Abstract
We report on a family with a history of venous thromboembolism associated with fibrinogen Paris V (fibrinogen A-Arg554→Cys). Ten members experienced thrombotic events, including 4 with fatal pulmonary emboli. Pulmonary embolism was the presenting feature in 4. Those with the mutation and a history of thrombosis had somewhat higher fibrinogen concentrations than those with the mutation and no thrombosis (294 ± 70 mg/dL vs 217 ± 37 mg/dL, respectively). The Paris V mutation consistently caused a prolongation of the reptilase time, and fibrin clots containing the abnormal fibrinogen were more translucent than normal clots. Given the early onset of symptoms and the initial presentation with pulmonary embolism in some family members, it was justifiable to offer prophylactic anticoagulation with warfarin to carriers of the mutation. Fibrinogen Paris V has now been reported in 4 apparently unrelated families, indicating that it is a relatively common cause of dysfibrinogenemia-associated thrombosis.
Dysfibrinogenemia is a rare disorder; only 300 or so cases have been reported to date.1,2 Approximately 20% are associated with thrombotic episodes,2,3 but a cause-and-effect relationship has not been clearly established in many instances. In 1995 Haverkate and Samama3 determined that convincing evidence for an association between dysfibrinogenemia and thrombophilia existed for only 26 reported cases. One of the best-characterized thrombosis-associated dysfibrinogenemias, fibrinogen Paris V, was first described as the Dusart syndrome in a French family in 1983.4 Another abnormal fibrinogen, Chapel Hill III,5 was subsequently demonstrated to result from the same mutation that causes the Dusart syndrome.6 Fibrinogen Paris V (fibrinogen Aα-Arg554→Cys) has properties that could facilitate thrombus formation, including poor plasminogen binding, impaired fibrin-dependent tissue plasminogen activator activation, and increased tendency to self-associate.7 8 We present a family with fibrinogen Paris V and a striking history of venous thromboembolism, and we discuss laboratory findings that facilitate the diagnosis of this disorder.
Patients, materials, and methods
Family history
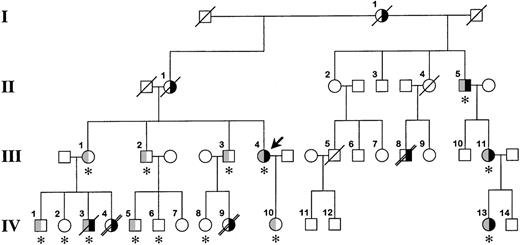
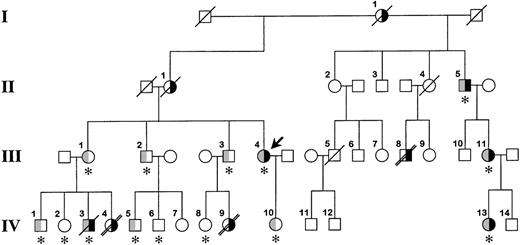
The family pedigree is shown in Figure1. The proband, a 47-year-old white woman (III.4 in Figure 1), had 2 episodes of axillary vein thrombosis at 21 years of age and was treated for 4 years with warfarin. She has been well since then. She requested consultation because of a family history of venous thromboembolism and early death. The proband's mother (II.1) and maternal grandmother (I.1) had recurrent lower-extremity deep vein thromboses. Her siblings (III.1, III.2, and III.3) are asymptomatic; however, several of their children were severely affected. A daughter (IV.4) of the proband's sister (III.1) died suddenly at 12 years of age. An autopsy determined pulmonary embolism to be the cause of death. Her brother (IV.3) experienced a deep vein thrombosis in his arm 1 year ago. He died at 30 years of age, 12 hours after a syncopal episode. No autopsy was performed. A daughter (IV.9) of the proband's brother (III.3) died at age 17 years after a respiratory illness; an autopsy revealed multiple pulmonary emboli. A half-uncle (II.5) has had multiple lower-extremity deep vein thromboses, and his daughter (III.11) and granddaughter (IV.13) have had pulmonary emboli, the latter at age 17 years. Dysfibrinogenemia (type undetermined) was diagnosed in all 3 of them several years ago, and they have remained asymptomatic on warfarin therapy. Finally, patient III.8 experienced multiple deep vein thromboses starting at age 14 years and died of a presumed pulmonary embolus at 45 years of age.
