Abstract
This study was designed to determine if macrophage inhibitory protein-1 (MIP-1), a recently described osteoclast (OCL) stimulatory factor,1 was present in marrow from patients with multiple myeloma (MM) and possibly involved in the bone destructive process. MIP-1, but not interleukin-1β (IL-1β), tumor necrosis factor-β (TNF-β), or interleukin-6 (IL-6), messenger RNA was elevated in freshly isolated bone marrow from 3 of 4 patients with MM compared to normal controls. Furthermore, enzyme-linked immunosorbent assays of freshly isolated bone marrow plasma detected increased concentrations of hMIP-1 (range, 75-7784 pg/mL) in 8 of 13 patients (62%) with active myeloma, in 3 of 18 patients (17%) with stable myeloma (range, 75-190.3), as well as in conditioned media from 4 of 5 lymphoblastoid cell lines (LCLs) derived from patients with MM. Mildly elevated levels of MIP-1 were detected in 3 of 14 patients (21%) with other hematologic diagnoses (range, 80.2-118.3, median value of 96 pg/mL) but not in normal controls (0 of 7). MIP-1 was not detected in the peripheral blood of any patients with MM. In addition, recombinant hMIP-1 induced OCL formation in human bone marrow cultures. Importantly, addition of a neutralizing antibody to MIP-1 to human bone marrow cultures treated with freshly isolated marrow plasma from patients with MM blocked the increased OCL formation induced by these marrow samples but had no effect on control levels of OCL formation. Thus, high levels of MIP-1 are expressed in marrow samples from patients with MM, but not in marrow from patients with other hematologic disorders or controls, and support an important role for MIP-1 as one of the major factors responsible for the increased OCL stimulatory activity in patients with active MM.
Multiple myeloma (MM) is an incurable plasma cell neoplasm that accounts for 13% of hematologic malignancies.1 Bone destruction is a common manifestation of the disease and is a major source of morbidity for these patients. Bone destruction results from increased osteoclastic bone resorption and decreased bone formation that occur only in areas of bone adjacent to myeloma cells.2 3 These data suggest that the bone disease results from local production of an osteoclast stimulatory factor (OSF) that is secreted by myeloma cells, marrow stromal cells, or both. The identity of this factor(s) in vivo is currently unknown.
In vitro studies have implicated several cytokines as responsible for bone destruction in myeloma,4 including interleukin-1β (IL-1β),5,6 interleukin-6 (IL-6),7 and lymphotoxin.8 Nevertheless, none of these cytokines has been found to be elevated consistently in the peripheral blood of patients with MM.9 Failure to detect these factors may reflect the possibility that they are only secreted locally in areas of the bone marrow that contain increased numbers of malignant plasma cells. Alternatively, other factors may be responsible for the bone destruction in patients with MM.
To try to identify the OSF present in patients with MM, we tested freshly isolated bone marrow plasma from heparinized marrow aspirates from patients with MM for their capacity to induce osteoclast (OCL) formation in human marrow cultures and have further characterized this activity. Our results show that bone marrow plasma from patients with MM significantly stimulated OCL formation in human bone marrow cultures when compared to normal controls. This OSF differed from the bone-resorbing cytokines previously implicated in myeloma bone disease. These data suggested that a different factor may be responsible for the bone destruction in patients with MM. Therefore, we determined if the chemokine macrophage inflammatory protein-1α (MIP-1α) was present in marrow samples from patients with MM.
Macrophage inflammatory protein-1α is a low molecular weight chemokine that can stimulate phagocyte activity,10 induce OCL formation in rat marrow cultures,11 and is chemotactic for OCLs.12 MIP-1α belongs to the RANTES family of chemokines in which the first 2 cysteine residues of a conserved 4 cysteine motif are not separated by an intervening amino acid (C-C). These chemokines act as chemoattractants and activators of monocytes. MIP-1α inhibits hematopoiesis by inhibiting the proliferation of CD34+ cells and has been implicated in the pathogenesis of anemia in patients with MM.13 In the current study we show that MIP-1α is an OCL-stimulating factor in human marrow cultures and that it is overexpressed in patients with MM but not in controls. More important, a neutralizing antibody to MIP-1α blocks the OSF activity present in bone marrow plasma from MM patients. These data suggest MIP-1α may be a major mediator of the bone destruction seen in patients with MM.
