Abstract
Tissue factor, which is expressed in vascular lesions, increases thrombin production, blood coagulation, and smooth muscle cell proliferation. We demonstrate that oxidized low-density lipoprotein (LDL) induces surface tissue factor pathway activity (ie, activity of the tissue factor:factor VIIa complex) on human and rat smooth muscle cells. Tissue factor messenger RNA (mRNA) was induced by oxidized LDL or native LDL; however, native LDL did not markedly increase tissue factor activity. We hypothesized that oxidized LDL mediated the activation of the tissue factor pathway via an oxidant-dependent mechanism, because antioxidants blocked the enhanced tissue factor pathway activity by oxidized LDL, but not the increased mRNA or protein induction. We separated total lipid extracts of oxidized LDL using high-performance liquid chromatography (HPLC). This yielded 2 major peaks that induced tissue factor activity. Of the known oxysterols contained in the first peak, 7α- or 7β-hydroxy or 7-ketocholesterol had no effect on tissue factor pathway activity; however, 7β-hydroperoxycholesterol increased tissue factor pathway activity without induction of tissue factor mRNA. Tertiary butyl hydroperoxide also increased tissue factor pathway activity, suggesting that lipid hydroperoxides, some of which exist in atherosclerotic lesions, activate the tissue factor pathway. We speculate that thrombin production could be elevated via a mechanism involving peroxidation of cellular lipids, contributing to arterial thrombosis after plaque rupture. Our data suggest a mechanism by which antioxidants may offer a clinical benefit in acute coronary syndrome and restenosis.
Introduction
Tissue factor is a 47-kd transmembrane cell surface protein that forms a complex with factor VII, initiating blood coagulation and leading to the local production of thrombin via the successive activation of factor IX or factor X and prothrombin.1-4 Studies using in situ hybridization and immunohistochemistry reveal significant tissue factor expression in cells of mesenchymal lineage within atherosclerotic lesions5 and in vascular smooth muscle cells after mechanical injury.6 The presence of tissue factor in coronary atherectomy specimens has been correlated with unstable angina,7 as has increased tissue factor expression in circulating blood monocytes in patients with unstable angina and acute myocardial infarction.8-10 These data indirectly support the hypothesis that the tissue factor is responsible for thrombosis in acute coronary syndrome.
We have recently demonstrated in vitro that native low-density lipoprotein (LDL) induces tissue factor messenger RNA (mRNA) and cell surface protein in smooth muscle cells without an increase in cell surface tissue factor pathway activity,11 defined in our assay system as the enzymatic activity of the tissue factor:factor VIIa complex of the blood coagulation cascade. We have also recently identified elements of the tissue factor promoter required for tissue factor gene induction by native and oxidized LDL.12 Interestingly, surface tissue factor protein made in response to LDL led to increased tissue factor pathway activity only after activation of the pathway by hydrogen peroxide, demonstrating a novel oxidative mechanism for regulating smooth muscle cells surface tissue factor pathway activity.11 Our observations on the induction of tissue factor by LDL in vitro may be related to the recent finding that in the atheroma of cholesterol-fed rabbits, where lipoproteins and oxidant-producing phagocytes are known to accumulate, tissue factor protein expression correlated with plasma cholesterol levels.13
We hypothesized that oxidized LDL alters the thrombogenicity of an atherosclerotic lesion via a tissue factor–dependent, oxidant-mediated mechanism. In this study, we evaluated in vitro the effects of oxidized LDL, a known component of atherosclerotic lesions,14 on smooth muscle cell surface tissue factor pathway activity and determined whether these effects were altered by antioxidants. From our results, we propose that oxidized LDL may alter multiple thrombosis-related events by 2 distinct and novel actions. Like native LDL, oxidized LDL induces tissue factor gene expression, synthesis, and presence on smooth muscle cell surfaces,12but unlike native LDL, oxidized LDL also can activate the tissue factor pathway on the smooth muscle cell surface. Our results further demonstrate that antioxidants blunt the second of these steps, the increased tissue factor pathway activity, but not the induction of gene expression. The activation of the cell surface tissue factor pathway by oxidized LDL can be linked to specific classes of oxidized lipids.
Materials and methods
Tissue culture
Smooth muscle cells were prepared from explants of excised aortas of Sprague Dawley rats or samples of human aorta obtained from transplant donors using procedures analogous to those previously described.15 Cultures from passages 4 to 10 were used in these studies. Twenty-four–well plates were seeded with approximately 25 000 cells per well in DMEM containing 10% fetal bovine serum (FBS). One day after seeding, the media was changed to serum-free DMEM after being washed twice with phosphate-buffered saline (PBS). All cells were in serum-free DMEM for 48 hours before the addition of the agonist of interest as previously described.11 16 In select experiments, cells were placed in DMEM containing 2% FBS, instead of serum-free DMEM, for the 48 hours before the addition of the agonist. Also in select experiments, (described in lipoprotein isolation and oxidation, below) cells were placed in DME/F12 on the day of plating, and then for the 48 hours before and after the addition of lipoproteins.
In those experiments involving antioxidant pretreatment, antioxidants (N, N′-diphenyl-1,4-phenylene-diamine, DPPD from Aldrich Chemical Co, Milwaukee, WI; Tiron, α-tocopherol, and deferoxamine from Sigma Chemical, St Louis, MO; ebselen from Cayman Chemical Co, Ann Arbor, MI) were added overnight immediately before the end of the 48-hour serum-free pretreatment. Antioxidants were also present during the exposure to oxidized LDL, native LDL, or control solvent. The α-tocopherol and ebselen were added in ethanol to a final ethanol concentration of 0.5%. Solvent concentration was matched in selected controls. In experiments using cycloheximide to block protein synthesis, cycloheximide (20 μmol/L) was added 30 minutes before and during the addition of lipoprotein or lipoprotein extract.
Lipoprotein isolation and oxidation
Human LDL was isolated from citrated plasma by differential ultracentrifugation between solvent density limits of 1.019 to 1.063 as previously described.17 EDTA was present (0.5 mmol/L) throughout the isolation procedure. Quality of all preparations was checked by assaying endotoxin level (less than 0.15 endotoxin units/mL) (Whittaker Bioproducts kit No. QCL-1000), electrophoretic mobility (Corning, Corning, NY) and thiobarbituric acid reactivity.18,19 Preparations were indexed by total cholesterol (Boehringer Mannheim Diagnostics kit No. 236691; [Indianapolis, IN]) and total protein20 measurements. Native LDL preparations were stored in 0.5 mM EDTA at 4°C until use.
