Abstract
Dendritic cells (DCs) genetically modified to continually express and present antigens may be potent physiologic adjuvants for induction of prophylactic or therapeutic immunity. We have previously shown that an env and nef deleted HIV-1 vector (HIV-1ΔEN) pseudotyped with VSV-G transduced monocyte-derived macrophages as well as CD34+ precursors of DCs. Here we extended these findings with HIV-1ΔEN to highly differentiated human DCs derived in culture from circulating monocytes (DCs). In addition, a new vector derived from HIV-1ΔEN but further deleted in its remaining accessory genes vif, vpr, and vpu(HIV-1ΔEN V3) was also tested. Both vectors efficiently transduced DCs. Transduction of DCs did not significantly alter their viability or their immunophenotype when compared with untransduced DCs. Furthermore, the phagocytic potential of immature DCs, as well as their ability to differentiate into mature DCs capable of stimulating T-cell proliferation, was not affected. Finally, DCs transduced by the HIV-1ΔEN vector were able to elicit a primary antiviral cytotoxic T-cell response in autologous CD8 T cells. These results suggest that HIV-1–based vectors expressing viral antigens may be useful for in vivo active immunization as well as ex vivo priming of cytotoxic T cells for adoptive T-cell therapy.
Introduction
There is compelling evidence supporting a role for HIV-specific cytotoxic T-lymphocytes (CTL) in the containment of HIV replication and for inducing memory CTL as part of a prophylactic vaccine strategy. The appearance of CTL during primary HIV-1 infection has been associated with the initial control of viremia and the resolution of symptoms.1 Individuals who were heavily exposed to HIV yet remained persistently uninfected have been shown to elicit strong HIV-specific proliferative responses, and in some cases, HIV-specific CTL responses, in the absence of anti-HIV humoral immunity.2 In HIV-infected persons who maintain low viral loads and stable CD4 counts despite many years of infection, termed “long-term nonprogressors,” a strong and broadly directed CTL response to HIV antigens has been detected.3 CTL induced by dendritic cells (DCs) are able to inhibit HIV replication by lysing infected cells and by releasing soluble antiviral factors before progeny virus have been produced.4 However, the host immune response elicited by HIV-1 is usually transient and insufficient to thwart the progressive destruction of the immune system in infected individuals.
Dendritic cells are promising targets for immunotherapy because of their ability to capture and present antigen to both CD8+and CD4+ lymphocytes, thereby inducing primary as well as secondary immune responses.5,6 Langerhans cells, which are immature DCs in the epidermis and genital mucosa, are among the first targets of HIV infection and may be involved in both virus transmission and priming of T cells to viral antigens.7 Transduction of DCs with replication-incompetent HIV vectors should confer DCs the ability to elicit antiviral immune responses without contributing to virus spread.
HIV-1 and other lentivirus-based vectors can achieve sustained transduction and expression of therapeutic genes in dividing as well as nondividing cells and may be superior to most other vectors for stable transduction of DCs.8.9
Recently, we reported the efficient transduction of nondividing macrophages10 and of hematopoietic stem/progenitor cells11 12 using replication-incompetent HIV-based vectors. In this study, we show that VSV-G pseudotyped HIV-1 vectors deficient in nef or all 4 accessory genes can efficiently transduce monocyte-derived DCs. We also examined the effect of transduction on DC morphology, immunophenotype, differentiation, and immune function. The results suggest that transduction by HIV-1–based vectors had no major impact on DC phenotypes, and the transduced DCs were able to elicit a primary antiviral cytotoxic T-cell response in autologous, naive CD8 T cells. This may provide a useful strategy for immune therapy and prevention for HIV infection.
Materials and methods
Generation of monocyte-derived dendritic cells and macrophages
DCs were generated from buffy coats or heparinized peripheral blood of healthy donors by using protocols similar to those previously reported.13 14 Peripheral blood mononuclear cells (PBMCs) were isolated by density centrifugation in Ficoll-Paque (Pharmacia, Uppsala, Sweden). Plastic-adherent monocytes were harvested by plating 1 × 108 PBMCs into 75 cm2 tissue culture flask (Costar, Cambridge, MA) in 9 mL of RPMI 1640 (Mediatech, Herndon, VA), supplemented with 10% heat-inactivated human serum AB (Gemini, Calabasas, CA) and 1% nonessential amino acids (Mediatech) (complete medium). After 3 hours, nonadherent cells were removed by vigorously washing 3 times with phosphate-buffered saline (PBS). Adherent cells were incubated for 7 days in complete medium, supplemented with granulocyte-macrophage colony-stimulating factor (GM-CSF) (100 IU/mL; Leukine; Immunex, Seattle, WA) and interleukin-4 (IL- 4) (1000 U/mL; R & D Systems, Minneapolis, MN). Cells were fed every 3 days with fresh cytokines at the same concentrations. On day 5, the nonattached immature DCs, being CCR5+, CD4+, CD86+, and HLA-DR+ but weak in CD83, were depleted of CD3, CD14, and CD20 cells either by cell sorting (FACStar plus, Becton Dickinson, Mountain View, CA) or by immunomagnetic bead depletion (Dynal, Lake Success, NY). Negatively-selected DCs (being CD14-negative and CD11c-positive, when tested on occasion) were transferred into 24-well cell culture plates (Costar) and incubated for 2 additional days in cytokine-containing medium. Mature DCs were generated by incubating immature DCs for l or 3 days in medium supplemented with LPS (10 ng/mL; Sigma Chemical, St Louis, MO) or TNF-α (100 U/mL; R & D Systems), respectively, in addition to GM-CSF and IL-4 supplementation. Macrophages were generated from plastic-adherent PBMCs after incubation in GM-CSF (100 IU/mL) containing medium for 7 days.