Materials and methods
Bone marrow samples
Bone marrow aspirates were collected into heparinized syringes from patients with MM (Durie-Salmon, stages I-III), normal controls, and patients with a variety of hematologic diseases involving the marrow. All patients gave informed consent, and these studies were approved by the Institutional Review Board of the University of Texas Health Science Center at San Antonio. Bone marrow (10 mL) was collected in a single aspiration from each subject, and an aliquot of the bone marrow sample was stained with Wright-Giemsa for histologic examination. The percentage of plasma cells present in the sample was determined by morphologic criteria. The bone marrow was pelleted by centrifugation at 1000 × g at 4°C immediately after collection, and the bone marrow plasma was collected and stored at −80°C for subsequent studies. The cells were resuspended in α-Minimal Essential Media (αMEM)/5% fetal calf serum (FCS), and the mononuclear cell fraction separated by gradient centrifugation.14Mononuclear cells were resuspended at 1 mL/5 × 106cells and RNA was extracted from the cells using RNAzol according to the manufacturer's protocol (Biotecx Laboratories, Houston, TX). Peripheral blood plasma samples from the patients were collected at the time of bone marrow examination.
Cell lines
Lymphoblastoid cell lines (LCLs) derived from patients with MM including IM9,15 RPMI-8226,16MCCAR,17 SKO,18 and ARH-7719 were grown in RPMI 1640 media containing 10% FCS (Gibco, Grand Island, NY) for 5 days. Initially, 5 × 106 cells were plated. The IM9, MCCAR, and ARH-77 cell lines are Epstein-Barr virus (EBV)-transformed cells. After 5 days of culture, conditioned media were collected and saved in aliquots at −80°C for enzyme-linked immunosorbent assays (ELISA).
Osteoclast formation assays
Nonadherent marrow mononuclear cells from normal donors were prepared as previously described14 and resuspended in αMEM/20% horse serum (αMEM, Gibco; horse serum, Hyclone, Logan, UT) at 106 cells/mL in quadruplicate. The normal marrow cells (1 × 105 cells/well) were plated in 96-well plates and treated with varying concentrations of bone marrow plasma from MM patients and controls or recombinant MIP-1α. In selected experiments, neutralizing antibodies to MIP-1α, tumor necrosis factor (TNF)-β, IL-6, or IL-1β (anti-MIP-1α and TNF-β: R & D, Minneapolis, MN; anti-IL-6 and IL-1β: Genzyme, Cambridge, MA) were added to cultures treated with bone marrow plasma. Cultures were maintained in a humidified atmosphere of 4% CO2 and air at 37°C for 3 weeks. The cultures were fed weekly by replacing half the media with an equal volume of fresh media. Cells were fixed with 2% formaldehyde in phosphate-buffered saline (PBS), and the number of OCL-like multinucleated cells scored with the 23c6 monoclonal antibody, which identifies OCLs (generously provided by Dr Michael Horton, St. Bartholomew's Hospital, London, UK).20 Binding of the 23c6 monoclonal antibody was assessed with biotin-conjugated rabbit antimouse IgG coupled to alkaline phosphatase (Vector Laboratories, Burlingame, CA). The cells were counterstained with methyl green.21 We have previously demonstrated that multinucleated cells that cross-react with the 23c6 monoclonal antibody (23c6Ab+ multinucleated cells) express calcitonin receptors, contract in response to calcitonin, and form resorption lacunae on calcified matrices, all phenotypic characteristics of OCLs.14 21-23 OCL-like cell formation in these cultures ranged from 50 to 150 23c6+ multinucleated cells per 105 marrow cells plated, depending on the donor marrow used.