LDL (4 to 8 mg cholesterol/ml) was oxidized during dialysis at 4°C or 22°C against isotonic saline without EDTA, pH 7.4, containing 3 μmol/L CuS04 or 6 μmol/L FeSO4 for up to 2 days, as previously described.18,21 No difference was noted in the tissue factor response of aortic smooth muscle cells to LDL oxidized using either CuS04 or FeSO4 (data not shown). During oxidation LDL underwent a characteristic color change from golden to yellow to translucent as previously reported.22 Numerous oxidized LDL preparations were used in these experiments. The ranges of typical oxidation levels for LDL using these oxidation protocols have been previously reported:18,21,23 Thiobarbituric acid reactivities are 5 to 10 nmol of malondialdehyde (as standard) per milligram LDL cholesterol and total lipid peroxide contents, 190 to 1200 nmol/mg LDL cholesterol. Preparations used in this study were within these ranges. After modification, oxidized LDL samples were assayed for cholesterol and/or protein, dialyzed against 0.5 mmol/L EDTA in isotonic saline, pH 8 to 9, and stored at 4°C until use. A less oxidized preparation of oxidized LDL was made by exposing LDL to smooth muscle cells in culture medium that contained higher amounts of metal ions than DMEM, ie, a mixture of DMEM and Ham F12 (DME/F12: Fe(NO3)3-50 μg/L, FeSO4-417 μg/L, CuSO4-0.13 μg/L vs DMEM: Fe(NO3)3-100 μg/L). We have previously characterized this preparation of oxidized LDL.24
Total lipid extracts were obtained and separated using high-performance liquid chromatography (HPLC), as described previously.25Briefly, 5 mg aliquots of either native or oxidized LDL were lyophilized overnight and extracted in acetone. The total lipid extracts were dried under nitrogen and redissolved in isopropanol/acetonitrile, 1:1 (vol/vol). The extracts were separated using reverse phase HPLC (Waters μBondapak C18 preparative column [Milford, MA]) over 1 hour using a 5 mL/min flow rate. The initial solvent elution gradient was water/acetonitrile, 1:1 (vol/vol), which was increased over 5 minutes to acetonitrile (100%), and then changed over 45 minutes to isopropanol (100%). Isopropanol (100%) was continued for the final 10 minutes. Fractions were collected every 3 minutes. Each fraction was then dried under nitrogen and stored at −70°C. Lipids in each fraction were then dissolved in acetone:ethanol, 1:1 (vol/vol) just before adding the lipids to the cell layers.
The 7β-hydroperoxycholesterol was synthesized by incubating cholesterol with soybean lipoxidase and ethyl lineoleate, as previously prescribed.26 Isolation, purification, and quantification was achieved by sequential HPLC steps, as reported previously.25 26 Other oxysterols were obtained commercially (Steraloids Inc, Wilton, NH).
SnCl2 reduction of total lipid extracts
Lipid hydroperoxides contained in total lipid extracts of lipoprotein preparations were reduced using SnCl2.27 Approximately 5 mg (protein) native or oxidized LDL were treated with SnCl2 (10 mmol/L final concentration) for 2 minutes at room temperature. Lipoproteins were treated with ethanol to serve as a control. The lipoproteins were then lyophilized, extracted in chloroform:methanol, aliquoted, and dried under nitrogen and then resuspended in ethanol:acetone (1:1) before use.
Tissue factor assay
Cell surface tissue factor pathway activity was assessed using a 2-step amidolytic assay previously described.28 After each well was washed twice with PBS, a reaction mixture containing 0.5 mL of phenol red-free M199, 50 μL of 2 mg/mL S-2222 (Pharmacia-ATPAR, Piscataway, NJ), and 20 μL of Proplex T (containing 1 unit of factor VII, plus factor X; Baxter Biotech, Glendale, CA) was added to each well. Standards containing the same reaction mixture with varying amounts of rabbit brain thromboplastin (Sigma) were also prepared. One milliunit (mU) of tissue factor activity was defined as the amount of activity contained in 1 μL of resuspended rabbit brain thromboplastin after a 1:10 dilution. The reaction mixture remained on cell layers for approximately 20 minutes. Aliquots of the media were pipetted into 96-well plates and read along with the standards on a spectrophotometer at 405 nm. The tissue factor activity in each well was then calculated using the standard curve.
Northern hybridization
Total cellular RNA was extracted by the guanidine isothiocyanate–cesium chloride method from rat arterial smooth muscles cells grown in 100-mm dishes.29 Samples of total RNA (10 μg) were separated on a 1% agarose/2.2 mol/L formaldehyde gel and subsequently blotted onto Magna nylon membranes with 20 × SSC by capillary transfer, according to previously published methods.30 The RNA was cross-linked to the membrane with an ultraviolet cross-linker (Stratagene, La Jolla, Ca). The blots were prehybridized for 2 to 6 hours at 42°C in 50% formamide, 1% SDS, 5 × SSC, 1 × Denhardt solution (0.02% Ficoll, 0.02% bovine serum albumin, 0.02% polyvinylpyrrolidone), 0.25 mg/mL denatured salmon sperm, and 50 μmol/L sodium phosphate (pH 6.5) and then hybridized with 2 × 106 cpm/mL of (α-32P) dCTP radiolabeled complementary DNA (cDNA) plasmid probe at 42°C for 16 to 24 hours. After hybridization, blots were washed with 0.1% SDS, 2 × SSC for 30 minutes at 65°C, followed by 2 washes with 0.1% SDS, 0.1 × SSC for 30 minutes at 65°C. The blots were then exposed to XAR-5 x-ray film with intensifying screens at −70°C. Expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA was used as an internal control for the quantity of total RNA on each lane of the gel and this control was applied in all experiments.
Western blot analysis
Total cellular protein was obtained from human smooth muscle cells grown in 100-mm dishes. At the end of each incubation cell layers were washed twice with PBS and protein was extracted in ice-cold RIPA buffer, containing protease inhibitors (leupeptin, PMSF, pepstatin; Sigma). Cellular DNA was removed by collecting the supernatant after centrifugation at 10 000 rpm in a microcentrifuge for 10 minutes. The total protein concentration in each sample was determined using bovine serum albumin as a standard.31 SDS-polyacrylamide gel electrophoresis using 10% acrylamide gels was performed with each lane loaded with 50 μg protein per sample under denaturing conditions. Broad range and low range molecular weight standards (Sigma) were run in parallel. Proteins were transferred to membrane paper using 2 mA/cm2 of current. Membranes were blocked with 5% milk powder for 30 minutes, followed by 45 minutes of primary antibody (0.5 μg/mL rabbit antihuman tissue factor, American Diagnostics, Greenwich, CT) in PBS, containing 5% milk powder and 0.1% Tween, then washed 3 times in PBS and 0.1% Tween for 10 minutes. A 1:5000 dilution of a peroxidase-labeled secondary antibody was then applied (Goat antirabbit IgG; Boehringer Mannheim) in 5% milk powder and 0.1% Tween for 30 minutes. The blots were then washed an additional 3 times. The signal was developed with exposure to film for 1 to 3 minutes using ECL (Amersham, Buckinghamshire, England).