For prolonged storage, cells were frozen down in human serum AB supplemented with 10% (v/v) DMSO, using an isopropyl alcohol-filled freezing container as recommended by the manufacturer (Nalgene, Rochester, NY) and finally placed in liquid nitrogen.
Construction and preparation of retroviral vectors
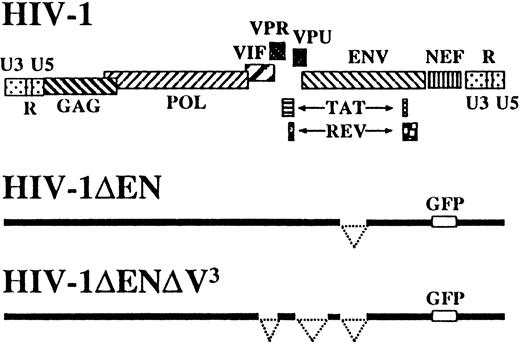
The HIV-1ΔEN vector was originally derived from the HIV-1 clone NL4-3.12 All nt numbering for HIV-1ΔEN V3 is based on the HIV-1ΔEN sequence. The HIV-1ΔEN vector plasmid was digested with ApaI and XhoI to release the 6.2-kiloase (kb) ApaI/XhoI fragment (nt 2005 through nt 8206), which was then subcloned into theApaI/XhoI sites of the pBluescript (pBS) plasmid (Stratagene, La Jolla, CA) as an intermediate to facilitate removal of accessory gene sequences. The entire vpu ORF and the 5′ end of the env sequence (nt 6054-6822) were removed by digestion with AflIII and StuI and religation. The 3′ half (nt 5297-5621) of the vif ORF and 5′ half (nt 5561-5741) of the vpr ORF were deleted by digestion withPflMI (nt 5297) and EcoRI (nt 5741), followed by religation. The truncated ApaI/XhoI fragment was then reinserted into the HIV-1ΔEN backbone to generate the HIV-1ΔEN V3 vector.
Vesicular stomatitis virus G protein (VSV-G) pseudotyped HIV- 1 vectors were prepared by calcium phosphate-based cotransfection of 293T cells (5 × 106 cells; 75 cm2 tissue culture flask, Costar) with plasmids expressing VSV-G and the modified HIV- 1 genome under investigation, containing the (enhanced) green fluorescent protein (EGFP) gene from Aequorea victoria. Cell culture supernatants were collected at 72 hours after transfection. The titers were determined on transduced CRFK cells (ATCC, Manassas, VA) as measured by GFP expression.
Transduction of monocyte-derived dendritic cells
Monocyte-derived DCs were transduced with HIV-1 vectors at multiplicities of infection (MOI) of 5, unless otherwise stated, in the presence of 4 μg/mL polybrene (Sigma), GM-CSF, and IL-4 for 16 hours at 37°C. DCs were then washed and incubated for 4 days in medium supplemented with GM-CSF and IL-4.
Immunofluorescence
Immunophenotyping of cells was accomplished by using phycoerythrin (PE)-conjugated, anti-CD4 (RPA-T4), anti-CD80 (L307.4), anti-CD86 (FUN-1), anti-HLA-DR (L243), isotype control antibody (all from Becton Dickinson), anti-CD40 (EA-5; Biosource International, Camarillo, CA), and anti-CD83 (HB 15A; Immunotech, Marseille, France). The analyses were carried out on a flow cytometer (Epics Elite, Coulter).
Viability assays
The number of viable cells was determined by trypan blue exclusion assay (Sigma). Cells were observed under a Nikon UFX-IIA microscope (Tokyo, Japan). Apoptotic cells were stained with annexin V-PE (PharMingen, San Diego, CA) and quantitated by FACS.
Phagocytosis of latex beads
Immature DCs at a concentration of 105 cells with or without prior transduction were coincubated with 5 × 106red fluorescent microspheres (diameter 1 μm, 2.5% solid, carboxylate-modified latex, Sigma) for varying periods. To distinguish nonspecifically bound beads from phagocytosed beads, the cells were poisoned with 1.0% (w/v) sodium azide before the addition of the red fluorescent microspheres. At the end of the assay, the cells were separated from unengulfed beads by density gradient centrifugation15 and analyzed by FACS.
Mixed leukocyte reaction assay
To assess the antigen-presenting cell function of DCs, irradiated mature DCs (2000 rad, cesium-137 source) at varying concentrations were coincubated with allogenic peripheral blood leukocytes (PBL; 1 × 105) in 96-well U-bottom tissue culture microplates (Costar) for 5 days. [3H]-thymidine (0.037 Mbq [1 μCi] per well; DuPont NEN, Boston, MA) was added 18 hours before harvest, using an automated cell harvester (Skatron, Sterling, VA). Incorporation of [3H]-thymidine into the cells was quantified using a β-counter (Beckman, Fullerton, CA).
Loading dendritic cells with tyrosinase peptide
DCs, with or without transduction counterpart, were loaded with the tyrosinase cytotoxic T-cell epitope (tyrosinase 369-377, YMNGTMSQV) at 20 μg/mL for 1 hour at 37°C. Excess peptide was removed by centrifugation and the cells were washed twice with fresh medium with 5% human serum. DCs were then labeled with 5 μL of [51Cr]-sodium chromate per 50 μL (0.5 mC/mL, Dupont NEN). After washing twice with fresh medium containing 5% human serum, 2 × 103 DCs were then added to each microwell as targets. The CD8+ tyrosinase peptide-specific T-cell line was established by priming with peptide pulsed DCs and restimulating weekly with peptide-pulsed monocytes according to the method of Tsai et al.16 The effector:target cell (E:T) ratios used were 40:1 to 10:1. The cells were cocultured at 37°C for 6 hours and the amount of 51Cr radioactivity released into the supernatant was determined in a Wallach Microbeta scintillation counter (Perkin Elmer, Gaithersburg, MD).
