca++ (Figure 7B; lanes 2, 3).
To further investigate the role of factor X and potentially PZ in the degradation of ZPI during coagulation, the XCP from Russell's Viper venom, phospholipids, and Ca++ were used to initiate coagulation in barium adsorbed plasma (BAP) with or without the addition of factor X and PZ (Figure 7B; lanes 4-6). No ZPI cleavage was detected in BAP alone, and only minimal ZPI cleavage occurred in BAP with added factor X. Complete ZPI cleavage, however, was found with the coagulation of BAP containing both factor X and PZ. In sum, the results suggest that factor Xa in concert with PZ is predominantly responsible for the consumption of ZPI that occurs when coagulation is initiated by means other than direct contact activation. With contact activation (eg, kaolin treatment), factor XIa also contributes significantly to ZPI degradation.
Discussion
The inhibition of a proteinase by a serpin involves the formation of an initial, reversible “encounter” interaction that proceeds to 1 of 2 alternative fates: (1) the production of a stable inhibitory complex or (2) an enzyme-substrate reaction with the production of cleaved inhibitor and free enzyme.17 The stable complex may also behave as a regular, albeit slow, enzyme-substrate reaction with the ultimate production of cleaved inhibitor and free enzyme. For certain enzyme-serpin interactions (eg, chymotrypsin-α2-antiplasmin), it has been shown that the inhibitory enzyme-serpin complex can dissociate to give active inhibitor and free enzyme.18 19
The stability of the factor Xa–ZPI inhibitory complex appears to be dramatically less than that of other enzyme-serpin complexes, eg, that of thrombin-antithrombin. In contrast to the thrombin-antithrombin interaction, the factor Xa–ZPI complex is not stable to SDS-PAGE (Figure 2), although it can be detected in the less denaturing conditions of alkaline-PAGE (Figure 3). Dissociation of the thrombin-antithrombin complex is very slow (approximately 2.5 × 10−6 seconds−1 at 37°) and appears to proceed exclusively through the cleavage of antithrombin.20 In the experiment depicted in Figure 6 in which the chelation of Ca++ (EDTA) was used to prevent factor Xa released from a factor Xa–ZPI complex from associating with PZ and interacting with additional ZPI, the initial rate of return of factor Xa activity is much more rapid (1.7 × 10−4seconds−1). This result is not due to a direct effect of PZ on the stability of the inhibitory factor Xa–ZPI complex, as previous studies have shown the same effect when high levels of the factor Xa chromogenic substrate Spectrazyme Xa (2.5 mmol/L), rather than EDTA, is added to the reactions.21 At this high concentration, the chromogenic substrate abrogates an interaction of the active site of the released factor Xa with ZPI (in the presence of PZ, phospholipids, and Ca++). From the present data, it cannot be determined whether the dissociation of factor Xa from ZPI is solely related to substratelike cleavage of ZPI, as is the case in the dissociation of thrombin from antithrombin,20 or whether true reversal of factor Xa–ZPI binding with the release of free factor Xa and active, uncleaved ZPI also occurs.
PZ serves as a critical protein cofactor for the inhibition of factor Xa by ZPI, enhancing the rate of factor Xa inhibition more than 1000-fold. Such enhancement of a serpin's inhibitory action by a protein cofactor has been described previously. Thrombomodulin increases the rate of thrombin inhibition by protein C inhibitor approximately 140-fold.22 This effect of thrombomodulin appears to depend primarily on the protein-protein interaction between thrombin and thrombomodulin.22 Vitronectin also serves as a protein cofactor, increasing the rate of thrombin inhibition by plasminogen activator inhibitor–1 (PAI-1) about 200-fold.23-25 Vitronectin appears to produce this enhancement by binding PAI-1 and inducing a conformational change at its reactive center and through a protein-protein interaction with thrombin as well.25-28 PZ appears to bind factor Xa in a Ca++-dependent manner at phospholipid surfaces,10 and preliminary data show that a portion of plasma ZPI circulates in a complex with PZ.29 Thus, it is likely that the enhancement in ZPI-mediated factor Xa inhibition produced by PZ involves interactions of PZ with both factor Xa and ZPI.
Besides inhibiting factor Xa in a PZ-, phospholipid-, and Ca++-dependent fashion, ZPI produces rapid factor XIa inhibition that does not require these cofactors. Whether this effect of ZPI is physiologically relevant is not yet known, but studies comparing the phenotypes of PZ- andZPI-gene–deleted mice may help address this issue. It is important to note, however, that an apparent interaction between factor XIa and ZPI is detectable in the plasma milieu, at least when relatively large quantities of factor XIa are produced through kaolin-induced contact activation (Figure 7A, lane 3; 7B, lane 2). Under these in vitro conditions, therefore, ZPI appears to compete effectively with the other factor XIa inhibitors (eg, α1-antitrypsin, C1 esterase inhibitor) and the substrate factor IX in plasma for the active site of factor XIa. As the interaction of factor XIa with ZPI produces cleaved, inactive ZPI, an additional effect of this interaction would be to reduce the level of active ZPI available for PZ-dependent factor Xa inhibition. The levels of factor XIa produced during normal, in contrast to kaolin-induced, coagulation of plasma are likely to be very low,30 however, suggesting that the potential reduction in ZPI activity produced by factor XIa could be physiologically important only at local sites of coagulation. On the other hand, the complete consumption of ZPI produced by factor Xa and PZ during tissue-factor–induced coagulation would prevent subsequent ZPI inhibition of factor XIa.
In the presence of PZ, ZPI inhibits not only factor Xa, but also factor Xa in the prothrombinase complex (Figure 4). PZ/ZPI inhibition of prothrombinase, however, is negated in the presence of plasma concentrations of prothrombin. Therefore, the anticoagulant action of PZ/ZPI must presumably precede the activation of factor V and/or perhaps follow the consumption of prothrombin at local sites. In reactions containing factor Xa, prothrombin, factor V, phospholipids, and Ca++, the combination of PZ and ZPI delays the initiation and the ultimate rate of thrombin generation (Figure 4). The results in this system that uses purified components are consistent with previous studies in an artificial plasma system that showed that the presence of PZ delays the initiation and reduces the rate and the final extent of thrombin generation when coagulation is initiated by small amounts of factor IXa.12 Moreover, the dampening of the coagulation process produced by PZ/ZPI is likely to be physiologically important as PZ deficiency dramatically enhances the thrombotic phenotype in mice with the factor VLeidengenotype.12
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
George J. Broze Jr, Division of Hematology, Barnes-Jewish Hospital of St Louis, 216 South Kingshighway Blvd, St Louis, MO 63110; e-mail: broze@im.wustl.edu.