Quantification of surface tissue factor protein levels was also performed using Western blot analysis, as previously described.11 After the 4-hour treatment with a defined agonist, cell layers were incubated with rabbit-antihuman tissue IgG antibody at 4°C for 60 minutes. Cell layers were harvested, as described previously. Cell lysate (50 μg protein per treatment) was incubated with Protein-A sepharose beads to immunoprecipitate IgG bound tissue factor.11 Immunoprecitates were then separated on 10% SDS-PAGE gels, as described previously.
Results
Effect of oxidized low-density lipoprotein on tissue factor messenger RNA and surface tissue factor pathway activity
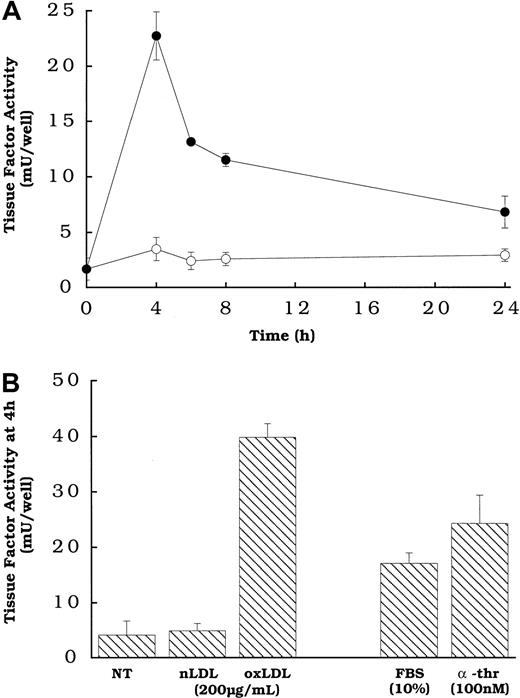
Figure 1A shows that the rat aortic smooth muscle cells treated with oxidized LDL, but not native LDL exhibited a transient increase in the expression of cell surface tissue factor pathway activity, with peak activity occurring at approximately 4 hours. The transience of the response to oxidized LDL, and the time to reach peak tissue factor pathway activity, are similar to those observed for smooth muscle cells exposed to other agonists.16 The level of cell surface tissue factor activity induced by oxidized LDL was comparable to that induced by 2 agonists previously shown to induce tissue factor gene expression and activity in smooth muscle cells (Figure 1B), FBS and α-thrombin,16 at concentrations of the agonists that we determined to be optimal (data not shown).
Oxidized LDL-induced tissue factor pathway activity as a function of time and compared with other known agonists at 4 hours in smooth muscle cells.
(A) Oxidized LDL (●) or native LDL (○), 150 μg cholesterol/mL, was added to rat aortic smooth muscle cells at various times before measuring tissue factor pathway activity. (B) Serum and α-thrombin or lipoprotein was added to wells of rat aortic smooth muscle cells at the specified concentrations 4 hours before tissue factor assay. NT indicates no treatment (control). All cells had been in serum-free DMEM for 48 hours at the time of assay. Data represent means ± SD of 4 wells per treatment. Data are from an experiment representative of multiple experiments.
Oxidized LDL-induced tissue factor pathway activity as a function of time and compared with other known agonists at 4 hours in smooth muscle cells.
(A) Oxidized LDL (●) or native LDL (○), 150 μg cholesterol/mL, was added to rat aortic smooth muscle cells at various times before measuring tissue factor pathway activity. (B) Serum and α-thrombin or lipoprotein was added to wells of rat aortic smooth muscle cells at the specified concentrations 4 hours before tissue factor assay. NT indicates no treatment (control). All cells had been in serum-free DMEM for 48 hours at the time of assay. Data represent means ± SD of 4 wells per treatment. Data are from an experiment representative of multiple experiments.
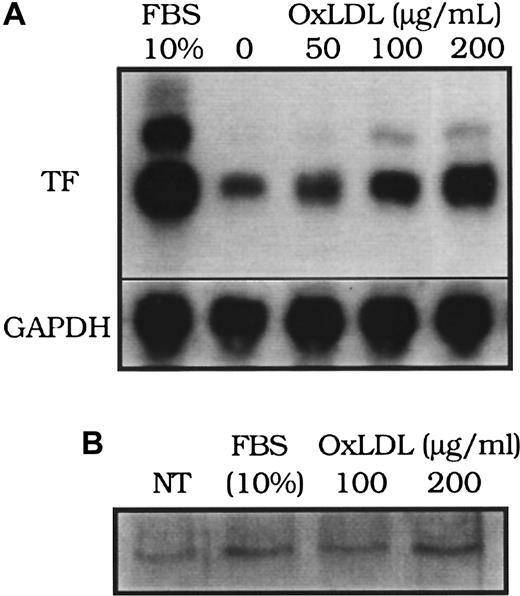
The induction of tissue factor mRNA in response to oxidized LDL was concentration dependent (Figure 2A). Peak tissue factor mRNA induction occurred at approximately 90 minutes after exposure to either native or oxidized LDL, as previously reported12 (data not shown), which is also similar to the time course of tissue factor mRNA increases in smooth muscle cells exposed to other agonists, including FBS.16 Interestingly, although the peak levels of tissue factor pathway activity induced by serum were comparable to those induced by oxidized LDL (Figure 1B), mRNA levels achieved after serum treatment were significantly higher than those after oxidized LDL treatment (Figure 2A). Immunoprecipitation of tissue factor protein on the cell surface analyzed by Western blot analysis revealed that the surface tissue factor protein was elevated above control levels after 4 hours of FBS or oxidized LDL stimulation (Figure 2B).
Effect of oxidized LDL on tissue factor mRNA and protein.