Messenger RNA analysis
Total RNA was analyzed by RNAse protection assay using probes derived from the human MIP-1α messenger RNA (mRNA) sequence. Radiolabeled human MIP-1α antisense transcripts were synthesized from linearized DNA templates, using 32P-UTP (800 Ci/mole; Amersham, Arlington Heights, IL) and the MIP-1α-specific primers [5′ ACA TTC CGT CAC CTG TCA AG 3′ (sense) and 5′ CGG TCG TCA CCA GAC GCG G 3′ (antisense)]. RNAse protection assays were performed using the RPA III kit (Ambion Inc, Austin, TX) with human reduced glyceraldehyde-phosphate dehydrogenase (GAPDH) as the internal control. RNA (10 μg) from normal donors and patients with MM were hybridized with 50 000 cpm of antisense RNA probe. After gel analysis followed by autoradiography, protected bands were quantified by densitometry.
Measurement of IL-6, IL-1β, lymphotoxin, and MIP-1 levels in bone marrow plasma from patients with MM and controls
The concentrations of IL-6, IL-1β, lymphotoxin, and MIP-1α in the bone marrow plasma from patients with MM, normal controls, and patients with other hematologic malignancies were determined using specific ELISAs, following the manufacturers' protocols (IL-6 and IL-1β, Genzyme; lymphotoxin and MIP-1α, R & D Systems). The concentration of MIP-1α was also measured in media conditioned for 5 days by 5 human LCLs. These assays can detect as little as 20 pg/mL of the respective cytokines. MIP-1α levels were considered significantly elevated if they were 2 SD above the upper limit of MIP-1α detected in bone marrow plasma from 7 normal individuals, that is, 42.49 + 2(15.9). Therefore, levels of MIP-1α greater than 75 pg/mL were considered to be significantly elevated.
Statistical analysis
Results are presented as the mean ± SEM. Differences between the means were compared by a 1-way analysis of variance and considered significant for P less than .05.
Results
Effects of bone marrow plasma from patients with MM and controls on OCL formation
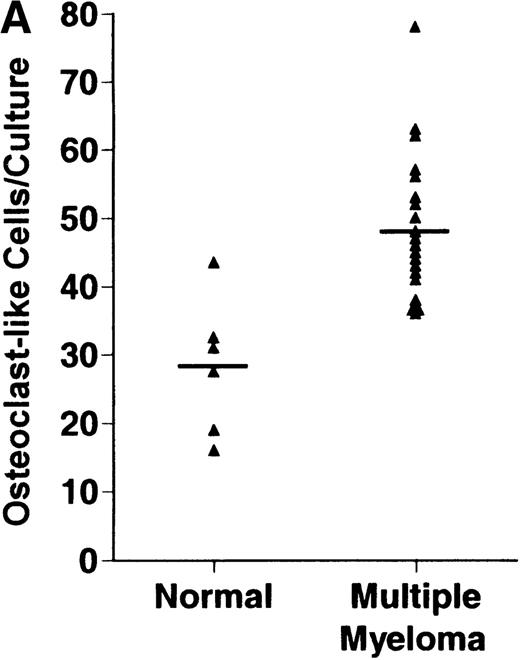
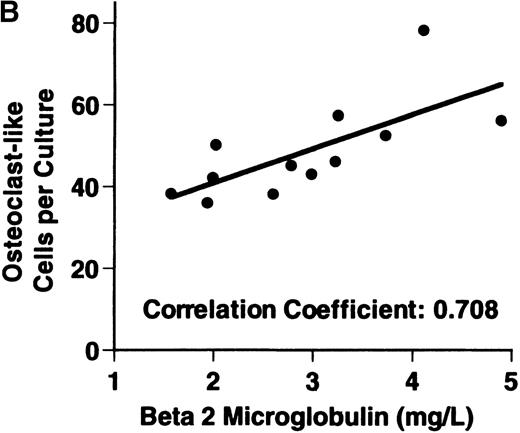
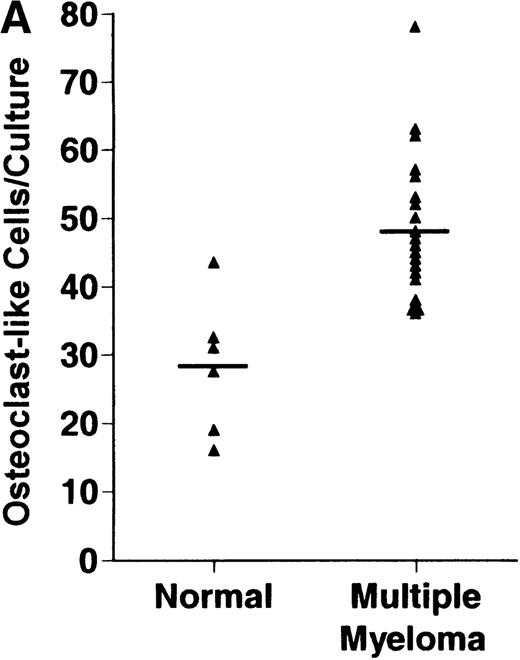
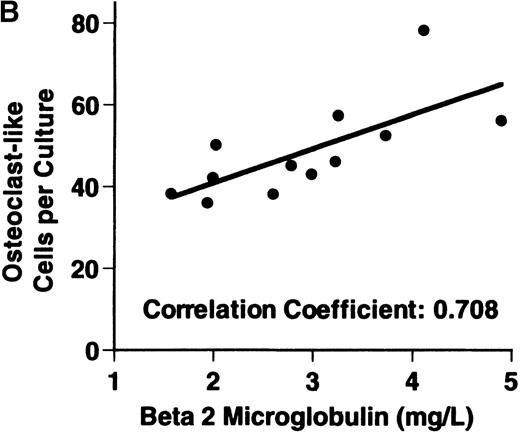
Bone marrow plasma from 24 of 27 patients with MM tested significantly stimulated the formation of OCLs in human bone marrow cultures when compared to controls (47.75 ± 6 [mean ± SEM for the 24 patients] versus 28.25 ± 5 23c6+multinucleated cells/105 cells plated [mean ± SEM] for the 6 controls; P < .05) (Figure1A). The relative levels of OCL formation induced by marrow plasma from patients with MM with normal renal function positively correlated with β2 microglobulin levels (Figure 1B; correlation coefficient = 0.708).
Stimulation of OCL formation.