In vitro priming of HIV-1–specific cytotoxic T cells with HIV-1ΔEN transduced dendritic cells
CD8+ T cells were positively selected using immunomagnetic beads coated with CD8 antibody (Dynabeads M-450 CD8, Dynal) and Detachabead (Dynal) according to the manufacturer's specifications. Free beads were removed magnetically and the CD8+ T cells counted and analyzed by flow cytometry to evaluate purity (more than 96%). Recovery of cells was typically more than 80% of CD8+ cells in circulation. This procedure does not activate CD8+ T cells nonspecifically. The purified CD8+ T cells were then mixed with HIV-1ΔEN–transduced DCs at a ratio of 10:1 and incubated for 4 days. Selective expansion of virus-specific T cells was carried out in the presence of low doses of IL-2 (20 U/mL) and IL-7 (30 U/mL), with weekly restimulation with irradiated HIV-transfected HLA-A2.1 Jurkat cells plus cytokines for up to 6 weeks. Virus-specific cytotoxicity was determined by a standard chromium (51Cr) release assay, using virus-infected (HIV-1 NL4-3) HLA A2.1-expressing Jurkat cells (A2.1-Jurkat) as positive targets and uninfected A2.1-Jurkat as negative control targets.
Statistics
Statistical significance was determined by using the Student t test. All comparisons were 2-tailed, and aP value of less than .05 was considered significant.
Results
Efficient transduction and transgene expression of monocyte-derived dendritic cells with pseudotyped HIV vectors
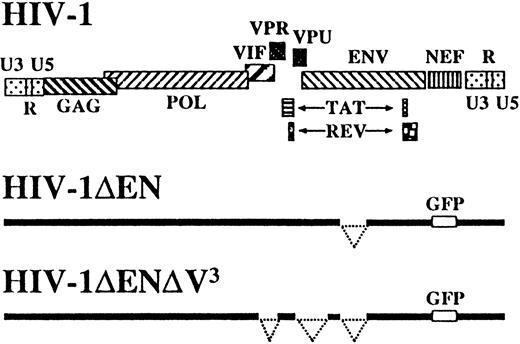
In addition to the previously described HIV-1 vector deleted inenv and nef,12 hence referred to as HIV-1ΔEN, we also constructed a vector deleted in all remaining accessory genes, namely, vpr, vif, andvpu (HIV-1ΔEN V3), to further reduce the possibility of generating pathogenic HIV-1 recombinants during vector production (Figure 1). HIV-1ΔEN V,3 produced at a titer of 1 to 4 × 107transducing units per milliliter on CRFK cells, efficiently transduced various human cell lines, regardless of their CD4 or coreceptor expression status (data not shown). Interestingly, HIV-1ΔEN V3 also transduced nondividing monocyte-derived macrophages, despite the absence of vpr, which was reported to be important for macrophage infection (Figure2A).17 The efficiency of macrophage transduction was comparable for HIV-1ΔEN V3and HIV-1ΔEN (Table 1).
Schematic representation of vector constructs HIV-1ΔEN and HIV-1ΔEN V3 derived from HIV-1 clone NL4-3.
Schematic representation of vector constructs HIV-1ΔEN and HIV-1ΔEN V3 derived from HIV-1 clone NL4-3.
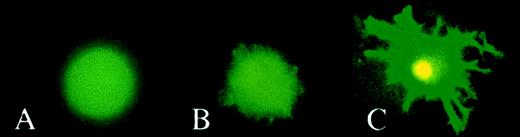
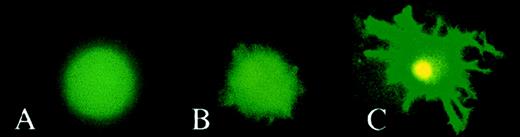
Fluorescence microscopy of transduced cells.
Monocytes were cultivated with GM-CSF or with GM-CSF and IL-4 to generate Mo macrophages (A) and DCs (B and C), respectively. On day 7, cells were transduced with HIV-1ΔEN V3 (A and B) or with HIV-1ΔEN (C) at a MOI of 5 and incubated further in cyokine(s) supplemented medium. On day 5 after transduction, cells were analyzed by fluorescence microscopy. DCs were either analyzed directly (immature DCs; B) or treated with LPS to generate mature DCs (C) before analysis by fluorescence microscopy.
Fluorescence microscopy of transduced cells.
Monocytes were cultivated with GM-CSF or with GM-CSF and IL-4 to generate Mo macrophages (A) and DCs (B and C), respectively. On day 7, cells were transduced with HIV-1ΔEN V3 (A and B) or with HIV-1ΔEN (C) at a MOI of 5 and incubated further in cyokine(s) supplemented medium. On day 5 after transduction, cells were analyzed by fluorescence microscopy. DCs were either analyzed directly (immature DCs; B) or treated with LPS to generate mature DCs (C) before analysis by fluorescence microscopy.
Monocyte-derived DCs, prepared as described in “Materials and methods” and verified to be more than 95% homogeneous, were transduced with the HIV-1 vectors at a MOI of 5 or 50. The percentage of GFP-expressing DCs, as a measure of transduction efficiency, was determined by counting under the fluorescence microscope (Figure 2, Table 1) or by flow cytometry (Figure 3). Transduction efficiencies by both HIV-1ΔEN and HIV-1ΔEN V3 were in the range of 20% to 50% (Table 1) and GFP-expression lasted for at least 14 days. Storage of DCs in liquid nitrogen before transduction did not affect transduction efficiency (data not shown). When the DCs were allowed to mature in the presence of TNF-α or LPS in culture, their susceptibility to transduction was reduced 5-fold (Table 1). Because detection of the fluorescence of GFP requires a high level of gene expression, we conclude that DCs have sufficient transcriptional factors to ensure high-level gene expression from the HIV-1 LTR promoter.