(A) Tissue factor (TF) mRNA from rat aortic smooth muscle cells was assessed by Northern blot analysis 90 minutes after the addition of fetal bovine serum (FBS, 10%), or oxidized LDL at 0, 50, 100, and 200 μg cholesterol/mL. (B) Tissue factor surface protein immunoprecipitated from 50 μg of cell lysate of human aortic smooth muscle cells was assessed by Western blot analysis 4 hours after “no treatment” (NT; control), FBS (10%), or oxidized LDL (100 or 200 μg cholesterol/mL). Glyceraldehye 3-phosphate dehydrogenase (GAPDH) was assessed as an internal control to confirm equal loading in each lane for Northern blot analysis.
Effect of oxidized LDL on tissue factor mRNA and protein.
(A) Tissue factor (TF) mRNA from rat aortic smooth muscle cells was assessed by Northern blot analysis 90 minutes after the addition of fetal bovine serum (FBS, 10%), or oxidized LDL at 0, 50, 100, and 200 μg cholesterol/mL. (B) Tissue factor surface protein immunoprecipitated from 50 μg of cell lysate of human aortic smooth muscle cells was assessed by Western blot analysis 4 hours after “no treatment” (NT; control), FBS (10%), or oxidized LDL (100 or 200 μg cholesterol/mL). Glyceraldehye 3-phosphate dehydrogenase (GAPDH) was assessed as an internal control to confirm equal loading in each lane for Northern blot analysis.
The protocol that we adapted for assessing agonist induction of tissue factor pathway activity included a 48-hour serum-free pretreatment before the addition of agonist.16 To evaluate the effects of this serum-free period on our results, we compared cells pretreated in serum-free DMEM with those in serum (2% FBS) for the 48 hours before the addition of lipoprotein. We observed similar levels of surface tissue factor mRNA induction at 90 minutes and surface tissue factor pathway activity at 4 hours in response to oxidized LDL in serum-free and 2% FBS conditions (data not shown). Furthermore, native LDL-induced tissue factor mRNA levels at 90 minutes were similar with either serum-free DMEM or FBS (2%) pretreatments (data not shown), and native LDL did not significantly induce tissue factor pathway activity whether cells were maintained in 2% FBS or serum-free DMEM.
Role of lipid peroxidation in oxidized low-density lipoprotein activation of cell surface tissue factor pathway
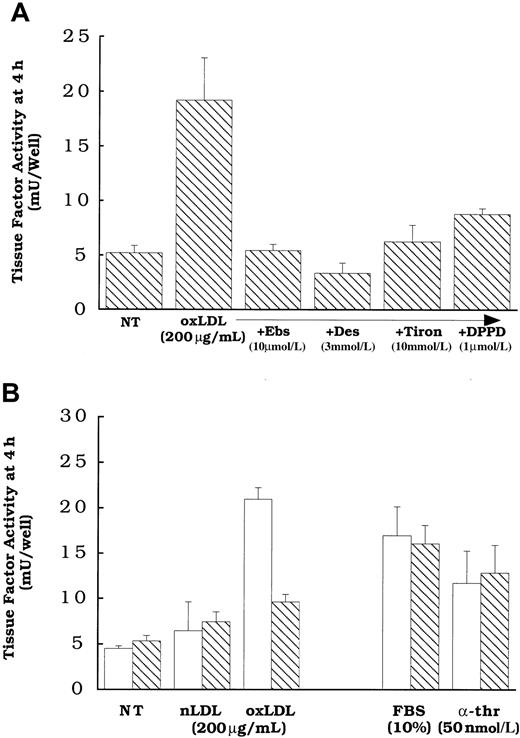
Because we have previously shown that oxidized LDL could induce cellular lipid peroxidation,22,26 and that oxidant stress in the form of exogenous H2O2 can enhance latent cell surface tissue factor pathway activity,11 we asked whether cellular lipid peroxidation induced by oxidized LDL is responsible for the activation of the tissue factor pathway involving latent cell surface tissue factor protein. Cells were pretreated overnight and during a 4-hour oxidized LDL treatment with multiple antioxidants (Figure 3) with different inhibitory actions, including N, N′-diphenyl-1,4-phenylene-diamine (DPPD) (1 μmol/L) or α-tocopherol (25 μmol/L), peroxyl radical scavengers;32 ebselen (10 μmol/L), an agent that reduces complex lipid hydroperoxides;33 or Tiron (10 mmol/L) or desferoximine (3 mmol/L), iron chelators that are active in cells or at cell membranes.34 35 Cells treated with these antioxidants were inhibited from the increased surface tissue factor pathway activity elicited by oxidized LDL (Figure 3A,B). Pretreatment of the cells with α-tocopherol or DPPD was required to achieve inhibition, because the presence of equivalent concentrations solely during the 4 hours of oxidized LDL exposure did not inhibit the activation of the tissue factor pathway (data not shown). Supporting the hypothesis that lipoproteins affect tissue factor activity by a novel pathway that is distinct from that of other known agonists, α-tocopherol had no effect on the induction of surface tissue factor pathway activity by α-thrombin or FBS (Figure 3B). Although antioxidants blocked the induction of surface tissue factor pathway activity, they did not inhibit the increases in tissue factor mRNA (Figure4A) or whole cell tissue factor protein expression (Figure 4B) induced in response to oxidized LDL.
Effect of antioxidant treatments on oxidized LDL induction of surface tissue factor pathway activity.
Oxidized LDL or native LDL (200 μg protein/mL), α-thrombin (50 nmol/L), or FBS (10%) was added to rat aortic smooth muscle cells for 4 hours before tissue factor assessment. (A) Cells were pretreated with DPPD (1 μmol/L), ebselen (Ebs, 10 μmol/L), Tiron (10 mmol/L), or desferoximine (Des, 3 mmol/L). (B) Cells were pretreated with ethanol alone (0.5%, ■) or vitamin E (25 μmol/L, ▧). All antioxidant treatments were overnight before and during the addition of the agonists. NT indicates no treatment (solvent control). All cells received 0.5% ethanol at the time of treatment with antioxidants to control for those antioxidants that required ethanol as a carrier. Data represent means ± SD of 4 wells per treatment.
Effect of antioxidant treatments on oxidized LDL induction of surface tissue factor pathway activity.
Oxidized LDL or native LDL (200 μg protein/mL), α-thrombin (50 nmol/L), or FBS (10%) was added to rat aortic smooth muscle cells for 4 hours before tissue factor assessment. (A) Cells were pretreated with DPPD (1 μmol/L), ebselen (Ebs, 10 μmol/L), Tiron (10 mmol/L), or desferoximine (Des, 3 mmol/L). (B) Cells were pretreated with ethanol alone (0.5%, ■) or vitamin E (25 μmol/L, ▧). All antioxidant treatments were overnight before and during the addition of the agonists. NT indicates no treatment (solvent control). All cells received 0.5% ethanol at the time of treatment with antioxidants to control for those antioxidants that required ethanol as a carrier. Data represent means ± SD of 4 wells per treatment.
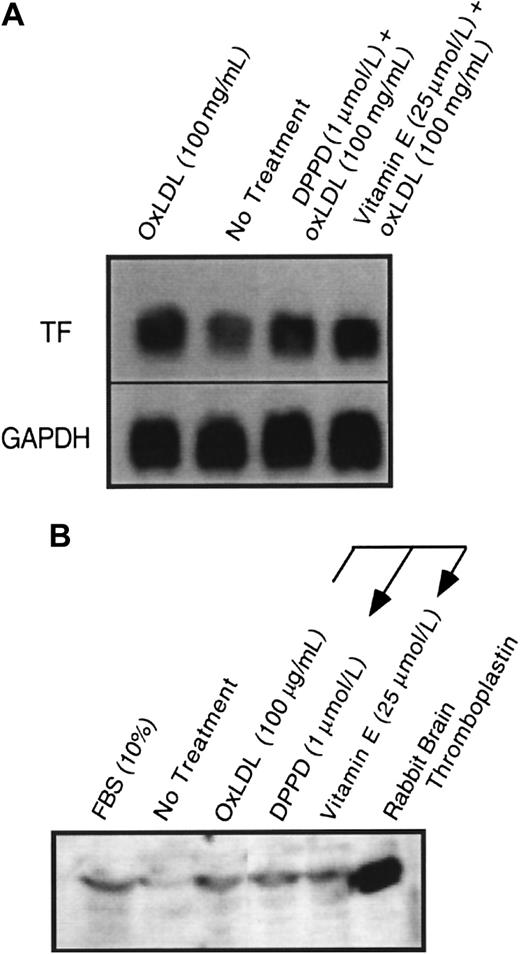
Effect of α-tocopherol and DPPD pretreatment on oxidized LDL induction of tissue factor mRNA and protein.
Tissue factor (A) mRNA by Northern blot at 90 minutes and (B) total cellular protein by Western blot at 4 hours was assessed after the addition of oxidized LDL at 100 μg protein/mL to plates of (A) rat or (B) human aortic smooth muscle cells. Cells were pretreated with either α-tocopherol (25 μmol/L) or DPPD (1 μmol/L) for 24 hours before the addition of oxidized LDL. GAPDH was assessed as an internal control to confirm equal loading in each lane for Northern blot analysis.
Effect of α-tocopherol and DPPD pretreatment on oxidized LDL induction of tissue factor mRNA and protein.
Tissue factor (A) mRNA by Northern blot at 90 minutes and (B) total cellular protein by Western blot at 4 hours was assessed after the addition of oxidized LDL at 100 μg protein/mL to plates of (A) rat or (B) human aortic smooth muscle cells. Cells were pretreated with either α-tocopherol (25 μmol/L) or DPPD (1 μmol/L) for 24 hours before the addition of oxidized LDL. GAPDH was assessed as an internal control to confirm equal loading in each lane for Northern blot analysis.
Multiple lipids in oxidized low-density lipoprotein induce surface tissue factor pathway activity
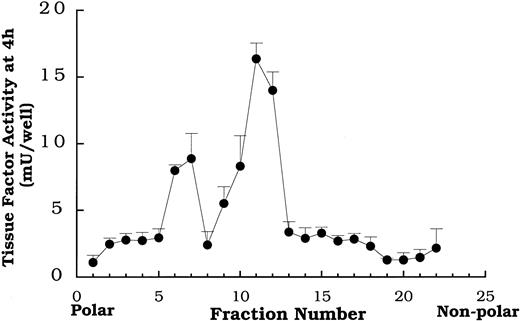
Total lipid extracts of oxidized LDL were separated by HPLC on a C-18 column and collected in 22 fractions. The data in Figure5 depict a typical tissue factor activity profile obtained by placing each of the 22 fractions on smooth muscle cells and measuring activity 4 hours later. Fractions 7 and 11-12 reproducibly yielded distinct peaks of tissue factor activity in the profiles from the lipid fractions of isolates from multiple preparations of oxidized LDL. Native LDL lipid fractions separated on HPLC failed to enhance tissue factor pathway activity (data not shown).
Tissue factor pathway activity at 4 hours induced by serial fractions of oxidized LDL lipids separated by reverse phase HPLC.
Fractions obtained from HPLC separation of the total lipid extract from oxidized LDL were added to rat aortic smooth muscle cells at 400 μg protein equivalents/mL 4 hours before tissue factor pathway assay. Data represent means ± SD of 4 wells per treatment. Data are from an experiment representative of multiple experiments.
Tissue factor pathway activity at 4 hours induced by serial fractions of oxidized LDL lipids separated by reverse phase HPLC.
Fractions obtained from HPLC separation of the total lipid extract from oxidized LDL were added to rat aortic smooth muscle cells at 400 μg protein equivalents/mL 4 hours before tissue factor pathway assay. Data represent means ± SD of 4 wells per treatment. Data are from an experiment representative of multiple experiments.
We focused attention on fraction 7, because we had previously identified several oxysterols that elute in it.26 The data in Figure 6 demonstrate that DPPD, tiron, ebselen, and desferoximine all inhibited the activation of the surface tissue factor pathway by the lipids contained in fraction 7 as they had for oxidized LDL. We know from prior studies in our laboratory that fraction 7 from this HPLC protocol contains, among other lipids, 7β-hydroperoxycholesterol,25,26 7β- and 7α-hydroxycholesterol, and 7-ketocholesterol.26
Effect of antioxidant treatments on fraction 7 induction of surface tissue factor pathway activity.
Fraction 7 (see Figure 5) at a level equivalent to 400 μg protein of intact oxidized LDL per milliliter was added to rat aortic smooth muscle cells for 4 hours before tissue factor assessment. Cells were pretreated with either DPPD (1 μmol/L), ebselen (10 μmol/L), Tiron (10 mmol/L), or desferoximine (3 mmol/L) overnight before and during the addition of fraction 7. NT indicates no treatment (solvent) control. Data represent means ± SD of 4 wells per treatment.
Effect of antioxidant treatments on fraction 7 induction of surface tissue factor pathway activity.
