Abstract
The development of inhibitory antibodies to factor VIII (FVIII) occurs in approximately 30% to 40% of patients with severe hemophilia A. Management options for patients with inhibitor include eradicating it via immune tolerance induction (ITI) or treating bleeding episodes with large quantities of hemostatic agents. ITI is costly, approaching $1 million for the average 5-year-old, but if successful results in improved clinical outcomes. We constructed a decision analysis using the Markov process to model expected clinical outcomes and costs over a lifetime for a typical 5-year-old hemophiliac with high inhibitor levels. Estimates of relevant variables were based on a thorough review of the medical literature. Outcomes modeled included total lifetime costs as well as life expectancy. The decision analytic model revealed that the ITI strategy was associated with an increase in projected life expectancy of 4.6 years. Total estimated lifetime costs for the ITI strategy were approximately $1.7 million less per patient. Sensitivity analyses over clinically and economically reasonable ranges did not change these findings. The insight that ITI can achieve an improved clinical outcome while being cost-saving is not reflected in many current treatment regimens. This example also illustrates that expensive therapy for patients with a chronic disease may be cost effective when analyzed from a societal perspective over the patient's lifetime. This finding has important policy implications for medical decision makers at many levels and reinforces the need to undertake pharmacoeconomic analyses and choose therapies from a long-term, societal perspective.
Introduction
Hemophilia A is an X-linked hereditary bleeding disorder with an incidence of approximately 1/5000 males that results from either reduced or absent levels of coagulation factor VIII (FVIII).1 FVIII replacement is the mainstay of management for patients with hemophilia A, and the life expectancy of individuals with this disorder, but not infected with human immunodeficiency virus (HIV), now approaches that of the general population. With aggressive factor replacement therapy these patients can also be expected to enjoy an improved quality of life due to a decrease in musculoskeletal disability associated with recurrent hemarthroses.2 The average cost of hemophilia care has been estimated to be $28,000 per year for a child under the age of 6 years.3 Thus, hemophilia serves as a model of a chronic disease for which efficacious therapy exists but for which benefits may not be realized until some future point.4
One important complication that occurs in approximately 30% of patients with severe hemophilia A is the formation of inhibitory antibodies to FVIII.5 These inhibitors sharply reduce the hemostatic effects of FVIII concentrate, resulting in an increase in morbidity and resource utilization.6 The utility of FVIII concentrates is a function of the titer of the inhibitor. In high responders, characterized by antibody titers more than 5 to 10 Bethesda units (BU), re-exposure to FVIII results in an anamnestic immunologic response. Low responders do not exhibit an anamnestic response and can generally be managed with FVIII concentrates, albeit with a significantly increased dosage compared to patients without inhibitors.
In high-responder patients, hemostatic agents other than human FVIII must be used to treat bleeding episodes. These include porcine factor VIII (pFVIII), and “bypassing” agents such as prothrombin complex concentrates (PCC), activated prothrombin complex concentrates (aPCC), and recombinant factor VIIa (rVIIa), each of which is more expensive to use than human FVIII. In addition, the clinical efficacy of bypassing agents is not as predictable as FVIII replacement.7
An alternative strategy is to attempt to eradicate the inhibitor and normalize pharmacokinetic parameters, so that patients can then be treated with human FVIII concentrates. This is accomplished through immune tolerance induction (ITI), which typically involves the daily infusion of large doses of FVIII over many months to years as well as the use of immunosuppressive agents and other procedures, depending on the protocol used. The efficacy rate of ITI, which depends in large part on the characteristics of the patient undergoing the treatment, ranges from 63% to 83% in most series.8-10 A major disadvantage of ITI is its high initial cost; for a single 5-year-old patient, the cost of factor concentrate required to induce tolerance is likely to approach $1 million. However, once achieved, tolerance is long lasting.9
The high initial cost of ITI has led some to question the cost-effectiveness of this strategy, and long-term data on the costs and efficacy of any individual ITI regimen are sparse.11 12 We therefore undertook a cost-effectiveness analysis to determine the optimal strategy for the management of patients with inhibitors by estimating the lifetime costs and survival for ITI followed by the use of FVIII concentrates, compared to a strategy using other hemostatic agents without immune tolerance induction.