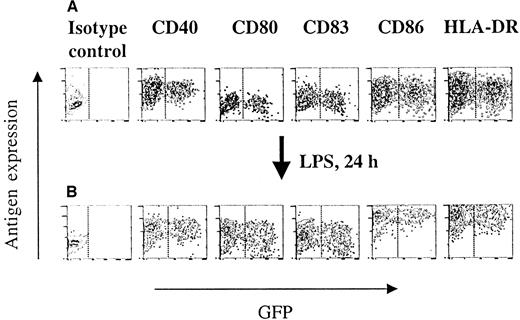
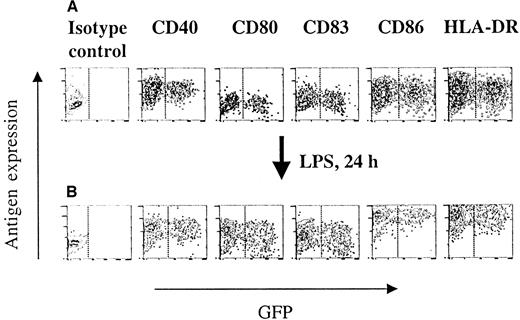
Immunophenotype and differentiation of HIV-1ΔEN V3 transduced immature DCs.
Immature DCs were transduced as described in the legend to Figure 2, and subsequently analyzed for their phenotype by direct immunofluorescence (A). The ability of transduced and untransduced immature DCs to differentiate into mature DCs was investigated by direct immunoflurescence of LPS (10 ng/mL) treated immature DCs (B). One representative experiment of at least 2 experiments is shown.
Immunophenotype and differentiation of HIV-1ΔEN V3 transduced immature DCs.
Immature DCs were transduced as described in the legend to Figure 2, and subsequently analyzed for their phenotype by direct immunofluorescence (A). The ability of transduced and untransduced immature DCs to differentiate into mature DCs was investigated by direct immunoflurescence of LPS (10 ng/mL) treated immature DCs (B). One representative experiment of at least 2 experiments is shown.
Immunophenotype and differentiation of transduced immature dendritic cells
To investigate whether transduction by the lentiviral vectors may have deleterious effects on DCs, flow cytometry was performed comparing the expression of several immunologically important DC surface markers in the transduced (GFP+) and untransduced (GFP−) populations. As shown in Figure 3A, transduction by HIV-1ΔEN V3 had no impact on the expression of HLA-DR, CD86, CD83, CD80, and CD40, as both GFP+ and GFP− cells from the same preparation expressed these markers at similar levels. Essentially identical results were obtained when using HIV-1ΔEN instead of HIV-1ΔEN V3 (data not shown). The majority of DCs cultured for 7 days with GM-CSF and IL-4 stained moderately to strongly for HLA-DR (mean fluorescence intensity, MFI: 15.7), CD86 (MFI: 10.8), CD40 (MFI: 17.7), and at lesser relative intensities for CD80 (MFI: 2.7) and CD83 (MFI: 3.1) when compared with isotype control (MFI: 1.7). This profile is characteristic of functionally immature DCs.18 Transduction of immature DCs did not significantly alter the expression level of CD40 (MFI: 20.8), CD80 (MFI: 3.13), CD83 (MFI: 3.76), CD86 (MFI: 13.4), and HLA-DR (MFI: 18.8) (Figure 3A). Induction of these immature DCs to differentiate into mature DCs was accompanied by an increase in HLA-DR (MFI: 149.4), CD86 (MFI: 139.1), CD83 (MFI: 5.91), and CD80 (MFI: 10.2) expression, whereas the level of CD40 expression stayed unchanged (MFI: 17.9). The expression level of the aforementioned DC surface antigens differed in less than 20% between the GFP+ and GFP− cells in the transduced cell populations (Figure 3B), as well as the untransduced population. Stimulating immature DCs for 72 hours with LPS resulted in an increase in intensity and percentage of CD83-positive DCs (MFI: 9.05; 94.5%), whereas, under the same conditions, TNF-α did not increase either the intensity or the percentage of CD83-positive DCs (MFI: 5.44; 48.4%).
Viability and functional properties of transduced dendritic cells
Because controversial reports exist on whether HIV-1 is cytopathic to DCs,19,20 we further investigated whether transduction of immature DCs by an HIV-1 vector may affect cell viability. We did not observe any changes in the percentage of necrotic or apoptotic DCs as a result of transduction by HIV-1ΔEN V,3 as determined by trypan blue exclusion assay and annexin V staining, respectively (Table 2). This was true even when transduction was carried out at the higher MOI of 50, resulting in 50% transduction of the immature DCs (Table 2).
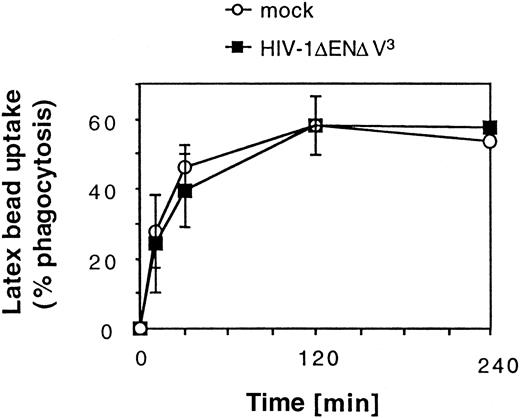
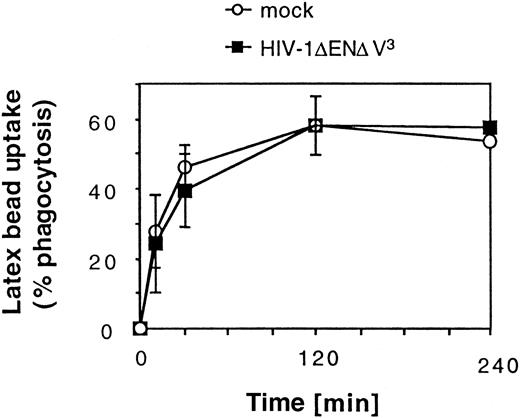
We next examined whether transduction had an impact on 2 characteristic functions of DCs. Phagocytosis of large particles is characteristic of immature DCs (Figure4).6 Our results show that after an incubation period of 2 hours, a plateau was reached at which about 60% of the DC population had phagocytosed more than 3 red fluorescent latex beads per cell. Transduction with HIV-1ΔEN V3 did not have any effect on the ability of DCs to phagocytose latex beads (Figure 4). We also tested whether transduced DCs were equally competent at eliciting an allogenic T-cell response in the mixed leukocyte reaction (MLR). Transduction of the immature DC (GFP+: 31% ± 4%) before maturation did not affect their ability to stimulate alloreactive T-cell proliferation (mean ± SD: 23 842 ± 5381 cpm, n = 2) when compared with untransduced DCs (25 716 ± 5665 cpm, n = 2) at a DC/T-cell ratio of 1:100. Similar results were obtained after sorting of transduced DCs (GFP+: more than or equal to 97%) (Figure5). Mature DCs, however, were approximately 2-fold more potent than immature DCs in stimulating T-cell proliferation, whereas macrophages showed no significant T-cell stimulation (data not shown).
Effect of HIV-1ΔEN V3 transduction on large particle uptake by immature DCs.
Immature DCs were transduced by HIV-1ΔEN V3 as described in the legend to Figure 2. Transduced as well as untransduced DCs were incubated with red fluorescent latex beads and analyzed after varying lengths of time by FACS. Background due to nonspecific binding of latex beads to DCs was determined by incubating sodium azide-treated DCs with latex beads. This value was subtracted from the data shown. Mean ± SD of at least 2 experiments are shown.
Effect of HIV-1ΔEN V3 transduction on large particle uptake by immature DCs.
Immature DCs were transduced by HIV-1ΔEN V3 as described in the legend to Figure 2. Transduced as well as untransduced DCs were incubated with red fluorescent latex beads and analyzed after varying lengths of time by FACS. Background due to nonspecific binding of latex beads to DCs was determined by incubating sodium azide-treated DCs with latex beads. This value was subtracted from the data shown. Mean ± SD of at least 2 experiments are shown.
Effect of HIV-1ΔEN V3 transduction on T-cell stimulation by mature DCs.
DCs were transduced and maturated as described in legends to Figure 2and Figure 3, respectively, and subsequently irradiated. Graded numbers of transduced and sorted (GFP+: more than or equal to 97%) or untransduced DCs were then incubated with 1 × 105allogenic peripheral blood leukocytes in the mixed leukocyte reaction. T-cell proliferation was determined after 5 days by [3H]-thymidine incorporation. Mean ± SD from 2 experiments are shown.
Effect of HIV-1ΔEN V3 transduction on T-cell stimulation by mature DCs.
DCs were transduced and maturated as described in legends to Figure 2and Figure 3, respectively, and subsequently irradiated. Graded numbers of transduced and sorted (GFP+: more than or equal to 97%) or untransduced DCs were then incubated with 1 × 105allogenic peripheral blood leukocytes in the mixed leukocyte reaction. T-cell proliferation was determined after 5 days by [3H]-thymidine incorporation. Mean ± SD from 2 experiments are shown.
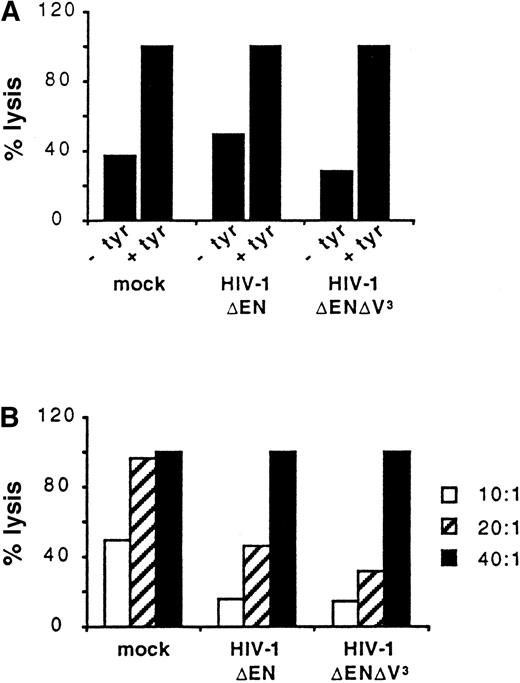
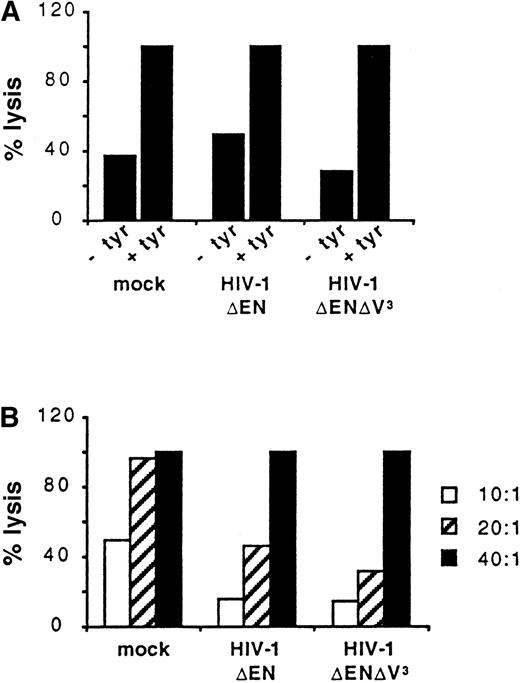
We further compared the ability of untransduced and transduced DCs to present foreign antigens to T cells. Untransduced DCs (mock) and DCs transduced with either HIV-1ΔEN or HIV-1ΔEN V3 were loaded with the tyrosinase cytotoxic T-cell epitope (tyrosinase 369-377, YMNGTMSQV) and used as target cells for a CD8+tyrosinase peptide-specific T-cell line (effector; Figure6). Panel A of Figure 6 shows the percentage lysis of the 3 DC populations at the E:T ratio of 40:1. DCs not pulsed with peptide were also included as controls. In all 3 DC preparations, peptide-specific lysis was detected, because less than 50% lysis was observed when the DCs were not pulsed with the tyrosinase cytotoxic T-cell epitope (Figure 6A). In addition, at the E:T ratio of 40:1, 100% lysis of all DC preparations was observed (Figure 6A,B). The DCs used for the experiment shown in Figure 6 were transduced by HIV-1ΔEN and HIV-1ΔEN V3 with an efficiency of 32% and 37%, respectively. The complete lysis of both preparations demonstrates that transduced DCs are capable of presenting the tyrosinase peptide and are recognized by the peptide-specific cytotoxic T-cell line. Panel B of Figure 6 shows that lysis of the 3 preparations of DCs is dependent on the E:T ratio used. It appeared that the nontransduced DCs are slightly more sensitive to lysis at the lower E:T ratios tested (Figure 6B).
Antigen-presentation by transduced DCs.
Untransduced DCs (mock) and DCs transduced with either HIV-1ΔEN or HIV-1ΔEN V3 were loaded with (+ tyr) or without (−tyr) the tyrosinase cytotoxic T-cell epitope and subsequently co-incubated with a CD8+ tyrosinase peptide-specific T-cell line at the E:T ratio of 40:1 (A). Tyrosinase-specific lysis of the 3 preparations of DCs, as determined by a standard chromium (51Cr) release assay, is dependent on the E:T ratio used (B). Data are from one representative experiment of 2.
Antigen-presentation by transduced DCs.
Untransduced DCs (mock) and DCs transduced with either HIV-1ΔEN or HIV-1ΔEN V3 were loaded with (+ tyr) or without (−tyr) the tyrosinase cytotoxic T-cell epitope and subsequently co-incubated with a CD8+ tyrosinase peptide-specific T-cell line at the E:T ratio of 40:1 (A). Tyrosinase-specific lysis of the 3 preparations of DCs, as determined by a standard chromium (51Cr) release assay, is dependent on the E:T ratio used (B). Data are from one representative experiment of 2.
In vitro priming of HIV-1–specific cytotoxic T cells with HIV-1ΔEN–transduced dendritic cells
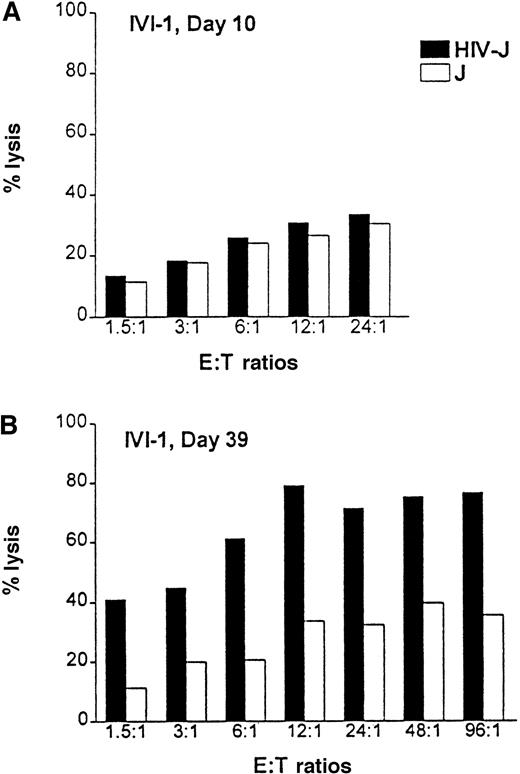
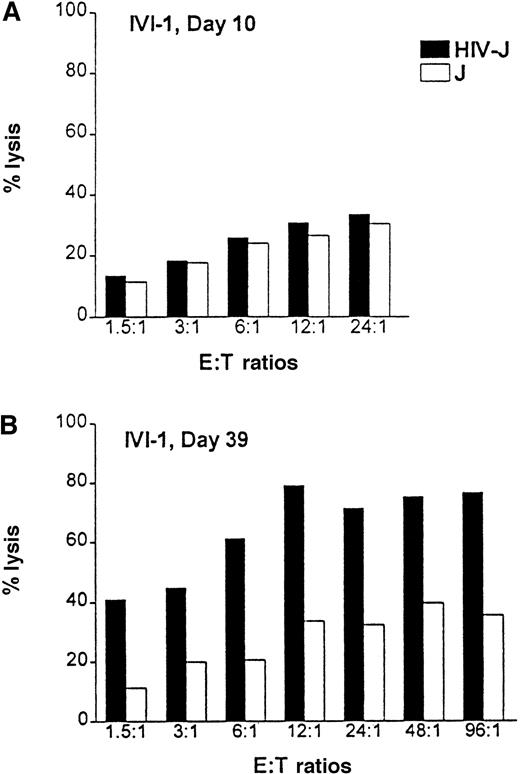
Positively selected CD8+ T cells from healthy HLA-A2.1 volunteers were primed in vitro with HIV-1ΔEN–transduced DCs. Purified CD8+ T cells were cocultured with HIV-transduced autologous DCs at a ratio of 10:1 in the presence of IL-7 and IL-2 to expand HIV-specific cytotoxic T-lymphocytes (CTL). The T-cell culture was restimulated weekly with irradiated HIV-transfected HLA-A2.1 Jurkat cells. HIV-specific cytotoxicity was measured by a standard chromium (51Cr) release assay, using HIV-1–infected or –uninfected Jurkat cells expressing the HLA-A2.1 epitope as target cells (T). One week after in vitro immunization, the CD8 T cells (effector cells, E) showed little specific killing of the infected cells at all the E:T ratios (1.5:1 to 24:1) (Figure7A). However, by day 39, the essentially homogeneous CD8+ T-cell cultures were highly cytotoxic to the HIV-1–infected HLA-A2.1 Jurkat cells at all E:T ratios tested (1.5:1 to 96:1). Lysis of the HIV-infected Jurkat cells was 40%, even at the low E:T ratio of 1.5:1 (Figure 7B). In contrast, lysis of the control Jurkat cells was at a background level of 10%. Maximal HIV-specific lysis (80%) was achieved at the E:T ratio of 12:1. Cytotoxicity was class I mediated, as evidenced by almost complete inhibition of lysis in the presence of a class I-specific blocking monoclonal antibody w6/32 (data not shown). A total of 3 donors have been studied with essentially the same results. In addition, transduced DCs themselves were lysed by the HIV-1–specific CTL (data not shown).
Priming of HIV-specific CTLs with HIV-1ΔEN–transduced DCs.
CD8+ T cells from HLA-A2.1 donors were cocultivated with HIV-1ΔEN–transduced DCs (transduction efficiency: more than or equal to 40%) for up to 5 weeks. After weekly restimulation of T cells with transduced DCs and expansion of CTLs (effector cells, E) by addition of IL-2 and IL-7, HIV-specific cytotoxicity was determined by a standard chromium (51Cr) release assay at week 1 (A) and week 5 (B) after coculture. HIV-1– infected (HIV-J) or –uninfected A2.1 Jurkat cells (J) were used as target cells (T). Data are from one representative experiment (donor IVI-1) of 3.
Priming of HIV-specific CTLs with HIV-1ΔEN–transduced DCs.
CD8+ T cells from HLA-A2.1 donors were cocultivated with HIV-1ΔEN–transduced DCs (transduction efficiency: more than or equal to 40%) for up to 5 weeks. After weekly restimulation of T cells with transduced DCs and expansion of CTLs (effector cells, E) by addition of IL-2 and IL-7, HIV-specific cytotoxicity was determined by a standard chromium (51Cr) release assay at week 1 (A) and week 5 (B) after coculture. HIV-1– infected (HIV-J) or –uninfected A2.1 Jurkat cells (J) were used as target cells (T). Data are from one representative experiment (donor IVI-1) of 3.
Discussion
Gene therapy of DCs offers the promise of new therapies for cancer, AIDS, and autoimmune diseases. The use of lentiviral vectors for gene delivery is superior to the use of oncogenic retroviruses, as lentiviral vectors enable the transduction of not only dividing but also nondividing cells, including the highly differentiated DCs. In this study, we show that immature DCs were efficiently transduced (up to 50% at the highest MOI tested) by HIV-1–based vectors pseudotyped by the VSV-G protein. These vectors are deleted in the envgene, as well as one or all of the accessory genes (vif,vpr, vpu and/or nef) of HIV-1. The efficient transduction of nondividing DCs by both vectors may be explained by the following: (1) replacement of the HIV-1 envelope protein by the VSV-G protein eliminates the requirement for Nef for enhanced HIV-infection21 and (2) vpr is dispensable for targeting the viral preintegration complex to the nucleus in nondividing cells due to functional redundancy of Vpr, matrix protein (MA), and integrase.22-24 In addition, it has been shown recently that the requirement of accessory genes for the transduction of growth-arrested or nondividing cells is not absolute and differs between individual cell types.25-27 However, in contrast to results of Connor et al,17 we did not findvpr to be important for transduction of macrophages. We also observed that cryopreserved DCs were transduced with an efficiency comparable to freshly cultivated immature DCs. Because cryopreservation also does not adversely affect the isolation/function of DCs derived from PBMCs,28 it is feasible then to store patient samples for later transduction and modification.
We also found that transduction did not affect the viability, immunophenotypes, and functions of DCs, or the ability of immature DCs to differentiate into mature DCs in vitro. In this regard, the HIV-1–based vectors described here differ from vaccinia virus vectors, which were reported to interfere with DC differentiation and cause cell toxicity.29 Interestingly, the ability to transduce mature DCs by HIV-1ΔEN V3 was markedly reduced when compared with transduction in immature DCs. This result is in accordance with previous observations that reverse transcription of HIV-1 is blocked in mature DCs, probably before the synthesis of plus-strand DNA.30 Finally, our data indicate that the transduced DCs are able to process and present HIV antigens and are capable of immunizing autologous, naive CD8+ T cells in vitro.
Live (replication-competent) attenuated AIDS vaccines, so far the most efficacious vaccines because of their ability to generate a sustained and broadly effective immunity,31 nonetheless present a safety concern because of their potential of inducing AIDS in a significant proportion of vaccinated animals.31-33 Because the vector systems used in this study allow only one round of infection, the virus load in vivo is unlikely to reach a pathogenic threshold. The deletion of env and all 4 accessory genes within the HIV-1 genome should further improve safety by eliminating the possibility of generating fully infectious HIV-1 during vector production. Further studies using self-inactivating vector systems and/or vectors based on less pathogenic lentiviruses, such as HIV-2 or FIV, are in progress. Retroviral vector systems based on Moloney murine leukemia virus (MMLV) would not be suitable for this approach because they are inefficient for transduction of terminally differentiated cells.34 Our data further suggest that HIV-1 vectors expressing viral antigens can target the critical antigen-presenting cells and may be useful for in vivo active immunization as well as ex vivo priming of cytotoxic T cells for adoptive T-cell therapy. The conservation as well as tolerance to substitutions of CTL epitopes allow cross-recognition among different HIV-1 clades,35thus ensuring the broad application of a vaccine derived from a single clade. Monocyte-derived DCs from HIV-infected subjects may be well suited for this immunotherapy approach because they have been shown to preserve their immunostimulatory functions while lacking HIV-1 DNA expression.36 Ex vivo–transduced DCs may either be directly reinfused into the patient or used for in vitro immunization of HIV-specific CTL for subsequent adoptive transfer. Defective Th-cell function resulting in the absence of a response to recall antigens could be circumvented by costimulating the PBMCs of these HIV-positive subjects with irradiated allogenic leukocytes from HIV-negative donors, as has been shown recently for influenza A virus (FLU).37In conclusion, DCs modified by lentiviral vectors expressing viral antigens may be considered as a promising avenue for active immunotherapy for HIV.
Acknowledgments
We would like to acknowledge the expert technical assistance of Tim Marsh, Maureen Ibanez, and May Yen. We are also thankful to Drs A. Wainstein and A. P. Luge (Karmanos Cancer Institute, Detroit, MI) for providing technical support and materials for the antigen presentation assay, Prof Dr E. O. Riecken (Freie Universität Berlin, Germany) for his support, and to the UCSD CFAR for use of core facilities.
Supported by NIH grants AI44372, AI45992; Deutsche Forschungsgemeinschaft (DFG) Bonn, Germany (A.G.); NIH AIDS training grant (K.K.); Japanese Foundation for AIDS Prevention (T.M.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Flossie Wong-Staal, UCSD School of Medicine, 9500 Gilman Dr, La Jolla, CA 92093-0665; e-mail: fwongstaal@ucsd.edu.

![Fig. 5. Effect of HIV-1ΔEN V3 transduction on T-cell stimulation by mature DCs. / DCs were transduced and maturated as described in legends to Figure 2and Figure 3, respectively, and subsequently irradiated. Graded numbers of transduced and sorted (GFP+: more than or equal to 97%) or untransduced DCs were then incubated with 1 × 105allogenic peripheral blood leukocytes in the mixed leukocyte reaction. T-cell proliferation was determined after 5 days by [3H]-thymidine incorporation. Mean ± SD from 2 experiments are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/4/10.1182_blood.v96.4.1327/4/m_h81600046005.jpeg?Expires=1769260905&Signature=zBU5pR-fMbs-S3pqaIz1QuGoi3Y0bEjTpuedZhbK1-GCwqLw0i8k0uwww22cr8FaRh4njy0bWkPK9vu5nHYV6lS7sg0zoKLKWih3MATr2wwYy7lIUcdvU-ZwM4DDtNX3ZWfppPRQtVYpltM6qzxis6wd~AvRvd0hrPwrZx3a5yKvkdrelF60zLXx4tahIVr6jy1tYN8bs8YICEDLNAw5QONwIbgciv0oPx0Asoxis3~vunUk-yHShXY6ShSjQ0LQIhtG4h7ivjzN64v3OG2XhIS3KkkWV462Jkj35ncwzlUGxltuHjLiSe6MAjwiVPhovT0rU67RUEORxlrnp62VIg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 5. Effect of HIV-1ΔEN V3 transduction on T-cell stimulation by mature DCs. / DCs were transduced and maturated as described in legends to Figure 2and Figure 3, respectively, and subsequently irradiated. Graded numbers of transduced and sorted (GFP+: more than or equal to 97%) or untransduced DCs were then incubated with 1 × 105allogenic peripheral blood leukocytes in the mixed leukocyte reaction. T-cell proliferation was determined after 5 days by [3H]-thymidine incorporation. Mean ± SD from 2 experiments are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/4/10.1182_blood.v96.4.1327/4/m_h81600046005.jpeg?Expires=1770202895&Signature=SUjGby0i3k1Xr9Kp2TgBCsnvCK2UEaieZrZfCq0pwk9oVPecf4s14KwXCH2348BKqmPvSmp5tPN8BGInRLKTEnb73VKCJ5HwXQ-pILsMKrzKsG92kRYEQoEucMqeVNwhp-wVEmFnxMH7QSIc7BfptnnQd75Z4~G~NHeSsHB6CTqIkP52nVCUBmvfAsbWbZJbOljmARU0wrJpM6~ynyQDgS19Dq~dSWrgkrUSAncdwFjE-hL-haGMlCUSO~3aT0pWFE~c01kUnBWgut6n~t9Mbi4mbdCZvuW0YMdtbytN2QFrsL-QPGxueEdUVp6v59lCDGZEt0~c0CIq6jkhvZOvVg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)