(A) Effects of bone marrow plasma from controls and patients with MM on OCL formation in human marrow cultures. Bone marrow plasma and marrow cultures were processed as described in “Materials and methods.” Results represent the mean ± SEM for all assays done in quadruplicate. Bone marrow plasma from patients with MM significantly stimulated the formation of OCLs in human bone marrow cultures when compared to controls (47.75 ± 6 versus 28.25 ± 5 multinucleated cells/105 cells plated, respectively,P < .05). (B) Correlation of OCL formation induced by marrow plasma from 12 patients with MM and their respective levels of β2 microglobulin. Bone marrow plasma and marrow cultures were processed as described in “Materials and methods.” The relative levels of OSF positively correlated with β2microglobulin levels in patients with MM with normal renal function (correlation coefficient = 0.708).
Stimulation of OCL formation.
(A) Effects of bone marrow plasma from controls and patients with MM on OCL formation in human marrow cultures. Bone marrow plasma and marrow cultures were processed as described in “Materials and methods.” Results represent the mean ± SEM for all assays done in quadruplicate. Bone marrow plasma from patients with MM significantly stimulated the formation of OCLs in human bone marrow cultures when compared to controls (47.75 ± 6 versus 28.25 ± 5 multinucleated cells/105 cells plated, respectively,P < .05). (B) Correlation of OCL formation induced by marrow plasma from 12 patients with MM and their respective levels of β2 microglobulin. Bone marrow plasma and marrow cultures were processed as described in “Materials and methods.” The relative levels of OSF positively correlated with β2microglobulin levels in patients with MM with normal renal function (correlation coefficient = 0.708).
Measurement of cytokine levels
Levels of IL-6, IL-1β, and lymphotoxin were measured using a sensitive ELISA that could detect a minimum of 18 pg/mL, 20 pg/mL, and 16 pg/mL of the cytokines, respectively. None of the 24 patients with MM or the 10 controls had detectable levels of IL-6, IL-1β, or lymphotoxin in their bone marrow plasma.
Levels of MIP-1α were then measured by ELISA in freshly isolated bone marrow plasma from 31 patients with MM, 14 patients with other hematologic diagnoses, 7 normal controls, and 5 LCLs. Two patients with MM had stage I disease and 29 had stage III disease (Table1). Disease activity was classified according to standard response criteria as inactive if disease parameters (β2 microglobulin, level of monoclonal protein, hemoglobin, Ca++ levels, and extent of bone disease) were consistent with stable or responsive disease, or active if patients were newly diagnosed or disease parameters were consistent with progressive disease.