Family pedigree.
The bold arrow indicates the proband. Roman numerals to the left of the figure indicate the generation, and Arabic numerals above each symbol indicate the family member. The right side of the symbol is black for family members who have experienced venous thrombosis. The left half of the symbol is gray for family members heterozygous for the fibrinogen Paris V mutation. The asterisk below the symbol indicates that DNA was available for identification of the fibrinogen Paris V mutation. A diagonal slash through the symbol indicates death, and a double slash indicates that death was from pulmonary embolism. It is possible that patient IV.3 died from a pulmonary embolus; however, this was not established during the hospital stay, and no autopsy was performed.
Family pedigree.
The bold arrow indicates the proband. Roman numerals to the left of the figure indicate the generation, and Arabic numerals above each symbol indicate the family member. The right side of the symbol is black for family members who have experienced venous thrombosis. The left half of the symbol is gray for family members heterozygous for the fibrinogen Paris V mutation. The asterisk below the symbol indicates that DNA was available for identification of the fibrinogen Paris V mutation. A diagonal slash through the symbol indicates death, and a double slash indicates that death was from pulmonary embolism. It is possible that patient IV.3 died from a pulmonary embolus; however, this was not established during the hospital stay, and no autopsy was performed.
Plasma assays
Blood for plasma and DNA preparation was collected into 3.2% sodium citrate for the patients listed in Table 1. Partial thromboplastin, prothrombin, thrombin, and reptilase times and fibrinogen level (Clauss method) were measured using an STA coagulation analyzer (Diagnostica Stago, Asnieres-sur-Seine, France). Fibrinogen antigen concentrations were determined by enzyme-linked immunosorbent assay (Accurate Chemicals, San Diego, CA) using standardized plasmas from George King (Overland Park, KS) to establish standard curves. Results of activity assays for protein C, protein S, and antithrombin III were normal in all persons, and no one had activated protein C resistance, a lupus anticoagulant, or elevated anticardiolipin antibodies.
DNA analysis
Exons of the fibrinogen Aα, Bβ, and γ genes from the proband and a healthy control subject were amplified by polymerase chain reaction (PCR), and the DNA sequence of each product was determined. The proband is heterozygous for a C-to-T substitution in exon 5 of the fibrinogen Aα gene, changing arginine 554 to cysteine. This is the fibrinogen Paris V mutation. To screen family members, a 518-bp fragment of the relevant part of fibrinogen Aα exon 5 was amplified by PCR and sequenced. The PCR primers are 5′-CGTTACTAAGACTGTTATTGGTC-CTG-3′ and 5′-AAGGAGAAGTGTGGATACCTCTG-3′. Neither the factor V Leiden nor prothrombin gene 20210 G→A polymorphisms were identified in this family.9 10
Plasma clot optical density measurements
The increase in optical density of liquid plasma as it changes to solid clot was measured as follows. Plasma samples (100 μL) were placed in 96-well microtiter plates, 50 μL of 50 mmol/L CaCl2 in 0.15 mol/L NaCl was added, and the change in optical density at 405 nm was followed until it reached a maximum. Reactions were performed at 37°C using a Thermomax microtiter plate reader (Molecular Devices, Sunnyvale, CA). The ΔOD 405 nm was determined by subtracting the maximum optical density of the clot from that of a 100 μL sample of liquid plasma supplemented with 50 μL of 0.15 mol/L NaCl.
Results and discussion
Ten family members had at least 1 venous thromboembolic event each, and 6 had diagnosed, or likely, pulmonary embolism (4 fatal cases). Pulmonary embolism was the presenting event in 4 of them. All patients with thrombosis for whom DNA was available were heterozygous for fibrinogen Paris V. In all, 11 family members have the mutation, and at least 5 deceased members were likely or obligate heterozygotes. A remarkable feature of this family is that some carriers of the mutation are asymptomatic, whereas their children are severely affected at young ages. In family members with fibrinogen Paris V, the fibrinogen concentration was higher in those with a history of thrombosis (Clauss 294 ± 70 mg/dL; antigen 356 ± 98) than in asymptomatic persons (Clauss 217 ± 37 mg/dL; antigen 229 ± 60). The presence of a thrombus or inflammation can acutely increase fibrinogen levels; however, no such process was present in any family member at the time blood samples were collected. Elevated plasma fibrinogen is clearly a risk factor for arterial thrombotic events,11,12 and an association has also been demonstrated for venous thrombosis.13 14 Our data suggest that fibrinogen concentration may be related to thrombosis in carriers of fibrinogen Paris V. No other common inherited or acquired factor predisposing to venous thrombosis was identified in this family. It must be noted, however, that the number of patients was too small for us to conclude definitively that fibrinogen concentration is a major determinant of thrombosis risk in patients with fibrinogen Paris V. It is common practice to withhold anticoagulation therapy from asymptomatic patients with a genetic predisposition to thrombosis or to reserve it for situations in which risk is increased (eg, during pregnancy, after surgery). Given the early onset of symptoms and the initial presentations of fatal pulmonary embolism in this family, we think it is justifiable to offer prophylactic anticoagulation with warfarin to family members with the mutation.
In the original study4 of fibrinogen Paris V (Dusart syndrome), it was noted that thrombin times were prolonged in affected persons to a greater extent than reptilase times. In contrast, in our study, reptilase times were prolonged in all patients with fibrinogen Paris V (heterozygotes, 47 ± 7 seconds; normals, 21 ± 1 second) and were prolonged to a greater extent than were thrombin times (heterozygotes, 30 ± 5 seconds; normals, 21 ± 1 second). Indeed, 2 carriers of the mutation had normal thrombin times. A possible explanation for this inconsistency is that human thrombin was used in our thrombin time assay, whereas bovine or equine thrombin was used in earlier studies.4 In our hands, the reptilase time appeared to be the better screening test for this condition. Clots from fibrinogen Paris V plasma have been noted to be more translucent than normal clots.4 Changes in clotting plasma optical density for carriers of fibrinogen Paris V in this family were substantially less than for normals (0.04 ± 0.06 OD units vs 0.60 ± 0.11 OD units). In fact, on visual inspection, the Paris V plasmas did not change appreciably during clotting. This finding, in conjunction with the reptilase time, may be useful for screening.
Dysfibrinogenemias are estimated to account for less than 1% of inherited thrombophilia.3,15 However, the incidence of these disorders is not known accurately because common screening tests, such as the thrombin and reptilase times, are unlikely to identify all fibrinogen variants. In 1994 Wada and Lord6 reported that fibrinogens Dusart and Chapel Hill III are identical. The responsible mutation was subsequently designated fibrinogen Paris V.16To our knowledge, this family is not closely related to either of the previously reported families. An additional patient with thrombosis and the Paris V mutation was identified recently in a German family.17 It is likely that the incidence of fibrinogen Paris V is low compared with that of other mutations predisposing to thromboembolism. However, the mutation has now been identified in 4 separate instances, raising the possibility that it is widespread and should be considered in the evaluation of a family with a history of thromboembolism. Reptilase time and measurement of plasma clot optical density are reasonable screening strategies for the disorder.
Acknowledgments
We thank Drs Don Gabriel (University of North Carolina, Chapel Hill, NC) and Michael Mosesson (Southeast Wisconsin Blood Center, Milwaukee, WI) for helpful discussions during manuscript preparation. We thank Jean McClure for artwork.
Supported by grant HL58837 from the National Heart, Lung, and Blood Institute. D.G. is an Established Investigator of the American Heart Association.
Reprints:David Gailani, Division of Hematology/Oncology, Vanderbilt University, 538 MRB II, 2220 Pierce Ave, Nashville, TN 37232-6305; e-mail: dave.gailani@mcmail.vanderbilt.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.