Methods
Using available data from the clinical literature and current estimates of the costs of relevant therapies and outcomes, we constructed a decision analysis (nonexperimental) model to describe the outcomes of 2 treatment strategies: ITI followed by standard FVIII replacement versus the indefinite use of alternative hemostatic agents. Outcomes studied included clinical events, costs, and life expectancy. We used the Markov process to approximate the typical clinical situation in which bleeding episodes and their sequelae occur recursively over a patient's lifetime. Assumptions regarding rates and probabilities used in the model are described, as are the sensitivity analyses performed to test the robustness of these assumptions.
The decision model
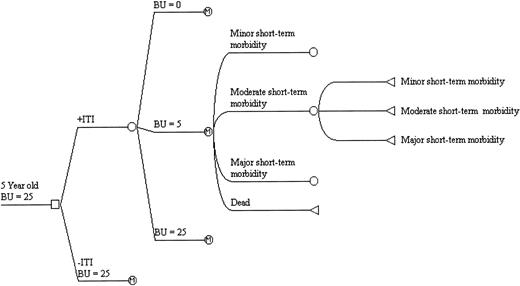
Figure 1 is a graphic representation of the model used for this decision analysis. Initially a decision is made to proceed under 1 of 2 scenarios: to manage all patients with ITI or to use alternative hemostatic agents without ITI. In the model, the hypothetical patient is a 5-year-old boy with severe hemophilia A and a high responding inhibitor of 25 BU. The upper part of the decision tree reflects the possible outcomes if ITI is chosen: complete response (CR) with eradication of the inhibitor, partial response (PR) with a decrease of the inhibitor to 5 BU, and failure. Each of these outcomes is assumed durable for the patient's lifetime. If ITI were completely successful in eradicating the inhibitor, we assumed the patients would be maintained on 20 U/kg tiw of FVIII for 1 year. If the decision is made to use alternative hemostatic agents, it was assumed that the titer of the antibody would remain stable at 25 BU.
A schematic representation of the decision analysis model.
The heavy bar represents the path taken by the patient for this particular cycle. Key to nodes: a ■ represents a decision node, a ○ represents a chance, and a ▹ represents a terminal node. For purposes of illustration, only 4 of the 7 possible Markov health states are shown.
A schematic representation of the decision analysis model.
The heavy bar represents the path taken by the patient for this particular cycle. Key to nodes: a ■ represents a decision node, a ○ represents a chance, and a ▹ represents a terminal node. For purposes of illustration, only 4 of the 7 possible Markov health states are shown.
The patient can then experience 1 of 7 possible health states, referred to as branches of a Markov node: 3 defined by short-term morbidity only, 3 defined by short-term morbidity superimposed on irreversible arthropathy, and death. Health states related to short-term morbidity were defined by the annual number and severity of acute bleeding episodes. Minor short-term morbidity corresponds to minor bleeding episodes treated at home with standard amounts of factor concentrate. Moderate short-term morbidity encompasses more severe bleeding episodes that require twice as many doses of factor concentrate. Major short-term morbidity refers to those bleeding episodes severe enough to require hospitalization. For each short-term morbidity state, it was assumed that events that occur in a less morbid state will continue to occur in addition to those events specific to the higher morbidity state. Long-term morbidity states were defined as any short-term morbidity state superimposed on irreversible arthropathy of at least one joint. The Markov cycle length was 1 year, at the end of which the patient would make a transition to 1 of the 7 possible health states.
The annual number of minor bleeding episodes was divided into 3 groups: low, average, and high. This was used to determine the transition of health states at the end of each cycle. If the patient suffered an average annual number of bleeding episodes, he remained in that short-term morbidity state for the next cycle. However, if he suffered a low rate of minor bleeds he would transition to the next least morbid state. Conversely, suffering the high rate of bleeding episodes would cause him to transition to the next most morbid state in the next cycle. This closely approximates clinical experience, in which patients who bleed most frequently progress more rapidly to states of higher morbidity.
For each health state and expected number of bleeding episodes in each cycle, we calculated resource utilization by applying the costs of factor concentrates to the amount of factor consumed. The amount of factor used for individual bleeding episodes depends on the severity of the bleeding episode and the titer of the inhibitor. As shown in Table1, all factor doses were based on the weight of the patient, which was assumed to be 20 kg at entry and increased linearly over 15 years to a final weight of 70 kg. The average cost of factor concentrate was calculated by taking the average wholesale price (AWP) for all products within a category (FVIII, aPCCs, etc) minus 10%. Costs were discounted at 3% per year.
Formulas for factor consumption based on inhibitor titer, patient weight, and severity of bleeding episode
| Inhibitor titer (BU) . | Severity of bleeding episode . | Factor (type and dosage) . |
|---|---|---|
| BU = O: | Minor | 40 U/kg FVIII |
| Moderate | 80 U/kg FVIII | |
| Major | 100 U/kg/d × 7d FVIII in hospital | |
| BU = 5: | Minor | 40 U/kg FVIII + 50 U/kg FVIII* |
| Moderate | 80 U/kg FVIII + 50 U/kg FVIII* | |
| Major | 100 U/kg/d × 7d FVIII + 50 U/kg FVIII* | |
| BU = 25: | Minor | aPCC: 180 U/kg† |
| pFVIII†: 1/4[30 U/kg bid × 2 d + 25*0.25*30 U/kg × 1 dose‡] | ||
| Moderate | aPCC: 360 U/kg† | |
| pFVIII†: 1/4[30 U/kg/bid × 4 d + 25*0.25*30 U/kg × 1 dose‡] | ||
| Major | pFVIII: 50,000 U/episode |
| Inhibitor titer (BU) . | Severity of bleeding episode . | Factor (type and dosage) . |
|---|---|---|
| BU = O: | Minor | 40 U/kg FVIII |
| Moderate | 80 U/kg FVIII | |
| Major | 100 U/kg/d × 7d FVIII in hospital | |
| BU = 5: | Minor | 40 U/kg FVIII + 50 U/kg FVIII* |
| Moderate | 80 U/kg FVIII + 50 U/kg FVIII* | |
| Major | 100 U/kg/d × 7d FVIII + 50 U/kg FVIII* | |
| BU = 25: | Minor | aPCC: 180 U/kg† |
| pFVIII†: 1/4[30 U/kg bid × 2 d + 25*0.25*30 U/kg × 1 dose‡] | ||
| Moderate | aPCC: 360 U/kg† | |
| pFVIII†: 1/4[30 U/kg/bid × 4 d + 25*0.25*30 U/kg × 1 dose‡] | ||
| Major | pFVIII: 50,000 U/episode |
One-time dose of FVIII to “neutralize” the inhibitor.
The “1/4” represents the 25% failure rate of aPCC and thus the use of pFVIII.
The initial dose for pFVIII was calculated by assuming an antiporcine titer approximately 25% as high as that of the antihuman titer.
Estimates and probabilities
ITI regimen and success rate.
The likelihood of success of ITI is best predicted by the amount of daily FVIII used to induce tolerance. Multivariate analysis from the International Registry on Immune Tolerance revealed that using100 U/kg/d or more conferred a relative risk for success of approximately 13.8 The ITI regimen in this model was defined as 100 U/kg/d of FVIII for 420 days. The majority of protocols used to date demonstrate similar efficacy (63%-83%) but differ appreciably with respect to dose and the time required for success.10 The regimen used in the model is consistent with practice in North America and a recently completed multicenter, prospective trial in the United States.9,13 14 Based on the clinical literature, we assumed that ITI would have an 80% probability of success, a 5% probability of partial response, and a 15% probability of failure. We also performed threshold analyses on the success rate and cost of ITI.
Bleeding episodes.
There is general agreement among hemophilia specialists that patients with inhibitors have the same annual number of bleeding episodes as do patients with severe hemophilia without inhibitors, albeit with a higher likelihood of complications.6 We assumed the average number of minor bleeding episodes was 15 per year with a low of 3 and a high of 27. 5,15-18 Similarly, we assumed that patients experiencing moderate short-term morbidity would suffer 3 moderate bleeding episodes per year. Those who experienced major short-term morbidity would have one major bleeding episode every 5 years. We performed a threshold analysis on the annual average rate of minor bleeding episodes.
Arthropathy.
The cumulative lifetime risk for the development of irreversible arthropathy in at least one joint is approximately 0.75 for both inhibitor and noninhibitor patients.19 In accordance with the clinical experience of an equal lifetime risk but more rapid development of arthropathy in inhibitor patients, we projected that a patient with a persistent inhibitor will reach this lifetime risk after 15 years compared to 20 years in a patient in whom the inhibitor is eradicated.
Mortality.
We used data from the literature to estimate the excess mortality associated both with severe hemophilia and with the development of an inhibitor.2 20-23 Based on this, noninhibitor patients were assigned a 2.5-fold increase in annual mortality rates compared to the general population, whereas inhibitor patients were assigned a 4-fold increase.
Factor utilization.