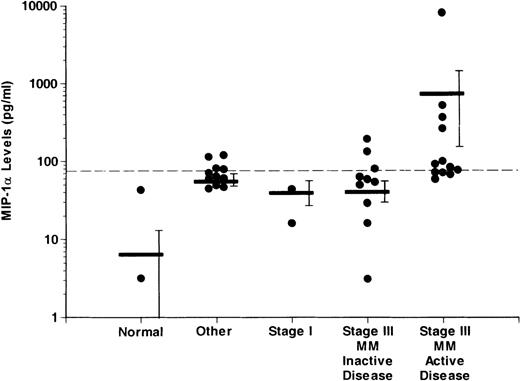
Overall, 11 of 31 patients with MM, 0 of 7 normal controls, and 3 of 14 patients with other hematologic disorders had increased levels of MIP-1α. A significantly larger proportion of MM patients with active disease (8 of 13) had increased levels of MIP-1α (median value; 178.1 pg/mL) compared to patients with MM with inactive disease (3 of 18). MIP-1α levels were more than 1500 pg/mL in the conditioned media from 4 of 5 of the LCLs tested.
Figure 2 shows the levels of MIP-1α in the different groups examined. MIP-1α levels were significantly higher in the patients with stage III MM with active disease (732.5 ± 588.98 pg/mL) when compared to normal controls (6.52 ± 6.01 pg/ml), patients with other hematologic diseases (55.7 ± 9.98 pg/mL), stage I MM patients (29.78 ± 13.81 pg/mL), and patients with stage III MM and inactive disease (40.58 ± 12.21 pg/mL) (P < .05). In the 3 patients with increased MIP-1α levels and non-MM hematologic diseases, the median MIP-1α level was 96.6 pg/mL.
Comparison of levels of MIP-1 in marrow plasma from controls and patients with different stages of MM
. MIP-1α levels were measured as described in “Materials and methods.” MIP-1α levels were significantly higher (P < .05) in the patients with active disease compared to patients with inactive disease, patients with stage I MM, or normal controls. The dashed horizontal line represents the upper limit for normals ± 2 SD (75 pg/mL). Levels above this value were considered to be significantly elevated. The horizontal bars represent the mean for each group. The vertical bars represent the SEM for each group. Because the values in the y-axis are in logarithm scale, MIP-1α levels of 0 pg/mL in each group do not appear on the graph.
Comparison of levels of MIP-1 in marrow plasma from controls and patients with different stages of MM
. MIP-1α levels were measured as described in “Materials and methods.” MIP-1α levels were significantly higher (P < .05) in the patients with active disease compared to patients with inactive disease, patients with stage I MM, or normal controls. The dashed horizontal line represents the upper limit for normals ± 2 SD (75 pg/mL). Levels above this value were considered to be significantly elevated. The horizontal bars represent the mean for each group. The vertical bars represent the SEM for each group. Because the values in the y-axis are in logarithm scale, MIP-1α levels of 0 pg/mL in each group do not appear on the graph.
Effects of MIP-1 on OCL formation in human bone marrow cultures
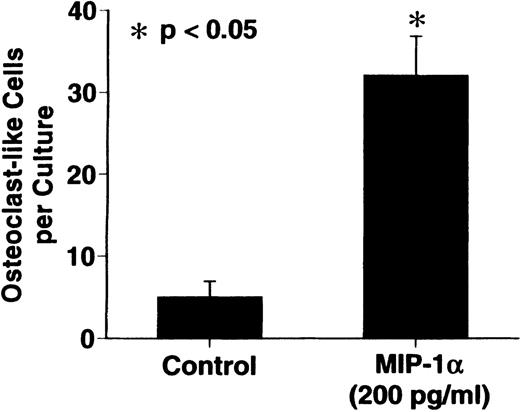
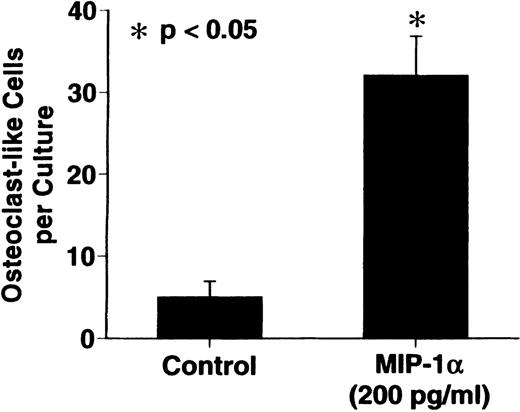
MIP-1α significantly stimulated OCL formation in human bone marrow cultures at concentrations of 100 to 200 pg/mL. The maximum effect was seen at 200 pg/mL (Figure 3).
Effects of recombinant human MIP-1 on OCL formation in human marrow cultures
. Human marrow cultures were processed as described in “Materials and methods.” MIP1-α significantly stimulated OCL formation in human bone marrow cultures when tested at concentrations of 100 to 200 pg/mL. Results represent the mean ± SEM of quadruplicate determinations for a typical experiment. A similar pattern of results was seen in 4 independent experiments.
Effects of recombinant human MIP-1 on OCL formation in human marrow cultures
. Human marrow cultures were processed as described in “Materials and methods.” MIP1-α significantly stimulated OCL formation in human bone marrow cultures when tested at concentrations of 100 to 200 pg/mL. Results represent the mean ± SEM of quadruplicate determinations for a typical experiment. A similar pattern of results was seen in 4 independent experiments.
Expression of MIP-1 mRNA in bone marrow samples from patients with MM
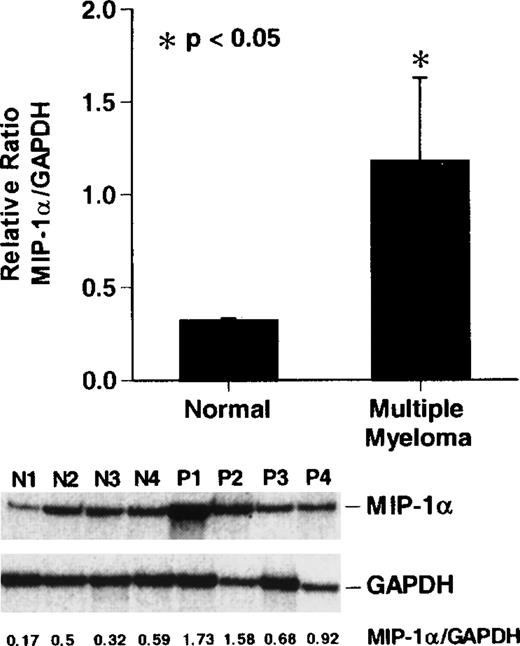
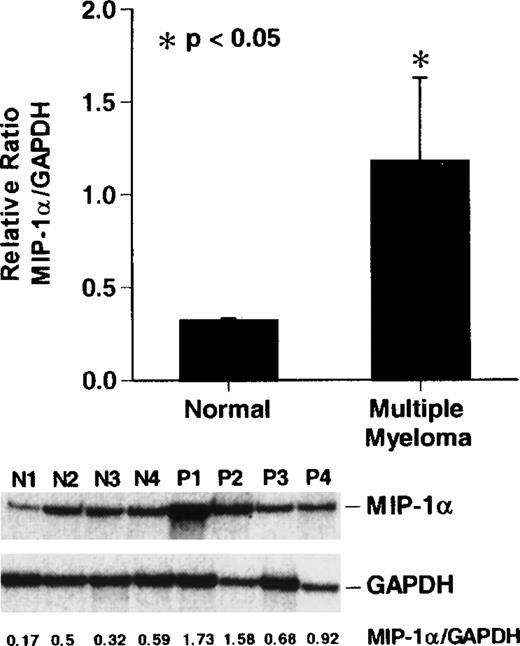
To compare the levels of expression of MIP-1α mRNA in patients with stage III MM and normal donor bone marrow, we performed RNAse protection assays for MIP-1α and GAPDH mRNA levels. MIP-1α mRNA expression was increased approximately 2- to 8-fold in 3 of 4 patients with stage III MM compared to the normal donors (P < .05) (Figure 4).
Reverse transcription-polymerase chain reaction (RT-PCR) analysis of MIP-1 mRNA expression in patients with MM
. Cycle-dependent RT-PCR was performed for 16, 20, or 24 cycles (lanes 1, 2, and 3) as described in “Materials and methods.” Results represent the mean ± SEM for 3 determinations for each patient. MIP-1α mRNA expression in patients with stage III MM was increased approximately 2- to 5-fold compared to controls in 3 of 4 patients with MM. A similar pattern of results was seen in 2 independent experiments.
Reverse transcription-polymerase chain reaction (RT-PCR) analysis of MIP-1 mRNA expression in patients with MM
. Cycle-dependent RT-PCR was performed for 16, 20, or 24 cycles (lanes 1, 2, and 3) as described in “Materials and methods.” Results represent the mean ± SEM for 3 determinations for each patient. MIP-1α mRNA expression in patients with stage III MM was increased approximately 2- to 5-fold compared to controls in 3 of 4 patients with MM. A similar pattern of results was seen in 2 independent experiments.
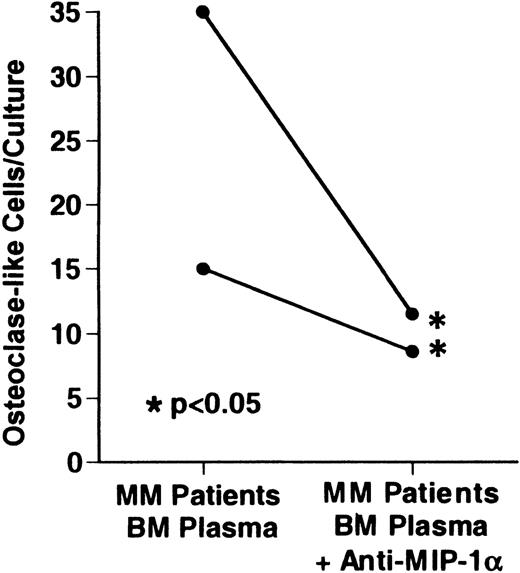
Effects of a neutralizing antibody to MIP-1 on OCL formation induced by bone marrow plasma from patients with MM
We tested the effects of anti–MIP-1α, anti–IL-6, anti–IL-1β, and anti–TNF-β on the OSF activity present in bone marrow plasma from patients with MM. Anti–MIP-1α at a concentration of 3 ng/mL, which can neutralize up to 10 ng/mL of MIP-1α, blocked OCL formation in human bone marrow cultures treated with bone marrow plasma from 2 patients with MM (Figure 5). Anti–MIP-1α had no effects on basal OCL formation in untreated cultures. Anti–IL-6, anti–IL-1β, and anti–TNFβ did not block OCL formation induced by marrow plasma from patients with MM (data not shown). Marrow plasma from normal controls did not stimulate OCL formation (data not shown).
Effects of anti-MIP-1 on OCL formation induced by marrow plasma from patients with MM
. Marrow plasma and marrow cultures were processed as described. Anti-MIP-1α at a concentration of 3 ng/mL, which neutralized up to 10 ng/mL of MIP-1α, blocked the OCL formation induced by the bone marrow plasma of 2 patients with MM in human bone marrow cultures. Results represent the mean ± SEM for a typical experiment. A similar pattern of results was seen in cultures from a total of 5 of 6 myeloma marrow plasma samples.
Effects of anti-MIP-1 on OCL formation induced by marrow plasma from patients with MM
. Marrow plasma and marrow cultures were processed as described. Anti-MIP-1α at a concentration of 3 ng/mL, which neutralized up to 10 ng/mL of MIP-1α, blocked the OCL formation induced by the bone marrow plasma of 2 patients with MM in human bone marrow cultures. Results represent the mean ± SEM for a typical experiment. A similar pattern of results was seen in cultures from a total of 5 of 6 myeloma marrow plasma samples.
Discussion
Bone destruction commonly occurs in patients with MM and is mediated by increased osteoclastic bone resorption and decreased bone formation in areas of bone adjacent to myeloma cells.24 The factors that mediate this abnormal bone remodeling in vivo are unknown. It is likely that myeloma cells secrete factor(s) that induce OCL activation either directly or indirectly, possibly through interactions with marrow stromal cells.
Several cytokines, in particular IL-1β,5,6IL-6,7 and lymphotoxin,8 have been implicated as OCL-stimulating factors in MM, based on in vitro studies, but in vivo studies have failed to confirm a role for these cytokines in the pathogenesis of myeloma bone disease. For example, IL-1β has been proposed as the major OCL-activating factor in MM, but the results have been controversial. Increased levels of IL-1β have been detected in cultures of freshly isolated myeloma cells by ELISA.25 Differential expression of IL-1β mRNA has been detected by in situ hybridization in bone marrow samples from patients with MM compared to samples from patients with monoclonal gammopathy of unknown significance.26 In contrast, normal levels of IL-1β have been reported in the plasma of patients with MM.9 Similarly, in the current study, IL-6, IL-1β, and lymphotoxin were undetectable in freshly isolated bone marrow plasma from 24 of 24 patients with stage III MM, using highly sensitive ELISA assays, which could detect cytokine concentrations as low as 20 pg/mL. Furthermore, although IL-1β was the only bone-resorbing cytokine detected in the bone marrow plasma obtained from our recently described in vivo model of myeloma bone disease,27 the levels of IL-1β were only 20 pg/mL, levels insufficient to stimulate OCL formation in vivo.
However, although IL-6, IL-1, or lymphotoxin was undetectable in marrow plasma from MM patients, freshly isolated bone marrow plasma from these patients significantly stimulated OCL formation in human bone marrow cultures. Furthermore, levels of OCL formation that were induced by the OSF in these marrow plasma correlated (r = 0.7) with tumor burden, as assessed by β2 microglobulin levels, in patients with normal renal function. These data suggest that factors other than IL-6, IL-1, and TNF-β are implicated in the pathogenesis of myeloma bone disease.
Our results and those of other workers suggest that MIP-1α is a good candidate for a possible OCL-stimulating factor in MM. In addition to its proinflammatory activities in vitro, MIP-1α increases OCL formation and OCL recruitment in rodent systems and is produced by LCLs.13 MIP-1α also inhibits CD34+ cell proliferation and stimulates phagocyte activity.28 We have shown that MIP-1α also stimulates OCL formation in human bone marrow cultures. We have demonstrated, using RNAse protection assay, that MIP-1α is overexpressed in 3 of 4 MM patients when compared to normal controls. Furthermore, the levels of MIP-1α were increased in the bone marrow plasma of the majority of patients with active MM, compared to patients with inactive disease. MIP-1α was not detected in the peripheral blood plasma of patients with MM with active disease, consistent with the observation that myeloma bone disease is a local process. Thus, bone marrow plasma should contain much higher levels of the factor(s) involved in myeloma bone disease than peripheral blood. In our studies, the bone marrow samples were obtained in a single aspiration and diluted with peripheral blood. Because peripheral blood did not have elevated levels of MIP-1α, the elevated marrow plasma levels of MIP-1α we found in these patients underestimate the actual levels of MIP-1α present in the marrow. Most importantly, anti–MIP-1α blocked the OSF present in bone marrow plasma from 2 patients with MM.
Taken together, these data demonstrate that MIP-1α is an OSF that is present in vivo in the marrow of the majority of patients with active myeloma bone disease, that it is a stimulator of human OCL formation, and that levels of MIP-1α correlate with the activity of myeloma bone disease in these patients. These results support a role for MIP-1α as a major mediator of the increased OCL activity in patients with MM.
Acknowledgments
We thank Bibi Cates for preparation of this manuscript and the staff of the General Clinical Research Center, Audie L. Murphy Veterans Administration Hospital, for their assistance in the care of our patients with multiple myeloma and the collection of samples.
Supported by Research Funds from the Veterans Administration (G.D.R.) and National Institutes of Health NCI Grants CA69136 (M.A.) and CA40035 (G.D.R.).
Reprints: Melissa Alsina, Research Service, 151 Audie L. Murphy Veterans Administration Hospital, 7400 Merton Minter Boulevard, San Antonio, TX 78284; e-mail: alsina@uthscsa.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.