Abstract
Multicentric Castleman disease (MCD) is a distinct type of lymphoproliferative disorder associated with inflammatory symptoms and interleukin-6 (IL-6) dysregulation. In the context of human immunodeficiency virus (HIV) infection, MCD is associated with human herpesvirus 8 (HHV8) infection. In a prospective study of 23 HIV-infected patients with MCD, clinical symptoms of MCD were present at 45 visits, whereas patients were in chemotherapy-induced clinical remission at 50 visits. Symptoms were associated with a high level of serum C reactive protein, high HHV8 viral load in peripheral blood mononuclear cells, and high plasma human IL-6 and IL-10 levels. Strong correlations between plasma IL-6 and plasma IL-10 with the HHV8 viral load suggest that both cytokines may be involved in the pathogenesis of this virus-associated lymphoproliferative disorder.
Introduction
The eighth human herpesvirus (HHV8) has been identified in a limited subset of lymphoproliferative disorders.1,2 Among these disorders are primary effusion lymphoma (PEL) and multicentric Castleman disease (MCD).3,4 MCD is characterized by lymphadenopathy with angiofollicular hyperplasia and plasma cell infiltration.5In the context of human immunodeficiency virus (HIV) infection, MCD may present as a distinct lymphoproliferative disorder, and the most effective therapy remains low-dose chemotherapy.6Virtually all HIV-positive cases of MCD and nearly 50% of HIV-negative cases are infected with HHV8.4,7 The dysregulated production of human interleukin 6 (huIL-6) has been considered as a pivotal factor in the pathogenesis of the disease,8,9 and the in vivo neutralization of huIL-6 by an anti–huIL-6 or anti–IL-6 receptor antibody alleviates systemic manifestations of MCD.10-12 A viral homologue of IL-6 (HHV8–vIL-6) exhibits many of the biological activities of huIL-6.13-15Furthermore, when expressed constitutively in mice, vIL-6 can induce symptoms similar to that observed in human MCD.15Immunohistochemical and in situ hybridization studies have shown that all HHV8-positive MCD tissues express high levels of vIL-6.7,16 Of note, the distribution of vIL-6– and huIL-6–positive cells in MCD tissues was nonoverlapping. The vIL-6-positive cells were plasmablastic HHV8-infected cells located in the mantle zone, whereas most huIL-6–positive cells were in the germinal centers.7,9,16,17 Interleukin 10 (IL-10) has also been shown to serve as a growth factor for acquired immunodeficiency syndrome (AIDS)-related B-cell lymphoma18,19 and to be produced by PELs HHV8-infected cell lines.20,21 In a previous report22 on a limited number of HIV-infected patients with MCD, changes in HHV8 viral load was associated with treatment response. We, therefore, evaluated plasma levels of IL-6, IL-10, and C reactive protein (CRP) in MCD by using 95 plasma samples from AIDS-MCD patients during the course of the illness. Results show that, in patients with MCD, clinical symptoms correlated with high levels of plasma human IL-6 and IL-10, accompanied by an average 1.7-log increment of HHV8 copy numbers in peripheral blood mononuclear cells (PBMCs).
Patients, materials, and methods
Twenty-three HIV-infected patients with histologically proven Castleman disease were followed in a single institution over a maximum 25-month period. At each visit, clinical symptoms were recorded. DNA extracted from PBMCs and plasma samples were collected and stored frozen. CD4 count was evaluated on fresh blood samples and CRP levels were analyzed from fresh serum samples. In parallel, 12 HIV-infected patients with asymptomatic HHV8 infection were also evaluated within a single visit.
A clinical attack of MCD was defined as the rapid onset of fever, splenomegaly and/or lymphadenopathy, sometimes associated with upper respiratory tract symptoms and peripheral edema, without evidence of acute infection.6 Clinical remission was defined by the absence of clinical symptoms and the absence of organomegaly.
HHV8 quantitative viral load evaluation in PBMCs
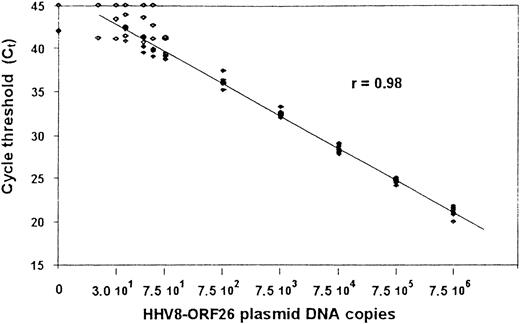
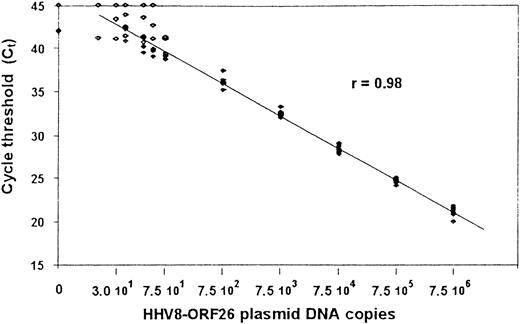
From 1 μg DNA extracted from PBMCs, quantitative polymerase chain reaction (PCR) detection of HHV8 was performed by using a Taqman technique derived from Kennedy et al.23 All samples were tested for amplification, using primers specific for β-globin. The primer set was designed in viral orf 26 (nt position: 47 318-337 and 47 401-422) and the internal probe (nt positions: 47 369-399) was synthesized and dual-labeled with fluorescent reporter dye (FAM) at the 5′ end and quencher dye (TAMRA) at the 3′ end (PerkinElmer, Courtabœuf, France). Quantitative PCR assay was performed on ABI PRISM sequence detection system 7700, using components supplied in the Taqman core Reagent kit (PerkinElmer). A reaction volume of 50 μL contained 5 μL of 10 × buffer; 15 pmoles of each primer; 5 mmol/L MgCl2 (optimal concentration); 200 mmol/L of dATP, dCTP, and dGTP; 1.25 U AmpliTaq Gold; 0.5 U AmpErase Uracil N-glycosylate (UNG); 10 pmoles of dual-labeled probe; and 1 μg DNA (5 μL) of each tested sample. Thermal cycling was initiated with 2 minutes of incubation at 50°C, followed by 10 minutes of denaturation at 95°C, and then 45 cycles of 95°C for 15 seconds and 60°C for 1 minute. A standard curve was based on PCRs performed twice in duplicate by using DNA copy numbers ranging from 1 to 7.5 × 106 copies calculated from a relevant cloned plasmid containing the same Kaposi sarcoma (KS)-associated herpesvirus sequence (KS 330 233). The cycle threshold values were plotted against given copy numbers and show a linear amplification between 30 and 7.5 × 106 copies with a coefficient of variation below 2% (Figure1).
Standard curve for quantitative PCR assays for HHV8 ORF26 gene.
Shown are the Ct values from 4 assays at which a given input of HHV8 DNA-containing plasmid was detected by PCR. Plasmid concentrations tested ranged from 1 to 7.5 × 106 copies per reaction mixture. A linear amplification was obtained for values between 30 and 7.5 × 106 copies. The Spearman rank coefficient of correlation (R) is given.
Standard curve for quantitative PCR assays for HHV8 ORF26 gene.
Shown are the Ct values from 4 assays at which a given input of HHV8 DNA-containing plasmid was detected by PCR. Plasmid concentrations tested ranged from 1 to 7.5 × 106 copies per reaction mixture. A linear amplification was obtained for values between 30 and 7.5 × 106 copies. The Spearman rank coefficient of correlation (R) is given.
Cytokine levels
Plasma samples were collected at the same time as the PBMCs used for the HHV8 quantitative PCR evaluation at each visit and were kept frozen for evaluation of cytokine levels. The quantitative determination of huIL-6 in plasma samples was performed with an enzyme immunoassay kit (Immunotech, Marseille, France). To test the specificity for huIL-6, a vIL-6 fusion protein that has an amino terminal tag of maltose-binding protein and amino acids 22-204 of vIL-6 (MBPvIL-6)15 was applied. No positive reaction was observed at a range between 40 and 3000 pg/mL of MBPvIL-6, corresponding to a range between 13 and 1000 pg/mL of vIL-6 (Y.A. et al, manuscript submitted). The quantitative determination of human IL-10 was performed with an enzyme immunoassay kit (Diaclone, Besançon, France).
Statistical analysis
Statistical analysis was performed by using Statview software. The Mann-Whitney U test was used for comparisons of CRP level, HIV and HHV8 viral load, and huIL-6 and IL-10 plasma levels according to the presence or absence of clinical symptoms. The Spearman correlation test was used to analyze the correlations between the following covariates: HHV8 viral load, plasma HIV-RNA, CD4 cell count, serum CRP, and plasma huIL-6 and IL-10.
Results
Patients
The 23 patients were followed for a total of 104 visits (1 to 15 per patient). However, complete data were not available for 9 visits, and only 95 visits were used for analysis. An analysis performed on the 104 visits with some missing values showed no significant differences. Demographic features of the patients are shown in Table1. Most patients were receiving intermittent low-dose chemotherapy, vinblastine or etoposide, every 2 to 3 weeks, at the onset of recurrent clinical symptoms. A clinical attack of MCD was present at 45 visits, whereas chemotherapy-induced clinical remission was noted in 50 visits. Twelve HHV8-seropositive HIV-infected male patients with asymptomatic HHV8 infection were used as control subjects (Table 2). Eleven of them were receiving antiretroviral therapy and 8 of 12 had a plasma HIV RNA level below 200 copies/ml. We were not able to pick control subjects with more matched CD4 cell counts and HIV loads. This inability may suggest that HHV8-infected patients with a low CD4 cell count and/or uncontrolled HIV viral replication quickly become symptomatic for HHV8 infection. Informed consent was required, and the study was approved by the institutional review board.
HHV8 viral load of PBMCs, cytokine levels, plasma HIV-RNA, and CD4 cell count
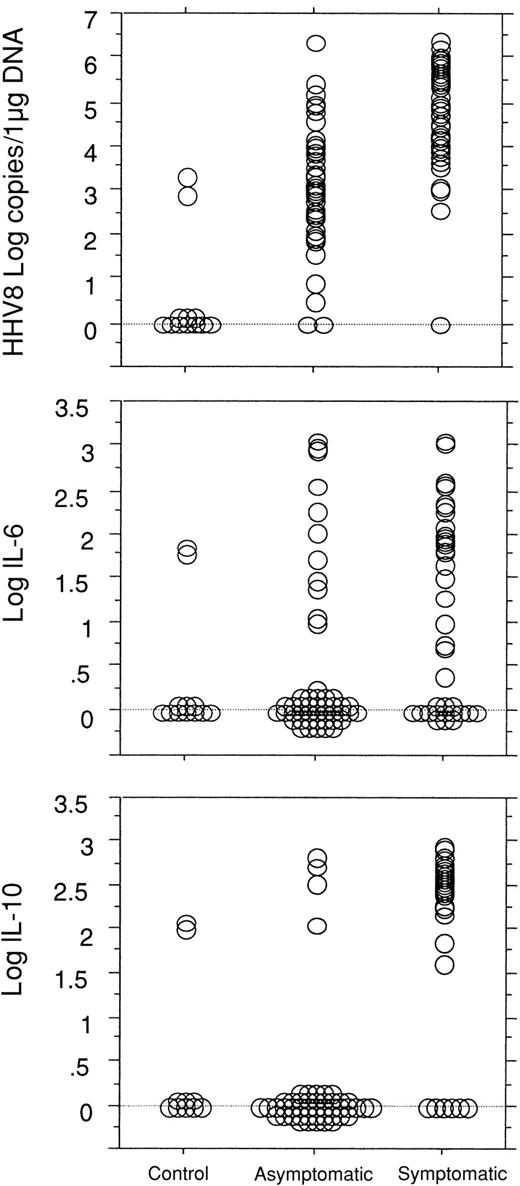
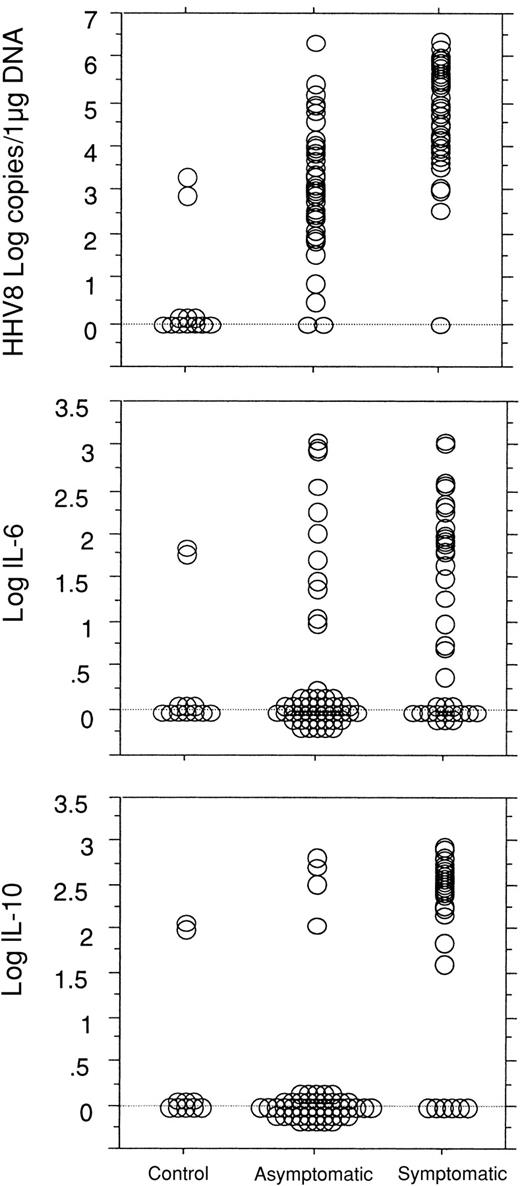
The presence of clinical symptoms of MCD was strongly associated with high HHV8 viral load in the PBMCs with a mean HHV8 viral load level of 4.77 (± 1.32) log copies/μg DNA in symptomatic patients and of 3.04 (± 1.34) log copies/μg DNA in asymptomatic patients (P < .0001). In comparison, only 2 of the 12 HHV8-seropositive control subjects had a detectable low level HHV8 DNA in the PBMCs (2.91 and 3.34 log copies, respectively). Clinical symptoms were also associated with high serum CRP levels with mean values of 2.12 (± 0.24) log mg/L versus 0.97 (± 0.38) log mg/L in asymptomatic patients (P < .0001), high plasma IL-6 levels with mean values of 1.25 (± 1.04) log pg/mL versus 0.51 (± 0.99) log pg/mL in asymptomatic patients (P < .0008), and high plasma IL-10 levels with mean values of 2.16 (± 0.97) log pg/mL versus 0.31 (± 0.86) log pg/mL in asymptomatic patients (P < .0001) (Figure 2).
Comparisons between the 3 groups of patients.
Comparisons between control HHV8-seropositive HIV-infected patients (n = 12, 1 visit/patient) and the HIV-infected patients with MCD (n = 23) in asymptomatic remission of the disease (50 visits) and with active symptomatic disease (45 visits) show a significant difference in the quantitative detection of HHV8 DNA copy numbers in PBMCs between the 3 groups (P < .0001) and a significant difference between the asymptomatic and the symptomatic groups in the level of plasma IL-6 (P = .0008) and IL-10 (P < .0001).
Comparisons between the 3 groups of patients.
Comparisons between control HHV8-seropositive HIV-infected patients (n = 12, 1 visit/patient) and the HIV-infected patients with MCD (n = 23) in asymptomatic remission of the disease (50 visits) and with active symptomatic disease (45 visits) show a significant difference in the quantitative detection of HHV8 DNA copy numbers in PBMCs between the 3 groups (P < .0001) and a significant difference between the asymptomatic and the symptomatic groups in the level of plasma IL-6 (P = .0008) and IL-10 (P < .0001).
Human IL-6 and IL-10 were both detectable in plasma from 29 of the 45 symptomatic patients, in plasma from only 2 of the 50 asymptomatic patients, and in 1 of the 12 control patients. A statistical link between IL-6 and IL-10 levels could be demonstrated in the patients with MCD (P < .0001).
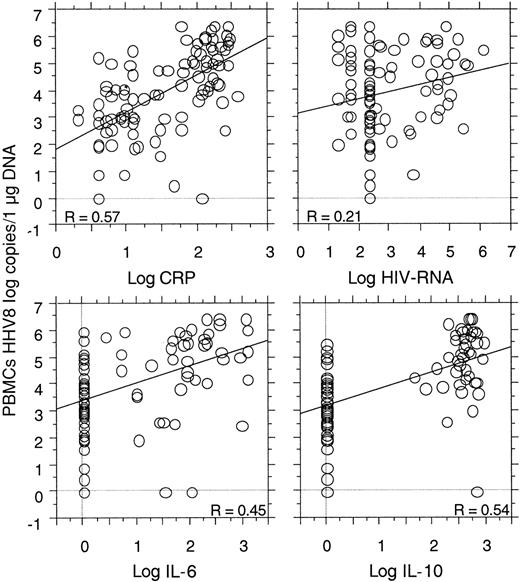
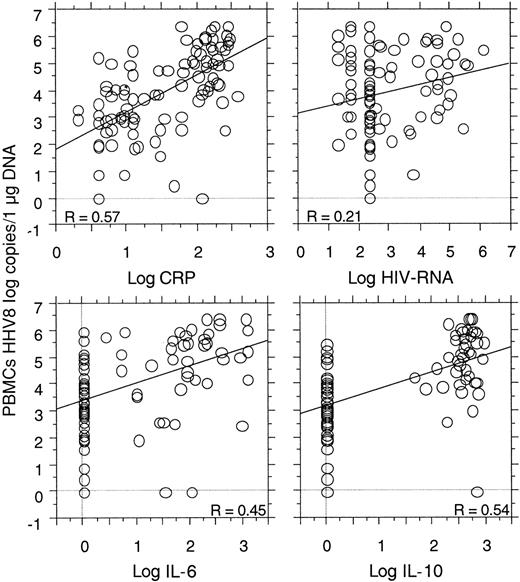
Strong correlations were shown between the HHV8 copy number in the PBMCs and the levels of serum CRP (P < .0001), plasma IL-6 (P = .0001), and plasma IL-10 (P < .0001). No correlation was found between the HHV8 copy numbers and the CD4 cell count (49 visits, P = .73). A correlation between plasma HIV-RNA and plasma IL-10 (P = .0002) and serum CRP (P = .03) was demonstrated, as well as a possible correlation between plasma HIV-RNA and HHV8 copy numbers (P = .08) (Figure3). No correlation was found between plasma HIV-RNA and plasma IL-6 (P = .31).
Correlations between the quantitative detection of HHV8 DNA copy numbers in PBMCs with the level of serum CRP (P < .0001), plasma IL-6 (P < .0001), and plasma IL-10 (P < .0001) in 23 HIV-infected patients (95 visits) with MCD.
A possible correlation between HHV8 copy numbers and plasma HIV-RNA was also noted (P = .08).
Correlations between the quantitative detection of HHV8 DNA copy numbers in PBMCs with the level of serum CRP (P < .0001), plasma IL-6 (P < .0001), and plasma IL-10 (P < .0001) in 23 HIV-infected patients (95 visits) with MCD.
A possible correlation between HHV8 copy numbers and plasma HIV-RNA was also noted (P = .08).
When the analysis was performed according to the presence or absence of KS, we found very similar results in both groups, except for the HHV8 viral load that was slightly higher in patients with MCD and KS [4.07 (± 1.36) log copies/μg DNA] than in patients with MCD alone: [3.57 (± 1.84) log copies/μg DNA; P = .35]. This result was true for the asymptomatic patients [3.23 (± 1.14) versus 2.76 (± 1.62) log copies/μg DNA] as well as for the symptomatic patients [4.99 (± 0.92) versus 4.56 (± 1.58) log copies/μg DNA]. The correlations between the HHV8 viral load and the cytokines and CRP levels remained statistically significant in both groups. The correlations between the HHV8 viral load and the HIV-RNA viral load reached statistical significance in the group of patients with MCD alone (P = .002), whereas no correlation was found in the group with both MCD and KS (P = .84). The absence of correlation between the HHV8 viral load and the CD4 cell count was confirmed in each group.
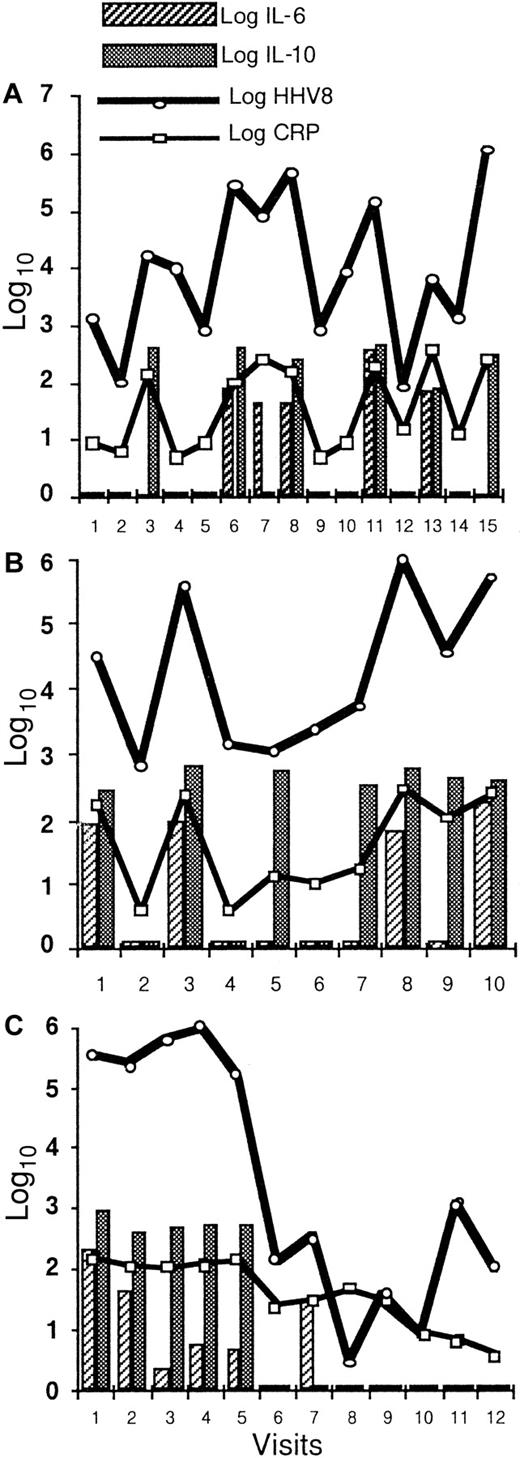
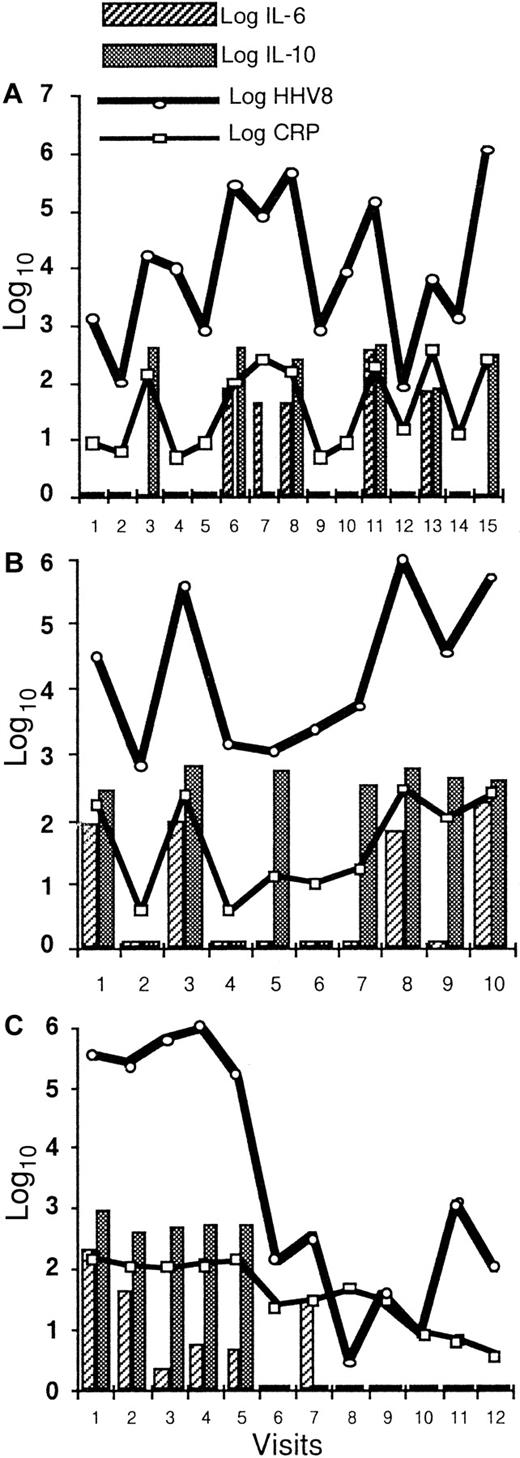
The longitudinal study of 7 patients with 5 visits or more demonstrates the close correlation between the serum CRP, plasma IL-6, plasma IL-10, and HHV8 viral load in the PBMCs (Figure4). High levels of these covariates were associated with clinical symptoms of the disease.
Longitudinal evaluation of HHV8 DNA copy numbers in PBMCs, serum CRP, plasma IL-6, and plasma IL-10.
Longitudinal evaluation of HHV8 DNA copy numbers in PBMCs, serum CRP, plasma IL-6, and plasma IL-10 in 3 patients shows a strong correlation between these 4 covariates in patient 2 (A) within 15 visits over a 25-month period and in patient 13 (B) within 10 visits over a 24-month period. Panel (C) shows a dramatic decrease in all biological markers during the first 4 weeks following the initiation of chemotherapy in patient 3.
Longitudinal evaluation of HHV8 DNA copy numbers in PBMCs, serum CRP, plasma IL-6, and plasma IL-10.
Longitudinal evaluation of HHV8 DNA copy numbers in PBMCs, serum CRP, plasma IL-6, and plasma IL-10 in 3 patients shows a strong correlation between these 4 covariates in patient 2 (A) within 15 visits over a 25-month period and in patient 13 (B) within 10 visits over a 24-month period. Panel (C) shows a dramatic decrease in all biological markers during the first 4 weeks following the initiation of chemotherapy in patient 3.
Discussion
HHV8-associated MCD is a new type of virus-associated lymphoproliferative disorder. huIL-6 and IL-10 may be involved as B-cell growth factors and be directly implicated in the pathogenesis and symptoms of the disease. Indeed, the present series shows that high plasma levels of both cytokines were associated with a 1- to 2-log increment of the circulating HHV8 copy numbers during clinical attacks of the disease. The HHV8 viral load observed during remission was in the same range as what was observed in 11 patients with active KS (data not shown), whereas during clinical attacks of MCD, the high HHV8 viral load suggests either a burst of circulating HHV8+ cells and/or active replication of the virus. However, the exact respective role of IL-6 and IL-10 in the pathogenesis of MCD remains to be determined as both cytokines may be involved in the genesis of the lesions and symptoms as well as secondary to the proliferation. In contrast to what has been observed in patients with KS,24the HHV8 viral load did not correlate with the CD4 cell count but was shown to be higher in patients with uncontrolled HIV-viral replication. In vitro experiments and transgenic models have shown that HIV-derived proteins can activate a number of cellular genes.25,26 The HIV transactivator protein Tat has been reported to promote expression of huIL-6 and huIL-10 in lymphoid cells.26 Here, we found a statistically significant correlation between HIV-RNA load and huIL-10 levels in plasma from patients with HIV-associated MCD. The same association has been observed in AIDS-PEL effusions.27 These findings suggest that the aggressive clinical course observed in these lymphoproliferative disorders may be due to viral protein-mediated enhancement of cellular cytokine transcription that stimulates the proliferation and differentiation of B-lineage cells. However, MCD may develop and progress as an independent devastating and fatal disorder in patients with undetectable plasma HIV-RNA, relatively high CD4 cell counts, and sometimes KS in remission.
Recent data have shown that, in MCD lymph nodes, the infected cells are monotypic lambda plasmablastic cells, suggesting that MCD may be considered as a microscopic plasmablastic lymphoma that can sometimes progress toward aggressive non-Hodgkin lymphoma.17Therefore, clinical attacks may be associated with expansion and circulation of these plasmablastic cells and/or active HHV8 replication in these cells.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Eric Oksenhendler, Service d'Immuno-Hématologie, Hôpital Saint-Louis, 1 Ave Claude Vellefaux, 75010, Paris, France; e-mail:oksenhendler@chu-stlouis.fr.