Factor utilization for each Markov cycle was calculated based on the weight of the patient, the number and severity of bleeding episodes, and the titer of inhibitor, as indicated by the formulas in Table1. Minor bleeding episodes would be treated at home with 2 doses of factor with the aim of achieving a 40% FVIII activity level. With respect to the acute management of bleeding episodes, we assumed that an aPCC would be used as initial treatment and that pFVIII would be used next for treatment failures. We assumed an efficacy rate for aPCCs of 75%24; in the 25% of instances in which aPCCs fail, pFVIII would be used. Moderate bleeding episodes would also be treated at home but would require twice as much factor as a minor bleeding episode. Major bleeding episodes would require hospitalization for 7 days. If the inhibitor levels allowed treatment with FVIII, we calculated a dose necessary to achieve factor activity levels of 100%. For patients in whom the titer of inhibitor precluded use of human FVIII, 50 000 U of pFVIII was used.25
All probabilities, annual number of bleeding episodes, costs, and life expectancy assumptions used in the model are summarized in Tables2 and 3. Baseline assumptions represent summary values from the literature. We performed sensitivity analyses over clinically reasonable ranges.
Probabilities used in ITI model
| Success rate of ITI* | Complete response = 0.8 |
| Partial response = 0.05 | |
| Failure = 0.15 | |
| Likelihood of annual number of minor bleeding episodes | Low = 0.1 Average = 0.8 High = 0.1 |
| Success rate of hemostatic agents | |
| aPCC | 0.75 |
| pFVIII | 1.0 |
| Lifetime risk and rate of developing arthropathy | 0.75 over 15 y with inhibitor 0.75 over 20 y without inhibitor |
| Success rate of ITI* | Complete response = 0.8 |
| Partial response = 0.05 | |
| Failure = 0.15 | |
| Likelihood of annual number of minor bleeding episodes | Low = 0.1 Average = 0.8 High = 0.1 |
| Success rate of hemostatic agents | |
| aPCC | 0.75 |
| pFVIII | 1.0 |
| Lifetime risk and rate of developing arthropathy | 0.75 over 15 y with inhibitor 0.75 over 20 y without inhibitor |
Complete response indicates inhibitor titer not detected immunologically, and normalization of FVIII pharmacokinetics. Partial response indicates inhibitor titer of 5 BU. Failure indicates no response, inhibitor titer remains 25 BU.
Data used in ITI model
| ITI regimen | Dose FVIII 100 U/kg/d |
| Duration 420 d | |
| Annual number bleeding episodes | Minor |
| Low = 3 | |
| Average = 15 | |
| High = 27 | |
| Moderate = 3 | |
| Major = 0.2 | |
| Weight of cohort members | |
| At entry (5 y old) | 20 kg |
| By 20 y old | 70 kg |
| Excess risk annual hazard rate for mortality | |
| With inhibitor | 4× |
| Without inhibitor | 2.5× |
| Factor concentrate cost | Cost (1997 Redbook AWP-10%) |
| FVIII (recombinant) | $1.11 |
| aPCC | $1.17 |
| pFVIII | $1.56 |
| Anti-pFVIII inhibitor titer | 25% antihuman titer |
| ITI regimen | Dose FVIII 100 U/kg/d |
| Duration 420 d | |
| Annual number bleeding episodes | Minor |
| Low = 3 | |
| Average = 15 | |
| High = 27 | |
| Moderate = 3 | |
| Major = 0.2 | |
| Weight of cohort members | |
| At entry (5 y old) | 20 kg |
| By 20 y old | 70 kg |
| Excess risk annual hazard rate for mortality | |
| With inhibitor | 4× |
| Without inhibitor | 2.5× |
| Factor concentrate cost | Cost (1997 Redbook AWP-10%) |
| FVIII (recombinant) | $1.11 |
| aPCC | $1.17 |
| pFVIII | $1.56 |
| Anti-pFVIII inhibitor titer | 25% antihuman titer |
Results
Life expectancy
The decision analytic model revealed that ITI was associated with a projected survival gain of 4.6 years (64.7 versus 60.1 years). When discounted at 3%, the difference in life expectancy is 0.85 years expressed as net present value.
Costs
The ITI strategy was found to be cost-saving as well as clinically superior. Using a discount rate of 3%, a patient undergoing ITI would be expected to consume approximately $2.9 million of factor concentrates, including almost $1.0 million for ITI and the remaining $1.9 million over the course of his lifetime. By contrast, a patient in whom ITI was not undertaken would consume approximately $4.6 million of alternative hemostatic agents over a lifetime. The $1.7 million excess in costs in the non-ITI approach was due primarily to the higher cost and decreased efficacy of alternative hemostatic agents used to treat frequent minor bleeding episodes over the patient's lifetime.