Fraction 7 (see Figure 5) at a level equivalent to 400 μg protein of intact oxidized LDL per milliliter was added to rat aortic smooth muscle cells for 4 hours before tissue factor assessment. Cells were pretreated with either DPPD (1 μmol/L), ebselen (10 μmol/L), Tiron (10 mmol/L), or desferoximine (3 mmol/L) overnight before and during the addition of fraction 7. NT indicates no treatment (solvent) control. Data represent means ± SD of 4 wells per treatment.
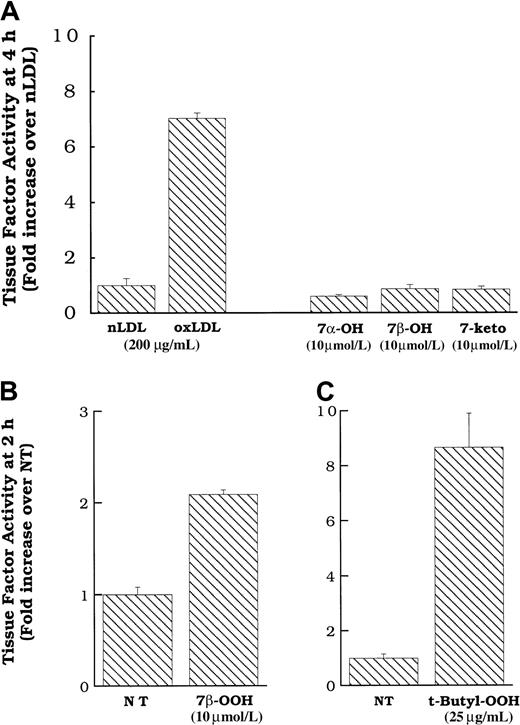
We directly added pure preparations of these oxysterols to smooth muscle cells in an attempt to determine whether they activated the tissue factor pathway on the smooth muscle cell surface. Figure7A shows that commercial preparations of 7α- and 7β-hydroxycholesterol, and 7-ketocholesterol, at concentrations approximating those in active levels of oxidized LDL,26 failed to induce surface tissue factor pathway activity in smooth muscle cells. In contrast, 7β-hydroperoxycholesterol did induce surface tissue factor protein expression at 2 hours (Figure 7B) and 4 hours (data not shown). Because only the hydroperoxide was active, we tested the effects of another hydroperoxide, t-butyl hydroperoxide (not found on oxidized LDL) at various times. The t-butyl hydroperoxide induced surface tissue factor pathway activity at 2 hours (Figure 7C) and 4 hours, with a peak at 2 hours (data not shown), similar to that which we observed previously for hydrogen peroxide.11 As we have previously demonstrated for hydrogen peroxide, neither t-butyl hydroperoxide nor 7β-hydroperoxycholesterol induced tissue factor mRNA expression (data not shown). However, similar to oxidized LDL and fraction 7, the induction of smooth muscle cell surface tissue factor pathway activity by t-butyl hydroperoxide and 7β-hydroperoxycholesterol was inhibited by treatment with multiple antioxidants (data not shown).
Cell surface tissue factor pathway activity in response to 7α- or 7β-hydroxycholesterol, 7-ketocholesterol, 7β-hydroperoxycholesterol, or t-butyl hydroperoxide.
(A) Oxidized LDL, native LDL (200 μg cholesterol/mL), 7α- or 7β-hydroxycholesterol (10 μmol/L) or 7-ketocholesterol (10 μmol/L) was added to rat aortic smooth muscle cells, and surface tissue factor pathway activity was measured 4 hours later. 7β-Hydroperoxycholesterol (10 μmol/L, B) or t-butyl hydroperoxide (25 μg/mL, C) was added to rat aortic smooth muscle cells, and surface tissue factor pathway activity was measured 2 hours later. Data represent means ± SD of 4 wells per treatment. Data are from experiments representative of multiple experiments at 2 and 4 hours.
Cell surface tissue factor pathway activity in response to 7α- or 7β-hydroxycholesterol, 7-ketocholesterol, 7β-hydroperoxycholesterol, or t-butyl hydroperoxide.
(A) Oxidized LDL, native LDL (200 μg cholesterol/mL), 7α- or 7β-hydroxycholesterol (10 μmol/L) or 7-ketocholesterol (10 μmol/L) was added to rat aortic smooth muscle cells, and surface tissue factor pathway activity was measured 4 hours later. 7β-Hydroperoxycholesterol (10 μmol/L, B) or t-butyl hydroperoxide (25 μg/mL, C) was added to rat aortic smooth muscle cells, and surface tissue factor pathway activity was measured 2 hours later. Data represent means ± SD of 4 wells per treatment. Data are from experiments representative of multiple experiments at 2 and 4 hours.
To verify the importance of the contribution of lipid hydroperoxides on the activation of the surface tissue factor pathway by oxidized LDL, we exposed rat aortic smooth muscle cells to SnCl2-treated total lipid extracts of native and oxidized LDL. SnCl2treatment results in the reduction of lipid hydroperoxides to their hydroxy-equivalents.27 Furthermore, SnCl2partitions to the aqueous phase during lipid extraction, thus it is not present when the lipid extract is added to the cells. SnCl2-treated oxidized LDL resulted in approximately 65% less tissue factor pathway activation than untreated oxidized LDL (data not shown).
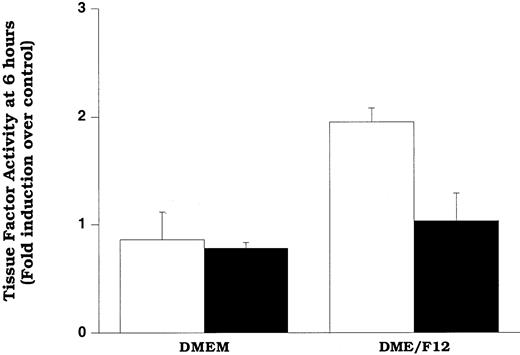
In an attempt to assess the extent of LDL oxidation that is required for induction of increased tissue factor pathway activity, we oxidized LDL relatively mildly by incubating it with smooth muscle cells for 6 hours in a culture medium containing DME/F12 (described in “Materials and methods”). In the other experiments described in this study, we used DME medium, devoid of copper and with minimal iron, to minimize spurious oxidation of LDL in experiments in which we wished to examine the effects of native LDL on smooth muscle cells. However, previously we showed that exposure of LDL to smooth muscle cells in DME/F12 facilitated smooth muscle cell oxidation of LDL.24 At relatively short times of exposure (eg, 6 hours), the oxidation was mild (minimal changes in thiobarbituric acid reactivity or electrophorectic mobility).24 The data in Figure8 show that cells exposed to LDL for 6 hours in DME/F12 exhibited significantly elevated levels of surface tissue factor pathway activity compared with those exposed to LDL in DME. Our data suggest that the increased activity in the cells exposed to DME/F12 was due to lipid hydroperoxides, because ebselen treatment significantly decreased tissue factor activity in the cells. Confirming that only mild oxidation of LDL occurred at 6 hours (as predicted by previous results),24 LDL recovered from these wells had the same electrophoretic mobility as LDL not exposed to cells (data not shown).
Mildly oxidized LDL can induce surface tissue factor pathway activity.
Cells in DMEM or DME/F12 were treated with LDL (200 μg protein/mL) for 6 hours before assessment of tissue factor pathway activity. Cells were pretreated with solvent (control, ■) or ebselen (10 μmol/L, ▪) overnight before and during the addition of LDL. Data represent fold induction over DMEM, lipoprotein-free control. DMEM and DME/F12 lipoprotein-free controls yielded identical tissue factor pathway activity. Data represent means ± SD of 4 wells per treatment.
Mildly oxidized LDL can induce surface tissue factor pathway activity.
Cells in DMEM or DME/F12 were treated with LDL (200 μg protein/mL) for 6 hours before assessment of tissue factor pathway activity. Cells were pretreated with solvent (control, ■) or ebselen (10 μmol/L, ▪) overnight before and during the addition of LDL. Data represent fold induction over DMEM, lipoprotein-free control. DMEM and DME/F12 lipoprotein-free controls yielded identical tissue factor pathway activity. Data represent means ± SD of 4 wells per treatment.
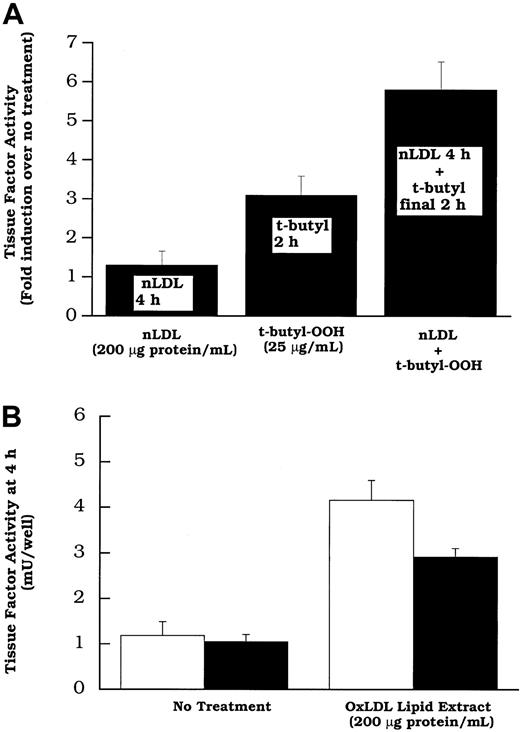
Because native and oxidized LDL both increased tissue factor mRNA, but 7β-hydroperoxycholesterol (up to 20 μmol/L) did not, it appeared that an unknown lipid (or lipids) in both native LDL and oxidized LDL, or perhaps a lipid common to both, induced the gene and that this action is distinct from lipid hydroperoxide activation. The concept of separate lipids inducing the tissue factor gene and activating its pathway was tested further in 2 ways. (1) We treated cells with native LDL to induce new tissue factor protein production11 before the addition of a lipid hydroperoxide “activator” of tissue factor pathway. The data in Figure9A reveal that more tissue factor pathway activity is observed in cells treated with native LDL before the addition of t-butyl hydroperoxide than in cells treated with t-butyl hydroperoxide alone. This shows that a lipid hydroperoxide is capable of “activating” the latent surface tissue factor pathway, including that involving tissue factor synthesized in response to native LDL. (2) The data in Figure 9B demonstrate that oxidized LDL induces surface tissue factor pathway activity even when new protein synthesis is decreased by the addition of cycloheximide. These data together are consistent with the concept that oxidized LDL increases surface tissue factor activity in 2 distinct steps: (1) increasing surface tissue factor protein and (2) activating the cell surface tissue factor pathway involving preexisting latent tissue factor protein. The lipoprotein component responsible for (1) is unknown; 7β-OOH cholesterol is one of the oxidized LDL components capable of (2).
t-Butyl hydroperoxide can activate the tissue factor pathway involving new synthesized protein induced by native LDL; oxidized LDL lipid extracts can activate surface tissue factor without new protein synthesis.
(A) Rat aortic smooth muscle cells were treated without or with LDL (200 μg protein/mL). Two hours later t-butyl hydroperoxide (25 μg/mL) was added to the wells of the groups indicated. After an additional 2 hours, surface tissue factor activity was assayed in all wells. (B) Total lipid extracts of oxidized LDL, at a concentration equivalent to 200 μg protein/mL of intact lipoprotein were added to rat aortic smooth muscle cell layers. Some were treated with cycloheximide (20 μmol/L; filled bars) 30 minutes before, and concurrent with lipid treatment. Tissue factor pathway activity was measured after 2 hours of lipid treatment. Data represent means ± SD of 4 wells per treatment.
t-Butyl hydroperoxide can activate the tissue factor pathway involving new synthesized protein induced by native LDL; oxidized LDL lipid extracts can activate surface tissue factor without new protein synthesis.
(A) Rat aortic smooth muscle cells were treated without or with LDL (200 μg protein/mL). Two hours later t-butyl hydroperoxide (25 μg/mL) was added to the wells of the groups indicated. After an additional 2 hours, surface tissue factor activity was assayed in all wells. (B) Total lipid extracts of oxidized LDL, at a concentration equivalent to 200 μg protein/mL of intact lipoprotein were added to rat aortic smooth muscle cell layers. Some were treated with cycloheximide (20 μmol/L; filled bars) 30 minutes before, and concurrent with lipid treatment. Tissue factor pathway activity was measured after 2 hours of lipid treatment. Data represent means ± SD of 4 wells per treatment.
Discussion
In this study, we have demonstrated that, like native LDL, oxidized LDL increases tissue factor protein expression on the surface of smooth muscle cells (Figure 2B); however, in contrast to native LDL, oxidized LDL also significantly increases tissue factor pathway activity on the cell surfaces (Figure 1A). Furthermore, we have demonstrated that the cellular pathway by which oxidized LDL increases tissue factor pathway activity on the cell surface is distinct from that reported for other agonists (Figure 3B), in that oxidative stress appears to be a required step in a mechanism in which the cell surface tissue factor complex exhibits increased activity. Finally, our data show that there are multiple lipids borne by oxidized LDL that enhance tissue factor pathway activity in smooth muscle cells. One of the lipids formed during LDL oxidation, which could be responsible for part of the activation of the tissue factor pathway by oxidized LDL, is 7β-hydroperoxycholesterol. That this may be a more general property of hydroperoxides is indirectly suggested by our findings that nonhydroperoxide oxysterols were ineffective in enhancing tissue factor pathway activity (Figure 7A), t-butyl hydroperoxide also activated the tissue factor pathway (Figure 7C), reduction of oxidized LDL lipids by SnCl2 significantly decreased the oxidized LDL tissue factor pathway activating capacity, and, as shown previously, hydrogen peroxide activated the tissue factor pathway.11
Our data further demonstrate that there exists a second peak of less polar lipids in oxidized LDL (fractions 11-12, Figure 5) that can also induce surface tissue factor pathway activity. Reduction of the total lipid extract of oxidized LDL by SnCl2 did not completely block the induction of surface tissue factor activity, which suggests there may be nonhydroperoxide lipids in oxidized LDL that are capable of inducing surface tissue factor pathway activity. However, because the increased cell surface tissue factor pathway activity induced by oxidized LDL was inhibitable by multiple antioxidants, it is likely that these other nonhydroperoxide lipids also activate the tissue factor pathway via inducing lipid peroxidation. Experiments are underway to identify more of the multiple lipids of oxidized LDL that activate tissue factor and to evaluate their relative potencies and mechanisms of action.
Our findings could have important pathophysiologic implications, because tissue factor is expressed in mesenchymal cells of vascular lesions5,7 and in smooth muscle cells after vascular injury,6 and because oxidized lipoproteins36and their constituents, including 7β-hydroperoxycholesterol,25 37 have been demonstrated in atherosclerotic lesions. If oxidized LDL in vivo induces tissue factor pathway activity, it could result in thrombosis as well as smooth muscle cell proliferation and thus contribute to atherosclerotic lesion growth, plaque rupture, or intimal smooth muscle cell proliferation observed in some instances of restenosis after angioplasty.
The pathway of tissue factor induction by lipoproteins in smooth muscle cells is at least in part distinct from that observed in endothelial cells and macrophages.38-43 Drake et al40reported an increase in tissue factor activity and mRNA accumulation in response to oxidized, but not native LDL in human umbilical vein endothelial cells. Pigeon macrophages in culture have been shown to have enhanced expression of tissue factor in response to oxidized LDL.41 Petit et al42 showed a significant increase in cell surface tissue factor activity in human monocyte–derived macrophages in response to cholesterol and oxidized LDL without an increase in cell surface–associated tissue factor protein. Brand et al,43 using human adherent monocyte cultures, found that neither native nor oxidized LDL altered tissue factor protein expression or activity; however, they found that oxidized LDL potentiated tissue factor expression induced by LPS. Despite the differing mechanisms by which oxidized LDL influences tissue factor expression in these various cell types, it is clear from these studies and ours that oxidized LDL can increase surface tissue factor pathway activity in endothelial cells, monocyte-derived macrophages, and smooth muscle cells, all of which exist in atherosclerotic lesions in close proximity to oxidized lipids.
That oxidized LDL, but not native LDL exposure, resulted in increased surface tissue factor pathway activity and antioxidants inhibited it (Figure 3A,B), suggests that oxidative stress causes activation of the tissue factor pathway, involving preexisting tissue factor protein on the cell surface that, before oxidant stress, could not participate in active complex formation. It has been proposed previously that tissue factor may exist in such a latent form on cell surfaces, and that can be induced to initiate tissue factor pathway activity.4Possible mechanisms proposed for tissue factor pathway latency on the cell surface, which could be potentially reversed by oxidized lipids include (1) sequestration of tissue factor in membrane crypts or caveolae;44,45 (2) surface tissue factor interaction with tissue factor pathway inhibitor;45-47 (3) changes in the quaternary structure of tissue factor on the cell surface; or (4) changes in cell membrane outer leaflet (eg, increased phosphatidylserine content) that enhances tissue factor:factor VIIa activity by, for example, increasing factor X associating with the membrane.48
The exact mechanism of surface tissue factor pathway activation by oxidant stress is unclear from these experiments; however, a general scheme of the propagation of lipid peroxidation could include (1) an initial interaction of cells with lipoprotein-borne lipid hydroperoxides, (2) reduction of lipid hydroperoxides by cellular iron to form alkoxyl radicals, and (3) formation of lipid and peroxyl radical intermediates leading to propagation of peroxidation of cellular lipids22,49 and tissue factor pathway activation. This interpretation is compatible with a report that copper transported via the carrier 8-hydroxyquinoline into human THP-1 monocytes caused cellular lipid peroxidation and significantly increased tissue factor pathway activity.50 Furthermore, Brisseau et al51 demonstrated that LPS-induced tissue factor pathway activity in macrophages and monocytes was inhibited by the antioxidants, N-acetyl-cysteine and pyrrolidine dithiocarbamate, with no decrease in LPS-induced tissue factor mRNA. It is likely that the mechanism of oxidant-induced activation of the tissue factor pathway involves cell surface or extracellular events because we have previously shown that H2O2-induced activation of tissue factor did not require the cytoplasmic tail of tissue factor.11
Our results suggest that oxidized lipoproteins, known to reside in vascular lesions,36 could be stimulants of tissue factor pathway activity in vivo. One of the oxidized LDL constituents, 7β-hydroperoxycholesterol, that can enhance tissue factor pathway activity, is also present in atherosclerotic tissue.25 37The lipid peroxidation responsible for tissue factor pathway activation is antioxidant inhibitable (Figures 3 and 5). Thus, tissue factor pathway activation by lipid hydroperoxides could explain ways in which antioxidants may inhibit atherosclerosis, acute coronary syndrome, and restenosis after angioplasty.
Acknowledgments
We thank Dr Robert D. Rosenberg, Massachusetts Institute of Technology, for encouragement and helpful discussions and Dr Scott Colles and Charles Kaul for the preparation of 7β-hydroperoxycholesterol.
Supported in part by National Institutes of Health grant HL 29582. M.S.P. is a recipient of a NRSA from the National Institutes of Health (HL 09911). M.-Z.C. is a recipient of a Scientist Development Grant from the American Heart Association (Dallas) (9730039N).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Guy M. Chisolm, Lerner Research Institute, NC10, Cleveland Clinic Foundation, 9500 Euclid Ave, Cleveland, OH 44195; e-mail:chisolg@ccf.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal