Abstract
A randomized trial was carried out comparing cyclosporin A (CsA) and short-term methotrexate (MTX) versus CsA alone for graft versus host disease (GVHD) prophylaxis in patients with severe aplastic anemia (SAA) undergoing allogeneic bone marrow transplantation (BMT) from a compatible sibling. Seventy-one patients (median age, 19 years; range, 4-46 years) were randomized to receive either CsA and MTX or CsA alone for the first 3 weeks after BMT. Subsequently, both groups received CsA orally, with gradual drug reduction until discontinuation 8 to 12 months after BMT. Patients randomized in both arms had comparable characteristics and received the same preparative regimen (ie, cyclophosphamide 200 mg/kg over 4 days). The median time for neutrophil engraftment was 17 days (range, 11-31 days) and 12 days (range, 4-45 days) for patients in the CsA/MTX group and the CsA alone group, respectively (P = .01). No significant difference was observed in the probability of either grade 2, grade 3, or grade 4 acute GVHD or chronic GVHD developing in the 2 groups. The Kaplan-Meier estimates of 1-year transplantation-related mortality rates for patients given either CsA/MTX or CsA alone were 3% and 15%, respectively (P = .07). With a median follow-up of 48 months from BMT, the 5-year survival probability is 94% for patients in the CsA/MTX group and 78% for those in the CsA alone group (P = .05). These data indicate that the use of CsA with MTX is associated with improved survival in patients with SAA who receive transplants from compatible siblings.
Introduction
Several studies have documented that many patients with acquired severe aplastic anemia (SAA) can be cured by allogeneic bone marrow transplantation (BMT) from a human leukocyte antigen (HLA)-identical sibling.1-5 Results have constantly improved over time,6 and several patient- and disease-related variables may have contributed to increase the survival rate of patients with SAA who undergo BMT from compatible siblings.
Earlier transplantation,6 more effective supportive care, and the addition of antithymocyte globulin (ATG) to cyclophosphamide in the preparative regimen7 have favorably influenced the outcome for patients with SAA who undergo transplantation. In particular, the introduction of cyclosporin A (CsA) instead of methotrexate (MTX) as graft versus host disease (GVHD) prophylaxis has been reported in some studies to decrease transplant-related mortality (TRM)6,8 and the risk for graft failure.4,8Previously published studies have documented that patients with SAA given CsA and short-term MTX for GVHD prevention enjoyed better results in comparison with those treated with MTX alone.6,7,9-11However, though several studies have compared the use of CsA versus CsA plus short-term MTX as GVHD prophylaxis in patients with leukemia,12-14 no prospective, controlled study has specifically addressed this issue in patients with SAA receiving BMT from compatible relatives.
On this basis, we evaluated—in a multicenter, randomized, prospective clinical trial—whether the use of CsA and short-term MTX for GVHD prevention could be associated with better survival than CsA alone in patients with acquired SAA who received allogeneic BMT from HLA-compatible siblings.
Patients and methods
Study design
The major endpoint of the study was to determine whether the combination of CsA and MTX could improve overall survival rates compared with CsA alone. Secondary endpoints were the occurrence of acute and chronic GVHD and the kinetics of neutrophil recovery in both randomization arms.
Eligibility criteria for patients to be enrolled in the study were age from 2 to 50 years, diagnosis of acquired SAA according to the criteria published by Camitta et al,15 availability of an HLA genotypically identical sibling, and written informed consent from the patient or the patient's parents.
To calculate the number of patients to be enrolled into this study, a 2-sided sample size evaluation method based on the log-rank test was used. To obtain a study significance level of 0.05 and a study power of 0.80 and supposing an estimated overall survival probability at 36 months of 0.90 for the first arm and 0.65 for the second arm of the study, 35 patients per arm with an accrual time of 5 years and an overall study duration of 7 years were required. To monitor the results of the trial, interim analysis was performed 3 years after the beginning of the study and, subsequently, every year. It was decided that patient enrollment should be closed when the difference in overall survival between the 2 arms reached P = .05.
Randomization was centralized at the Department of Hematology, Ospedale San Martino, Genoa, Italy, by one of the investigators (B.B.) not involved in the clinical management of the patients, and it was performed using a 1:1 allocation ratio. Patients were stratified according to the center where they underwent transplantation. Analysis was based on the intention-to-treat principle. Institutions participating in the study were not blind to the GVHD prophylaxis used. Grading of acute and chronic GVHD was performed by a single observer, who was expected to be blind to the randomization arm.
Treatment protocol
The pretransplant conditioning regimen consisted of cyclophosphamide (50 mg/kg recipient body weight intravenously on each of 4 successive days). All patients received unmanipulated marrow within 36 hours of the last dose of cyclophosphamide.
The day of transplantation was designated as day 0. Between days −7 and −3, patients were randomized to receive CsA alone at a dosage of 3 mg/kg per day (CsA group) or CsA at the same dosage together with short-term MTX (8 mg/m2 on days 1, 3, 6, and 11; CsA/MTX group) starting from day −1. Subsequently, when patients were able to tolerate oral intake, they were given CsA at a dosage of 6 mg/kg per day in 2 divided doses, with gradual reduction until it could be discontinued, in the absence of chronic GVHD, 6 to 12 months after BMT. No other agent was used for GVHD prophylaxis. Compared with the original schedule described by Storb et al,12 the reduced dosage of MTX was chosen on the hypothesis that a lower drug dose could shorten the period of neutropenia and diminish mucosal breakdown, thus decreasing the risk for infection. Moreover, Zikos et al16documented that the combination of CsA with low-dose MTX (10 mg/m2 on day 1; 8 mg/m2 on days 3, 6, 11) was able to reduce significantly the risk for acute GVHD in patients with acute leukemia who received transplants from compatible relatives.16
Dose modification of CsA was allowed when serum creatinine levels were greater than twice the baseline value. Physicians were free to increase the dose of CsA for patients experiencing acute GVHD. All but 5 patients received the 4 scheduled doses of MTX.
Supportive care was standardized within each center and was uniformly applied to patients in both groups. Usually, empirical broad-spectrum antibiotic therapy was started when patients became febrile, and antifungal therapy was used in the presence of either clinical evidence of fungal infection or fever persisting after 3 days of antibiotic therapy. Patients were given oral cotrimoxazole as prophylaxis forPneumocystis carinii pneumonia starting on the day of engraftment.
Cytomegalovirus (CMV) serologic status was studied before transplantation in all patients and donors. In all patients,the expression of pp65 human CMV matrix protein was monitored to detect CMV reactivation.17 Patients experiencing reactivation of CMV infection were treated with ganciclovir or foscarnet at a conventional dosage.
Transplant centers were free to use colony-stimulating factors to accelerate hematopoietic recovery after BMT. Twenty of 71 patients were given recombinant human granulocyte colony-stimulating factor (rHuG-CSF) after transplantation. In all these patients, G-CSF was started within the first 4 days of marrow infusion. Two more patients were given recombinant human erythropoietin. A higher proportion of subjects given CSA alone received rHuG-CSF than patients treated with CsA/MTX (P = .06; Table 1).
Development of acute and chronic GVHD was monitored throughout the study and graded by investigators at each participating center. Tissue biopsy samples were obtained to confirm the diagnosis of GVHD whenever clinically indicated and feasible. Treatment of acute and chronic GVHD was administered according to the protocols in use at each institution.
Patient demographics and characteristics
From May 1991 to August 1998, 71 patients (45 males, 26 females) with SAA who underwent allogeneic BMT from an HLA-identical sibling were centrally randomized and assigned to either of the treatment arms. Patients were treated in the centers listed in the . All randomized subjects underwent BMT and were included in the data analysis. At time of BMT, the patients' median age was 19 years (range, 4-46 years). Sixty-six of the 71 patients enrolled had idiopathic SAA, whereas the remaining 5 had posthepatitis SAA (Table1). Most patients had not received any treatment before BMT.
HLA class I and II antigen serologic typing of donors and recipients was performed by standard National Institutes of Health (NIH) microlymphocytotoxicity; low-resolution generic oligotyping was used to confirm the DRB1 identity in most donor/recipient pairs. The median number of cells infused was comparable in the 2 randomization arms (Table 1).
Additional details on patient and donor characteristics, pretransplant disease history, and comparison between the 2 arms of the study are reported in Table 1. The 2 groups were comparable for all the variables analyzed.
Definitions
Hematopoietic and lymphoid engraftment for each patient enrolled in this study was documented at each participating center by chromosome analysis of marrow cells and peripheral blood lymphocytes or by study of the genetic polymorphism of variable numbers of tandem repeat short DNA sequences. Moreover, 43 patients from 11 centers had chimeric status centrally assessed in one of the participating centers (Dublin) on at least 3, and as many as 15 occasions, after BMT. Assays were carried out on bone marrow or peripheral blood slides or on cell suspensions using polymerase chain reaction of short tandem repeats according to a previously described method.18 These patients were part of a study on chimerism in patients with SAA given an allograft (M.L. and S.M., unpublished data).
Myeloid and platelet engraftment were defined as the first of 3 consecutive days with an absolute neutrophil count greater than 0.5 × 109/L and unsupported platelets greater than 50 × 109/L, respectively. Patients were considered assessable for engraftment if they survived at least 14 days after transplantation.
Acute and chronic GVHD were classified according to previously published criteria.19 20 Patients with sustained donor engraftment who survived more than 14 days and more than 3 months after transplantation were assessable for occurrence and severity of acute and chronic GVHD, respectively.
Overall survival time was the time between transplantation and death from any cause. Because some surviving patients had autologous recovery of hematopoiesis, the probability of overall survival with transfusion independence and with complete or mixed donor chimerism was also calculated.
Statistical analysis
Data were analyzed as of March 15, 1999. Overall survival, TRM, GVHD occurrence, and curves for neutrophil and platelet engraftment after transplantation (starting point) were calculated by the Kaplan–Meier method21 and were compared using the log-rank test. Results of Kaplan-Meier analysis were reported as probability (%) and 95% confidence intervals (CI).
The Student t test, the Mann-Whitney U rank-sum test, and the Kruskal-Wallis test were used to compare differences in continuous variables between groups, and either χ2analysis or the Fisher exact test were used to compare percentages, as appropriate. All probability values were 2-sided, P < .05 was considered statistically significant. P > .1 was reported as not significant (NS), whereas values between .05 and .1 were reported in detail. The SAS package (SAS Institute, Cary, NC) was used for analysis of the data.
Results
The median follow-up for patients in the CsA and the CsA/MTX groups is 50 months (range, 7-86 months) and 52 months (range, 8-94 months) for survivors (P = NS) and 3 months (range, 7 days-46 months) and 10 months (range, 5-17 months) for deceased patients (P = NS), respectively.
Engraftment and hematopoietic recovery
All patients but 2, both in the CsA group, who died within the first 2 weeks after BMT, were evaluable for donor engraftment. Because only one patient, in the CsA/MTX group, had primary rejection of donor hematopoiesis, there was no difference in the engraftment rate of patients randomized into the 2 arms (100% vs 97%, respectively). The patient who experienced primary rejection underwent successful retransplantation using the same donor. The median time for neutrophil engraftment was 12 days (range, 8-45 days) and 17 days (range, 11-31 days) for patients belonging to the CsA and CsA/MTX groups, respectively (P = .01). The median time for neutrophil recovery in CsA patients who did or did not receive G-CSF was 12 and 14 days, respectively, whereas in the CsA/MTX group, myeloid engraftment in patients who were or were not given G-CSF was achieved at a median of 14 and 17 days, respectively (P = .06). Platelet engraftment was reached in 66 of the 71 patients analyzed: 3 patients died before platelet recovery, one had primary rejection, and one had secondary marrow failure. No significant difference for the kinetics of platelet recovery was observed between the 2 groups. The median time for reaching a self-sustained platelet count greater than 50 × 109/L was 22 days (range, 13-127 days) and 22 days (range, 13-96) for patients in CsA alone and the CsA/MTX group, respectively.
Five patients experienced secondary graft failure at a median of 92 days after BMT (range, 75-455 days). Three belonged to the CsA/MTX group and 2 to the CsA group (P = NS). Four of these 5 patients are alive and transfusion independent. One received a second allograft from the same donor, which resulted in a complete donor chimerism.
Graft versus host disease
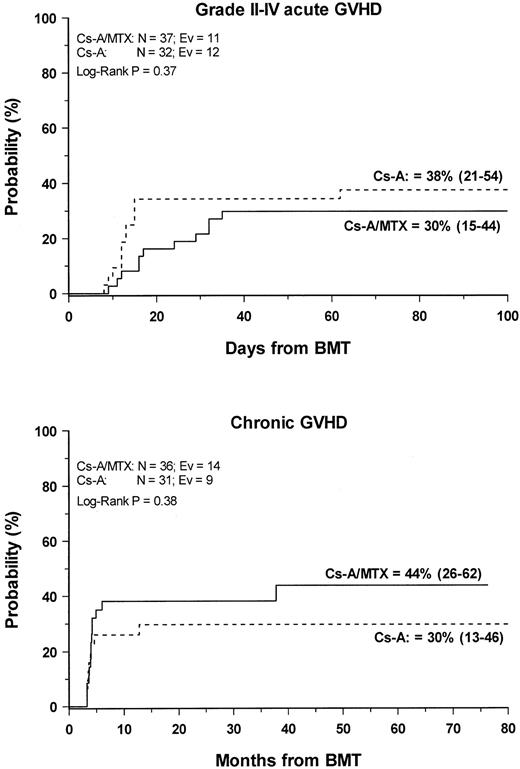
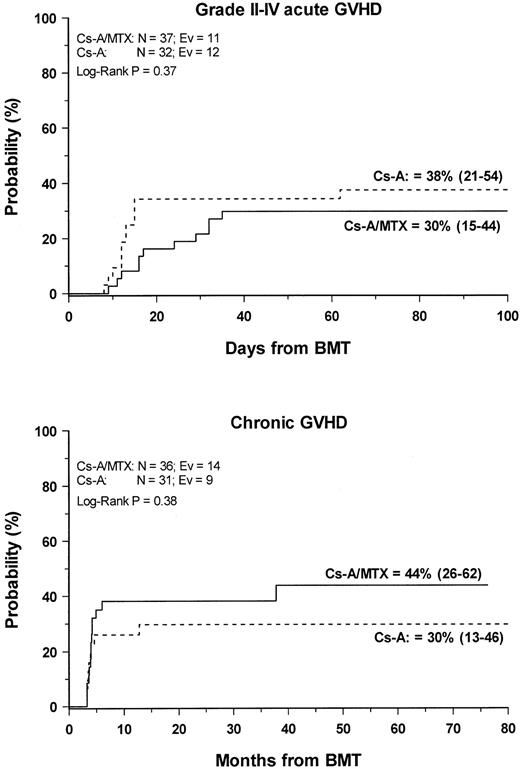
Grade 2 to 4 acute GVHD developed in 23 (33%) of the 69 evaluable patients. The cumulative probability of developing grade 2 to grade 4 acute GVHD was 38% (21–54) and 30% (15–44) for patients in the CsA and the CSA/MTX group, respectively (P = NS) (Figure1). No patients in either treatment group experienced grade 4 acute GVHD. Acute GVHD developed in patients who were randomized to the CsA or the CsA/MTX arms at a median of 13 (range, 6-62 days) and 23 (range, 9-45 days) after transplantation, respectively (P = .07). Table2 shows the details of organ involvement by acute GVHD. The differences between the 2 arms were not statistically significant.
Development of GVHD.
Cumulative probability of grade 2 to 4 acute (top) and chronic (bottom) GVHD for the CsA group (dotted line) and the CsA/MTX group (continuous line) is shown. N, number of patients in each arm of randomization; EV, number of events occurring in each arm of randomization.
Development of GVHD.
Cumulative probability of grade 2 to 4 acute (top) and chronic (bottom) GVHD for the CsA group (dotted line) and the CsA/MTX group (continuous line) is shown. N, number of patients in each arm of randomization; EV, number of events occurring in each arm of randomization.
Chronic GVHD developed in 23 of the 66 (35%) assessable patients. In all patients, chronic GVHD followed acute GVHD, and no case of de novo disease was observed. Nine patients had progressive chronic GVHD, whereas in the remaining 14 the disease developed after a period of quiescence, with no difference between the 2 randomization arms. Seventeen patients, 6 in the CsA group and 11 in the CsA/MTX group, had limited skin chronic GVHD, and 6 patients—3 in the CsA group and 3 in the CsA/MTX group—had the extensive form of the disease. The Kaplan-Meier estimate of chronic GVHD occurrence was 30% (13–46) for the CsA group and 44% (26–62) for the CsA/MTX group (P = NS; Figure 1). Chronic GVHD developed in patients randomized to the CsA and the CsA/MTX groups at a median of 3.5 months (range, 3-13 months) and 4 months (range, 3-37 months) after BMT, respectively (P = NS). When CsA tapering was started it did not have any influence on the development of chronic GVHD. Median Karnofsky score of surviving patients with chronic GVHD was 100% (50–100), with no difference between the 2 randomization arms. One patient in the CsA arm died of chronic GVHD.
Transplantation-related death
Nine patients died a median of 5 months after transplantation (range, 7 days-48 months). Seven patients were in the CsA group and the remaining 2 were in the CsA/MTX group. Details on the causes of death are reported in Table 3. Infectious complications accounted for 5 of the deaths observed in the study population. One child randomized to the CsA arm died of acute myeloid leukemia in the donor cells 15 months after transplantation. Grades 2 and 3 GVHD had been diagnosed in 6 of 7 patients evaluable for GVHD and who died.
The 100-day TRM probability was comparable in the 2 randomization arms (Table 4). The 1-year TRM probability was 9% (2–18) for the entire study population, whereas it was 15% (3–27) and 3% (0–15) for the CsA and the CsA/MTX group, respectively (P = .07). Because 2 more patients died beyond 1 year after BMT as a result of chronic GVHD and viral encephalitis (Table 4), ultimately the 5-year probability of dying was 6% (0–15) and 19% (5–34) for patients in the CsA/MTX or the CsA alone group, respectively (P = .09).
Survival, survival with transfusion independence, and complete or mixed donor chimerism
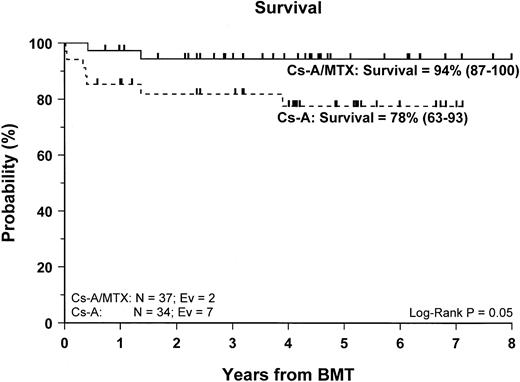
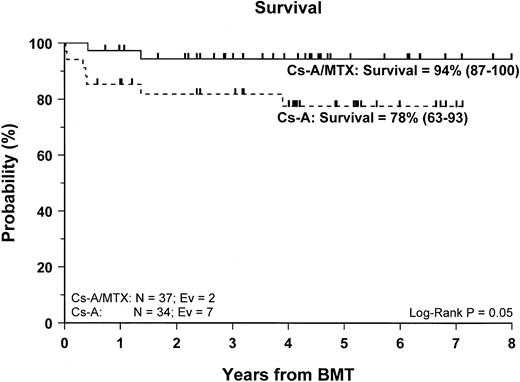
Sixty-two of the 71 enrolled patients (87%) are alive. The 5-year Kaplan-Meier estimate of survival is 86% (78–95) for the entire cohort of patients studied, whereas it is 94% (87–100) for patients in the CsA/MTX group, a value significantly better than the 78% (63–93) estimate for patients randomized in the CsA group (P = .05) (Figure 2).
Probability of survival.
Cumulative probability of survival for the CsA group (dotted line) and the CsA/MTX group (continuous line) is shown. N, number of patients in each arm of randomization; EV, number of events occurring in each arm of randomization.
Probability of survival.
Cumulative probability of survival for the CsA group (dotted line) and the CsA/MTX group (continuous line) is shown. N, number of patients in each arm of randomization; EV, number of events occurring in each arm of randomization.
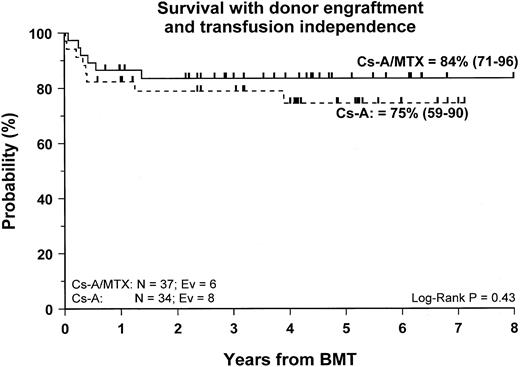
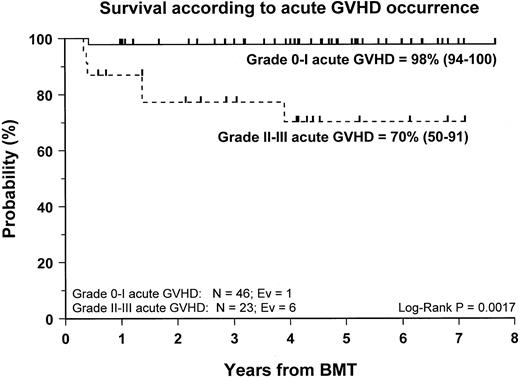
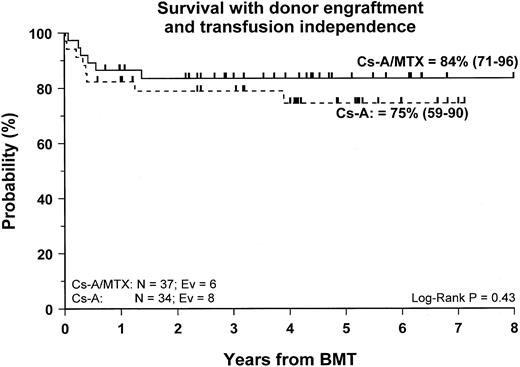
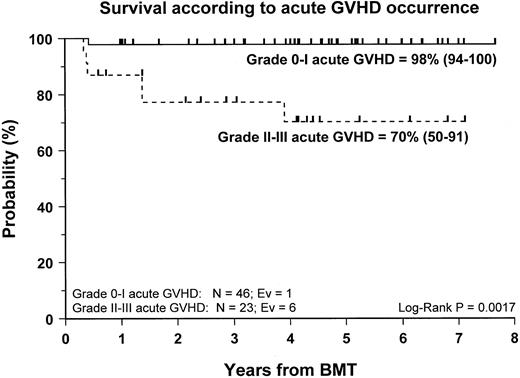
Fifty-nine patients (83%) are alive and transfusion independent and have complete or mixed donor chimerism. Of these, 2 patients in the CsA/MTX group achieved donor chimerism after a second allograft from the same donor. The 5-year Kaplan-Meier estimates of survival, with transfusion independence and mixed or complete donor chimerism, are comparable in the 2 randomization groups and are shown in Figure3. Details on donor chimerism according to the randomization arm are reported in Table 4. Three more patients, one in the CsA group and 2 in the CsA/MTX group, are alive and transfusion independent with autologous recovery of hematopoiesis (Table 4). Overall survival was 98% (94–100) and 70% (50–91) for evaluable subjects without or with grade 1 acute GVHD and for those with grade 2 or 3 acute GVHD, respectively (P < .005; Figure 4).
Probability of survival with transfusion independence and mixed/complete donor chimerism.
Cumulative probability of survival with transfusion independence and mixed/complete donor chimerism for the CsA group (dotted line) and the CsA/MTX group (continuous line) is shown. N, number of patients in each arm of randomization; EV, number of events occurring in each arm of randomization.
Probability of survival with transfusion independence and mixed/complete donor chimerism.
Cumulative probability of survival with transfusion independence and mixed/complete donor chimerism for the CsA group (dotted line) and the CsA/MTX group (continuous line) is shown. N, number of patients in each arm of randomization; EV, number of events occurring in each arm of randomization.
Probability of survival according to the development of acute GVHD.
Cumulative probability of survival for patients without or with grade 1 acute GVHD (continuous line) and for patients with grade 2 or 3 acute GVHD (dotted line) is shown. N, number of patients in each arm of randomization; EV, number of events occurring in each arm of randomization.
Probability of survival according to the development of acute GVHD.
Cumulative probability of survival for patients without or with grade 1 acute GVHD (continuous line) and for patients with grade 2 or 3 acute GVHD (dotted line) is shown. N, number of patients in each arm of randomization; EV, number of events occurring in each arm of randomization.
The cumulative probability of survival for patients younger or older than 18 years was 91% (80–100) and 83% (70–96), respectively (P = NS). The advantage in terms of survival offered by adding MTX to GVHD prophylaxis was comparable in children and adults (data not shown). Overall survival of patients who did or did not receive G-CSF was 89% (75–100) and 85% (75–95), respectively (P = NS). None of the other factors possibly influencing outcomes for patients with SAA after BMT—including absolute neutrophil count and platelet count at diagnosis, number of pre-BMT transfusions, refractoriness to platelet transfusion, prior treatment, number of cells infused, and interval between diagnosis and BMT—was associated with an increased risk for treatment failure (data not shown).
Discussion
This randomized, multicenter study documents that the combination of CsA and short-term MTX for GVHD prevention offers a survival advantage in comparison to CsA alone in patients with acquired SAA who receive an allograft from an HLA-identical sibling. Our data differ from those reported in a retrospective study from the International Bone Marrow Transplant Registry (IBMTR),6 which failed to demonstrate a significant improvement in survival rate by adding MTX to CsA. In contrast, our data confirm previously published reports showing a beneficial effect of the combination of CsA/MTX for GVHD prophylaxis on SAA patient survival, mainly because of acute GVHD prevention.9-11 In our population the combination of CsA and MTX did not affect the incidence of acute or chronic GVHD; hence, the advantage offered by adding MTX seems to be related to other factors, such as lower risk for infectious complications or organ toxicity. The later development (ie, in a phase of better immune competence) of acute GVHD, observed in patients given CsA and MTX, may have favorably influenced the mortality rate, reducing the risk for life-threatening infections. However, other unknown factors might have been at work to improve the survival rate of patients given the combination treatment.
The advantage in terms of survival offered by adding short-term MTX is lost when stable engraftment of donor hematopoiesis and transfusion independence are assessed. The Kaplan-Meier estimates of survival with transfusion-independence and complete or mixed donor chimerism were comparable in the 2 randomization arms.
The incidence of either primary or late graft failure in our cohort was 8%. This value is comparable to or better than those previously reported by retrospective studies of the IBMTR6,8 and slightly higher than the 5% documented by Storb et al,7 who added antithymocyte globulin to cyclophosphamide during the preparative regimen.
Nine of the 63 surviving patients with stable engraftment of donor hematopoiesis were mixed chimeras (Table 1). This confirms that mixed chimerism is relatively frequent among patients who undergo transplantation for aplastic anemia and that it is not uniformly associated with graft failure.22 Similar results are seen in patients with thalassemia given BMT from a compatible sibling.23
Three patients survive with autologous hematopoietic recovery and transfusion independence. This finding is in agreement with previously published results, documenting the efficacy of high-dose cyclophosphamide without BMT in restoring normal hematopoiesis,24-26 and it provides further support to the hypothesis that SAA is a disorder in which damage to hematopoietic stem cells may lead to an autoimmune response directed against the bone marrow.5 27
The earlier neutrophil recovery documented in our patients given CsA alone can be attributed both to the detrimental effect of MTX on the kinetics of myeloid recovery12 and to the higher percentage of patients treated with hematopoietic growth factors, which have been shown to accelerate neutrophil reconstitution in allogeneic BMT recipients.28,29 However, the delay in neutrophil recovery for our patients given CsA/MTX was not accompanied by an increased risk for either fungal or bacterial infections. Moreover, we have been unable to document any detrimental effect played by MTX on platelet recovery, confirming the results previously published by Storb et al12 in a randomized trial comparing CsA/MTX to CsA alone in patients with acute leukemia.
As mentioned above, patients receiving combination treatment did not show a lower probability for acute or chronic GVHD. However, it must be noted that the incidence of severe acute GVHD was particularly low in the overall population. No patient had grade 4 GVHD, and only one patient in the CsA/MTX group had grade 3 acute GVHD. This low incidence and severity of GVHD might be attributed to the young age of our population. In fact, approximately half the patients were children, and previously published studies have reported a low incidence of GVHD in pediatric patients given transplants from compatible siblings.11,30 We cannot exclude that the large number of centers involved in this study and the relatively low number of patients who underwent transplantation in each center might have led to an underestimation of the real incidence of GVHD. The persistence of residual host cells and the low-intensity preparative regimen used might have also contributed to a decrease in the probability of GVHD. Animal models have demonstrated that mixed chimerism is associated with reduced susceptibility to GVHD, probably through mechanisms of central tolerance with negative selection of host-reactive and donor-reactive T cells.31,32 A reduced incidence of acute GVHD associated with mixed chimerism was also reported in patients with SAA receiving BMT from compatible relatives.33 Moreover, it has been hypothesized that the cytokine storm, secondary to the intensity of the conditioning regimen and its related tissue damage, triggers the development of GVHD.34
Despite the negligible incidence of severe acute GVHD in our study population, our data confirm the previously reported9,35 36detrimental effect played by grade 2 to 4 acute GVHD on the outcome of patients with SAA given an allograft. The survival probability rates of patients with grade 0 to 1 and grade 2 to 4 acute GVHD were 98% and 70%, respectively (P < .005). This finding supports the concept that the most effective pharmacologic strategies for GVHD prevention should be used in patients with SAA given unmanipulated BMT.
We conclude that the combination of CsA and short-term MTX for GVHD prevention improves the probability for survival in patients with SAA given allogeneic BMT from compatible siblings. Whether this advantage, in terms of overall survival, results in better survival with transfusion independence and mixed or complete donor chimerism remains to be demonstrated.
GITMO-EBMT participating centers
Alessandrino EP, Bernasconi C, Institute of Hematology, IRCCS Pol. S. Matteo, University of Pavia, Italy; Argiolu F, Cao A. Pediatic Clinic, Ospedale Regionale Microcitemie, Cagliari, Italy; Amadori S, Department of Hematology, Tor Vergata University, St. Eugenio Hospital, Rome, Italy; Arcese W, Mandelli F, Institute of Hematology, La Sapienza University, Rome, Italy; Bacigalupo A, Van Lint MT, Bruno B, Division of Hematology II, Ospedale S Martino, Genoa, Italy; Dallorso S, Dini G, Istituto Giannina Gaslini, Genoa, Italy; DiBartolomeo P, Unità Terapia Intensiva Ematologica per il Trapianto Emopoietico, Dipartimento di Ematologia, Ospedale Civile, Pescara, Italy; Falda M, Locatelli F, Division of Hematology, Ospedale Molinette, Turin, Italy; Fagioli F, Madon E, Department of Pediatrics, University of Torino, Ospedale Regina Margherita, Turin, Italy; Fanin R, Baccarani M, Division of Hematology, University of Udine, Italy; Iacopino P, Morabito F, Division of Hematology, Az. Osp. Bianchi-Melacrino-Morelli, Reggio Calabria, Italy; Izzi T, Department of Medicine, Spedali Civili, Brescia, Italy; Lambertenghi Deliliers G, Soligo D, BMT Unit, Osp. Maggiore, University of Milano, Milan, Italy; Locasciulli A, Masera G, Pediatric Clinic, Osp. Nuovo S Gerardo, University of Milano, Monza, Italy; Locatelli F, Zecca M, Pediatric Clinic, IRCCS Pol. S. Matteo University of Pavia, Pavia, Italy; McCann S, Lawler M, Department of Hematology, St James Hospital, Trinity College, Dublin, Ireland; Maiolino I, Department of Hematology, Ospedale V Cervello, Palermo, Italy; Pession A, Paolucci G, Pediatric Clinic, Ospedale S. Orsola, University of Bologna, Italy; Rotoli B, Division of Hematology, Federico II, Medical School, University of Napoli, Naples, Italy; Tichelli A, Gratwohl A, Department of Hematology, Kantonsspital, Basel, Switzerland.
Supported in part by grants from Associazione Italiana Ricerca sul Cancro and IRCCS Policlinico San Matteo (F.L.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Franco Locatelli, Dipartimento di Scienze Pediatriche, Università di Pavia, IRCCS Policlinico San Matteo, P. le Golgi, 2, I-27100 Pavia, Italy; e-mail:f.locatelli@smatteo.pv.it.