Sensitivity analyses
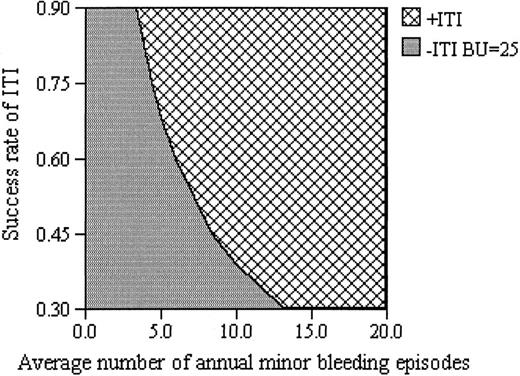
The conclusions of the model regarding costs were not influenced by variations in the price of pFVIII, as shown in Table4. The model was only modestly sensitive to the costs of FVIII and aPCC, in that if FVIII price alone increased by 78% or the aPCC price decreased by 45%, the long-term use of alternative hemostatic agents in the face of an unresolved FVIII inhibitor would be favored over ITI, although such price shifts are unlikely. The threshold level of annual rate of minor bleeding episodes was 4 per year. Thus, ITI would not be favored if a patient were expected to have fewer than 4 bleeding episodes per year, a very low level of bleeding rarely encountered in patients with severe hemophilia A. With respect to likelihood of success, threshold analysis revealed that if ITI is successful only 27% of the time (or more), it is the preferred strategy. This rate is well below the predicted overall success rate of 85% used in this model. Similarly, threshold analysis indicated that ITI remains the optimal choice for induction costs up to $2.6 million, an amount that is more than twice as much as the estimated $937,000 used as the reference case in our model. Figure2 presents a 2-way sensitivity analysis of mean number of bleeding episodes versus the likelihood of success of ITI. The greater the likelihood of success of ITI or of the number of bleeding episodes, the more strongly ITI emerges as the preferred strategy.
Threshold analyses
| Variable . | Reference case value . | Threshold level favoring ITI . | Comments . |
|---|---|---|---|
| Probability of success of ITI | 0.8 | > 0.26 | Range in literature 63-83% |
| Induction costs | $937,000 | < $2.6M | |
| Discount rate | 3% | < 9% | |
| Price FVIII | $1.11/U | < $1.98/U | |
| Price aPCC | $1.17/U | > $0.65/U | |
| Price pFVIII | $1.56/U | — | Not sensitive to price of pFVIII |
| Annual rate of minor bleeding episodes | 15 | > 4 |
| Variable . | Reference case value . | Threshold level favoring ITI . | Comments . |
|---|---|---|---|
| Probability of success of ITI | 0.8 | > 0.26 | Range in literature 63-83% |
| Induction costs | $937,000 | < $2.6M | |
| Discount rate | 3% | < 9% | |
| Price FVIII | $1.11/U | < $1.98/U | |
| Price aPCC | $1.17/U | > $0.65/U | |
| Price pFVIII | $1.56/U | — | Not sensitive to price of pFVIII |
| Annual rate of minor bleeding episodes | 15 | > 4 |
The threshold level for a given variable represents the level at which the preferred strategy shifts to ITI followed by conventional doses of FVIII from the indefinite use of alternative hemostatic agents.
Sensitivity analysis on annual rate of bleeding episodes and probability of success of ITI.
A 2-way sensitivity analysis illustrates potential outcomes as the annual number of minor bleeding episodes is varied along with the probability of successfully inducing immune tolerance. ITI is the favored strategy for the combination of values represented by the cross-hatched shading, whereas the darker shading represents the combinations of values for which long-term treatment with alternative hemostatic agents are favored.
Sensitivity analysis on annual rate of bleeding episodes and probability of success of ITI.
A 2-way sensitivity analysis illustrates potential outcomes as the annual number of minor bleeding episodes is varied along with the probability of successfully inducing immune tolerance. ITI is the favored strategy for the combination of values represented by the cross-hatched shading, whereas the darker shading represents the combinations of values for which long-term treatment with alternative hemostatic agents are favored.
Finally, the findings were not sensitive to the discount rate over the generally recommended range (0%-5%). Thus, despite the initial high cost of ITI, over the course of a lifetime this strategy resulted in a net savings of well over $1 million while improving life expectancy.
Discussion
The development of inhibitors in patients with hemophilia is a serious complication of factor replacement therapy that is associated with increased morbidity and cost. Eradication of the inhibitor, when possible, is a highly desirable and widely accepted goal of hemophilia care. However, the up-front costs that are associated with currently used ITI regimens present a financial challenge to patients and payers that must be addressed.
Our analysis resulted in the surprising finding that ITI is the preferred strategy for managing patients with hemophilia A with high titer inhibitors, resulting in both $1.7 million lower costs and 4.6 years increased life expectancy when compared with the use of alternative hemostatic agents. The dominance of ITI was not altered when we used sensitivity analyses to vary the success rate or cost of ITI, the annual rate of bleeding episodes, the cost of factor concentrates, or the discount rate over clinically plausible ranges.
An alternative strategy of using pFVIII as the initial therapeutic strategy in the subset of patients in which the anti-pFVIII titer is low was not explored but would not be expected to appreciably affect these results. The use of rVIIa is predicted to increase in the treatment of patients with high-titer inhibitors following the recent licensure of this product in the United States.26 Although at present no clinical studies directly compare the clinical efficacy of this product to aPCCs, published treatment regimens suggest that the use of rVIIa would be no less costly than that of aPCCs and would thus tend to favor the ITI strategy.
Observational clinical data describing single-institution experiences and published reports from large registries with pooled data from multiple centers using disparate ITI regimens suggest that higher daily doses of FVIII are associated with shorter mean times to induction.27-33 Thus, ITI would be the preferred strategy even if larger doses of FVIII (200-300 U/kg/d) were used so long as there was a compensatory decrease in the duration of the induction period.
In the present analysis, only costs of factor concentrates were considered. Other costs such as hospitalization or the placement and maintenance of central venous catheters were not included, because numerous studies have demonstrated that factor concentrates account for 80% to 90% of the total cost of managing hemophilia patients in both outpatient and inpatient settings.34 Because patients not receiving ITI are likely to incur more costs because of their overall increased morbidity, including these costs would likely only further strengthen the finding favoring ITI.
In clinical practice, most patients in whom ITI is successful are placed indefinitely on full prophylactic doses of FVIII (20-40 U/kg tiw) with the goal of preventing or retarding the development of hemophilic arthropathy. Regular and frequent exposure to FVIII may also be important in maintaining the state of immune tolerance although there is a paucity of data on this point. We have included in our model the cost of 1 year of prophylaxis following the completion of ITI and conservatively assumed that no additional clinical benefit would be derived from this mode of therapy. If we had defined the completion of ITI as the time of disappearance of the inhibitory antibody or had included in the analysis the decreased disability associated with prophylaxis, the cost effectiveness of the ITI strategy would have been further increased.3 35
The findings of this study demonstrate that ITI is cost saving over the course of a lifetime and results in an increase in life expectancy, presenting the fortunate confluence of clinical superiority with reduced cost. Two aspects of the perspective used for this study are crucial in this regard. First, the perspective taken was that of society as a whole. Secondly, the temporal perspective was that of the patient's entire life span, and not just the period during which ITI was accomplished. In the current health care environment, however, many decisions are undertaken by policymakers using different perspectives and timeframes. Immediate economic pressures may discourage the use of an expensive strategy for which cost savings may not be realized in the short term, or that may benefit another economic entity. This study can serve as a paradigm for such a situation and is not unique, because the treatment of many chronic and childhood diseases share these important characteristics. In an era of cost constraints, this insight can have broad policy implications as consumers, providers, and payers of health care often assume different perspectives when approaching medical decisions. Our findings reinforce the necessity of taking a long-term view and a more global perspective rather than more narrow or parochial views when assessing the worth of competing strategies for clinical interventions.
Acknowledgment
The authors wish to thank Karen M. Kuntz for her generous advice on the development of the Markov model used in this analysis.
Supported in part by a grant from the Kettering Family Foundation (A.B.C.), an Agency for Health Care Policy and Research institutional training grant (A.B.C.), and core support to the Division of Pharmacoepidemiology and Pharmacoeconomics from the Brigham and Women's Hospital. No support for this work was received from any pharmaceutical company.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jerry Avorn, Division of Pharmacoepidemiology and Pharmacoeconomics, Brigham and Women's Hospital, 221 Longwood Ave, Suite 341, Boston, MA 02115.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal