Abstract
The Shc adaptor protein possesses 2 distinct phosphotyrosine (pTyr) recognition modules—the pTyr binding (PTB) domain and the Src homology 2 (SH2) domain—and multiple potential sites for tyrosine (Tyr) phosphorylation (Tyr residues 239, 240, and 317). On stimulation of hematopoietic cells with interleukin 3 (IL-3), Shc becomes phosphorylated and may therefore contribute to IL-3 signaling. We investigated the interactions mediated by the Shc modular domains and pTyr sites in IL-3–dependent IC2 premast cells. The Shc PTB domain, rather than the SH2 domain, associated both in vitro and in vivo with the Tyr-phosphorylated β subunit of the IL-3 receptor and with the SH2-containing 5′ inositol phosphatase (SHIP), and it recognized specific NXXpY phosphopeptides from these binding partners. In IL-3–stimulated mast cells, Shc phosphorylation occurred primarily on Tyr239 and 317 and was dependent on a functional PTB domain. Phosphorylated Tyr317, and to a lesser extent, Tyr239, bound the Grb2 adaptor and SHIP. Furthermore, a pTyr317 Shc phosphopeptide selectively recognized Grb2, Sos1, SHIP, and the p85 subunit of phosphatidylinositol 3′ kinase from mast cells, as characterized by mass spectrometry. These results indicate that Shc undergoes an interdependent series of pTyr-mediated interactions in IL-3–stimulated mast cells, resulting in the recruitment of proteins that regulate the Ras pathway and phospholipid metabolism.
Cytokines play an important role in hematopoiesis by regulating the proliferation, differentiation, and cellular functions of various hematopoietic cell lineages at different stages of development.1 Among these cytokines, interleukin 3 (IL-3) can stimulate the growth, survival, and differentiation of multipotent progenitors and more mature cells, including those of erythroid, mast, and granulocyte cell lineages. The high-affinity IL-3 receptor is composed of a specific α subunit of 60 to 70 kd and a β subunit of 130 to 140 kd, which is also shared by the granulocyte-macrophage colony-stimulating factor (GM-CSF) and the interleukin 5 receptors, termed the β common (βc) chain.2 Both subunits belong to the cytokine receptor superfamily and are required for high-affinity binding, but the β subunit plays the pivotal role in signal transduction.
The β subunit lacks an intrinsic catalytic domain, but on stimulation with IL-3, it associates with and activates cytoplasmic tyrosine (Tyr) kinases of the JAK and Src families.3,4 Once activated, these kinases phosphorylate the βc chain on multiple tyrosines, which serve as docking sites for signaling molecules containing the Src homology 2 (SH2) domain, the phosphotyrosine (pTyr) binding (PTB) domain, or both.5 6
The adaptor protein Shc has been implicated in linking growth factor, cytokine, and antigen receptors to Ras signaling.7 Shc contains an amino-terminal PTB domain, a central region (CH1), and a carboxyl-terminal SH2 domain.8-11 The Shc SH2 and PTB domains have quite distinct binding specificities for pTyr-containing motifs. The PTB domain requires residues amino-terminal to the pTyr with the consensus sequence ψXNXXY (where ψ is a hydrophobic residue).9,11-14 In contrast, the SH2 domain requires residues carboxy-terminal to the pTyr.15 Shc binds directly to a range of activated cell-surface receptors and consequently becomes phosphorylated in the central CH1 region at 3 Tyr sites, residues 239, 240, and 317.11,12,16 Once these sites are phosphorylated, they can be recognized by SH2-containing proteins. In particular, Tyr-phosphorylated Shc associates with the Grb2 adaptor protein through direct binding of the Grb2 SH2 domain to Shc pTyr site Y239 or Y317.12,17,18 Grb2 can in turn associate with the guanosine diphosphate–guanosine triphosphate exchange factor Sos1, possibly leading to Ras activation.17-20
In addition to interacting with activated receptors and Grb2, Shc also associates with the SH2-containing 5′ inositol phosphatase (SHIP).21-23 This 145-kd protein contains an SH2 domain at its amino-terminal, a central catalytic domain containing 2 motifs highly conserved among inositol polyphosphate 5 phosphatases, and a carboxy-terminal tail with 2 potential PTB-binding motifs (NXXY sequences) and several proline-rich sequences with the potential to bind SH3 domains.21,22 SHIP becomes Tyr phosphorylated on activation of several receptors in hematopoietic cells and subsequently forms a complex with Shc.24-27 SHIP has been implicated in inhibitory signaling by means of FcγRIIB on mast and B cells,28,29 mitogenic signaling by means of macrophage colony-stimulating factor,22 and induction of apoptosis by means of IL-3.30 Studies using targeted disruption of the mouse SHIP gene indicated that the SHIP protein is an important negative regulator of cytokine signaling in cells in the hematopoietic system.31
Structure-function analyses of the βc chain have helped to map specific intracellular signaling pathways to defined regions of the cytoplasmic domain of the βc chain. However, the connection between these biochemical pathways and specific biologic responses is less well understood. Cell proliferation has been associated with the membrane-proximal region of the βc subunit and with activation of JAK2 and induction of c-myc, pim-1, and cisexpression.32,33 But whether this cellular response is mediated only by the JAK/STAT pathway or by another, undetermined pathway is not known. On the other hand, the membrane-distal region has been found to be necessary for Shc phosphorylation, activation of the Ras/MAPK cascade, and induction of c-fos and c-jun, and was also observed to be involved in suppression of apoptosis and differentiation.33-37
The precise role of Shc in mediating IL-3–specific pathways and biologic events has remained unclear. In this study, we used the immature murine IC2 premast cell line, which depends on IL-3 for its proliferation and viability, to examine the role of the different modular domains and phosphorylation sites of Shc in IL-3 signaling by analyzing wild-type and mutant forms of Shc. We found both in vitro and in vivo that the PTB domain plays a central role in Shc phosphorylation and consequently in Grb2 binding and association with SHIP. Analysis of the binding properties of the Y239/240 and Y317 Shc phosphorylation sites indicated that the pY317 site interacts preferentially with Grb2-Sos1 and SHIP, whereas the pY239 site bound more weakly to Grb2. Our data suggest that Shc may be involved in multiple signaling pathways leading to the different biologic responses stimulated by IL-3.
Materials and methods
Cell lines and antibodies
The IL-3–dependent immature mast cell line IC238 was cultured in RPMI 1640 containing 10% fetal bovine serum (FBS), 50 μmol/L β-mercaptoethanol (β-ME), and 1% X63 cell supernatant as a source of IL-3. The IC2/Shc transfectants were cultured in Dulbecco-Vogt modified Eagle medium (DMEM) containing 10% FBS, 50 μmol/L β-ME, and 1% IL-3–conditioned medium. NIH-3T3 cells were grown in DMEM containing 10% FBS. Polyclonal anti-Shc serum was raised as described previously.8 Polyclonal anti-Grb2 C-23 and anti-IL-3Rβ K-19 sera were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal anti-pTyr 4G10 and monoclonal anti-FLAG antibodies were purchased from UBI (Lake Placid, NY) and Eastman Kodak Company (Rochester, NY), respectively. Polyclonal anti-SHIP serum was generously provided by Dr Gerald Krystal (British Columbia Cancer Research Centre, Vancouver, BC, Canada).
Generation of IC2 cells expressing FLAG-tagged mutant forms of Shc
A FLAG-tagged form of wild-type Shc was generated by fusing the FLAG epitope in frame to the first amino acid of the human p52 Shc complementary DNA (cDNA) in the mammalian expression vector pcDNA3. Mutant Shc cDNAs were then generated by using a method based on polymerase chain reaction and subsequently sequenced to ensure fidelity. All Shc constructs were cloned back into the pMCSV retroviral vector containing the puromycin resistance genepac.39 Because of the high homology between human and murine Shc, the human constructs were used for transfection into murine cell lines. All plasmids were transfected with use of calcium phosphate into the packaging cell line, GP+E. Forty-eight hours later, selection of cells in 2 μg/mL puromycin began and continued for 10 days. Retroviral supernatants were then collected, filtered, and used to infect either NIH-3T3 (for titering) or IC2 cells. The viral titers were approximately 1 × 106 colony-forming units/mL. IC2 cells were infected with 1 mL viral supernatant for 16 hours and selected for 2 weeks in 2 μg/mL puromycin. Resistant cells were then pooled and analyzed for Shc expression by Western blot analysis with anti-Shc antibodies.
In vitro binding assays
pGEX glutathione-S-transferase (GST)–Shc fusion constructs were generated and prepared as described previously.11 IC2 cells (4 × 107) were starved in RPMI-1640 medium containing 0.5% FBS and 1% bovine serum albumin (pH 7) (Sigma, St Louis, MO) for 6 hours and subsequently treated with 100 ng/mL recombinant mouse IL-3 (PharMingen, San Diego, CA) at 37°C for 5 minutes. Cells were washed once with cold phosphate-buffered saline (PBS) and then solubilized on ice with lysis buffer (0.1% Triton X-100, 150 mmol/L sodium chloride [NaCl], 50 mmol/L Tris [pH 7.6], 10% glycerol, 0.1 mmol/L sodium vanadate, 1 mmol/L phenylmethylsulfonyl fluoride, 10 μg/mL aprotinin, and 10 μg/mL leupeptin) for 30 minutes. Lysates were cleared by centrifugation at 12 000 rpm for 5 minutes and incubated for 2 hours at 4°C on a rocker with 10 μL glutathione–Sepharose beads containing 5 μg fusion protein. Beads were washed 3 times with lysis buffer and once with PBS buffer. Bound proteins were analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting.
Immunoprecipitations and Western blotting
Cell lysates of IC2 or IC2 infected with Shc were prepared as described above. The supernatants were then incubated in the presence of the designated serum or monoclonal antibody and 50% Protein A– or antimouse IgG–Sepharose beads (Sigma) for 2 hours at 4°C. Immune complexes were washed as described above and boiled for 3 minutes with sample buffer for SDS-PAGE. Eluted proteins were separated by electrophoresis and transferred to supported nitrocellulose (Schleicher & Schuell, Dassel, Germany). For immunoblotting, membranes were blocked for 1 hour at room temperature in PBS–0.2% Tween 20 (PBST) buffer containing 1% gelatin and then incubated with the designated antibodies in PBST with 0.1% gelatin for 1 hour at 4°C. Blots were washed 3 times for 10 minutes each time with PBST. Membranes were incubated for 1 hour with horseradish peroxidase conjugated either to Protein A or goat antimouse antibodies (Bio-Rad, Hercules, CA) in PBST with 0.5% gelatin. Reactive proteins were visualized with an enhanced chemiluminescence detection system (Amersham, England) as directed by the manufacturer. Densitometric scanning was performed with a high-resolution charged coupled device (CCD) camera (ImageQuant, Molecular Dynamics, Sunnyvale, CA).
Phosphopeptide inhibition assays
The phosphopeptides used for the inhibition studies were the 11-mers EMINPNpYIGMG (p145-1), MFENPLpYGSVS (p145-2), and IISDPEpYLLDQ (p145-3), corresponding to the sequence flanking Y917, Y1020, and Y798, respectively, within SHIP, and the 16-mer QLPSFDFNGPpYLGPPQ (pIL-3Rβ) corresponding to the sequence flanking Y577 within the βc chain of the IL-3 receptor. Unstimulated IC2 cell lysates or IC2 lysates stimulated with IL-3 for 5 minutes were incubated with GST-Shc-PTB fusion protein and the appropriate concentration of phosphopeptide and rotated at 4°C for 2 hours. GST beads were then washed as described above, and the bound proteins were eluted by boiling for 3 minutes in SDS-sample buffer and subjected to Western blot analysis with anti-pTyr 4G10 monoclonal antibodies.
Affinity chromatography and protein identification by quadrupole/time-of-flight mass spectrometry
The synthetic phosphopeptides used to identify associated proteins were biotin-ε-aminocaproic acid (Aca)-DHQpYYNDFPGKE, biotin-Aca-DHQYpYNDFPGKE, biotin-Aca-DHQpYpYNDFPGKE, and biotin-Aca-DPSpYVNIQNLD, corresponding to the sequences flanking Y239, Y240, Y239/240, and Y317, respectively, within Shc. For each affinity reagent, a 100-μL bed volume of streptavidin agarose resin (Sigma) was washed with PBS and then resuspended in an excess of biotinylated peptide. After mixing at room temperature for 15 minutes, the resins were poured into a 1.5-mL chromatography column (Bio-Spin disposable chromatography column; Bio-Rad) and washed extensively with cold Tris buffer (50 mmol/L Tris–hydrochloric acid [pH 7.5], 50 mmol/L NaCl, 1 mmol/L EDTA, 2 mmol/L benzamidine, and 4 mmol/L dithiothreitol). IC2 cell lysate from 1.3 × 109 cells was prepared as described above, diluted with 2 parts volume Tris buffer, and applied to each column. The columns were washed with 3 mL Tris buffer and eluted with 1 mL of 50 mmol/L phenylphosphate in Tris buffer. Eluted proteins were precipitated by adding trichloroacetic acid to a final concentration of 12%, and the resulting pellets were washed with anhydrous ether and ethanol (80/20 vol/vol). Proteins were resolved by using 9% SDS-PAGE and visualized with use of silver staining as described by Shevchenko et al.40 Bands selected for mass spectrometry (MS) analysis were excised from the gel and subjected to tryptic digestion.40 MS measurements were carried out on a Q-TOF apparatus (MDS-Sciex, Concord, ON, Canada).41
Results
The PTB domain of Shc interacts with specific pTyr-containing proteins in mast cells stimulated with IL-3
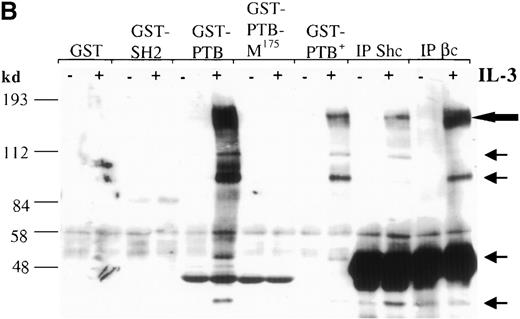
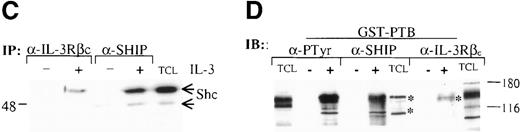
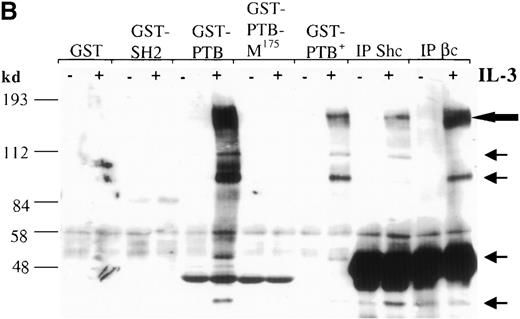
The Shc adaptor protein can potentially interact with Tyr-phosphorylated proteins through its amino-terminal PTB domain, its carboxy-terminal SH2 domain, or both. To examine whether these distinct pTyr-recognition modules of Shc associate with different proteins in IL-3–stimulated cells, we incubated lysates from IC2 premast cells treated with IL-3 with GST fusion proteins containing the Shc PTB or SH2 domains (Figure 1A). Several pTyr-containing proteins of approximately 150 to 120, 115, 98, 56, 52, and 41 kd associated with the GST–PTB-domain fusion protein after IL-3 treatment, whereas neither GST alone nor the GST–SH2-domain fusion protein formed detectable complexes with phosphorylated proteins (Figure 1B). In addition, a mutant form of the PTB domain in which arginine 175, which is critical for pTyr recognition, is substituted with methionine,42,43 failed to recognize IL-3–induced pTyr-containing proteins. Two of the proteins known to coimmunoprecipitate with Shc after IL-3 stimulation are the IL-3 βc subunit and the p145 inositol phosphatase SHIP21 (Figure1B, C). Interestingly, a prominent phosphoprotein of approximate 150-120 kd was present in the GST-PTB precipitate (Figure 1B). To investigate the identity of this band, PTB-associated proteins from IL-3–stimulated cells were immunoblotted with anti-SHIP or anti-βc antiserum. Both SHIP and the βc proteins were identified in the GST-PTB complex (Figure 1D).
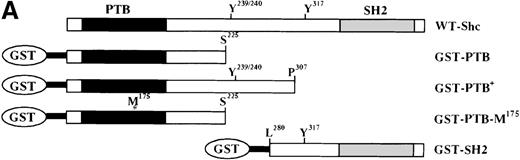
The Shc phosphotyrosine (pTyr) binding (PTB) domain interacts with the SH2-containing 5′ inositol phosphatase (SHIP) and the β common (βc) chain of the IL-3 receptor.
(A) Schematic representation of Shc domains expressed as glutathione-S-transferase (GST) fusion proteins. GST–PTB-M175 contains an arginine to methionine mutation in residue 175. (B) IC2 cells (4 × 107) were stimulated (+) with 200 ng/mL IL-3 for 5 minutes, lysed, and then incubated with GST alone or with different GST-Shc fusion proteins. Immunoprecipitation with anti-Shc and anti-βc chain antibodies in the same experiment is also shown. The bound proteins were analyzed with anti-pTyr antibodies. Arrows mark phosphorylated proteins in cells stimulated with IL-3. (C) IC2 cells were stimulated with 100 ng/mL IL-3 for 5 minutes, lysed, and then immunoprecipitated with either anti-βc or anti-SHIP antiserum. Western blot analysis was performed with anti-Shc antibodies. (D) GST precipitates from unstimulated or stimulated cells were analyzed with anti-SHIP and anti-βc immunoblotting. TCL indicates total cell lysate. Asterisks mark the position of the SHIP and βc chain isoforms.
The Shc phosphotyrosine (pTyr) binding (PTB) domain interacts with the SH2-containing 5′ inositol phosphatase (SHIP) and the β common (βc) chain of the IL-3 receptor.
(A) Schematic representation of Shc domains expressed as glutathione-S-transferase (GST) fusion proteins. GST–PTB-M175 contains an arginine to methionine mutation in residue 175. (B) IC2 cells (4 × 107) were stimulated (+) with 200 ng/mL IL-3 for 5 minutes, lysed, and then incubated with GST alone or with different GST-Shc fusion proteins. Immunoprecipitation with anti-Shc and anti-βc chain antibodies in the same experiment is also shown. The bound proteins were analyzed with anti-pTyr antibodies. Arrows mark phosphorylated proteins in cells stimulated with IL-3. (C) IC2 cells were stimulated with 100 ng/mL IL-3 for 5 minutes, lysed, and then immunoprecipitated with either anti-βc or anti-SHIP antiserum. Western blot analysis was performed with anti-Shc antibodies. (D) GST precipitates from unstimulated or stimulated cells were analyzed with anti-SHIP and anti-βc immunoblotting. TCL indicates total cell lysate. Asterisks mark the position of the SHIP and βc chain isoforms.
Specific phosphopeptides containing the NXXY motifs of SHIP and βc chain inhibit Shc PTB-domain interactions
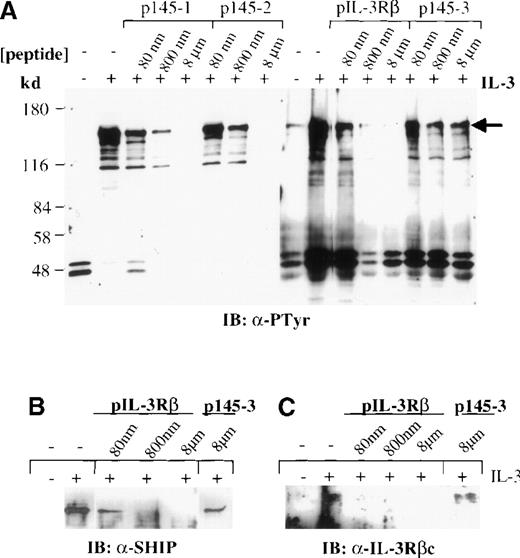
It has been shown that SHIP contains 2 NXXpY motifs required for binding to the PTB domain of Shc in cells stimulated with T-cell receptor.44 On the other hand, the βc chain of the IL-3 receptor contains the sequence PSFDFNGPYLGPP within its membrane-proximal domain, which provides a potential Shc PTB binding site.45 We investigated whether binding of the Shc PTB domain to SHIP or the βc subunit of the IL-3 receptor in mast cell lysates stimulated with IL-3 is mediated specifically by these NXXpY motifs. Lysates from IC2 cells treated with IL-3 for 5 minutes were incubated with GST–PTB domain fusion protein in the presence of increasing concentrations of phosphopeptides corresponding to the NXXpY sequences of SHIP (p145-1 and p145-2) or of the IL-3 receptor βc chain (pIL-3Rβ). All 3 NXXpY phosphopeptides, but not a control phosphopeptide (p145-3), inhibited binding of the Shc PTB domain to SHIP, βc, and other pTyr proteins in the mast cell lysates (Figure2A). Reprobing the blots with anti-SHIP and anti-βc sera confirmed these results (Figure 2B, C, and data not shown). These results indicate that the Shc PTB domain is primarily responsible for the recognition of pTyr-containing proteins by the Shc adaptor observed in IL-3–stimulated IC2 cells. The data also suggest that these associations depend on the presence of a phosphorylated NXXY motif in the Shc binding partners.
Association of the βc chain and SHIP with Shc is mediated by interaction of NXXpY motifs and the PTB domain.
Lysates from untreated IC2 cells (−) or IC2 cells treated (+) with IL-3 (100 ng/mL) for 5 minutes were precipitated with the GST–PTB-domain fusion protein in the presence and absence of phosphopeptides corresponding to the NXXpY motifs of SHIP (p145-1 and p145-2) and the βc chain (pIL-3Rβ) or a control phosphopeptide (p145-3), at the indicated concentrations. The precipitates were subjected to Western blot analysis with anti-pTyr antibodies (A). (B, C) Peptide competition with phosphopeptides pIL-3Rβ and control p145-3 was reprobed with anti-SHIP or anti-βc antibodies. Similar results were obtained with p145-1 and p145-2 phosphopeptides (data not shown).
Association of the βc chain and SHIP with Shc is mediated by interaction of NXXpY motifs and the PTB domain.
Lysates from untreated IC2 cells (−) or IC2 cells treated (+) with IL-3 (100 ng/mL) for 5 minutes were precipitated with the GST–PTB-domain fusion protein in the presence and absence of phosphopeptides corresponding to the NXXpY motifs of SHIP (p145-1 and p145-2) and the βc chain (pIL-3Rβ) or a control phosphopeptide (p145-3), at the indicated concentrations. The precipitates were subjected to Western blot analysis with anti-pTyr antibodies (A). (B, C) Peptide competition with phosphopeptides pIL-3Rβ and control p145-3 was reprobed with anti-SHIP or anti-βc antibodies. Similar results were obtained with p145-1 and p145-2 phosphopeptides (data not shown).
Mutations in Shc affect its Tyr phosphorylation in vivo in response to IL-3
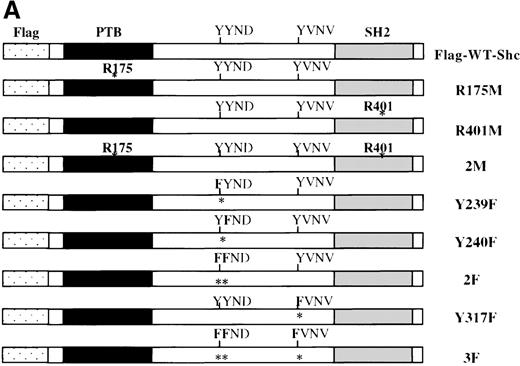
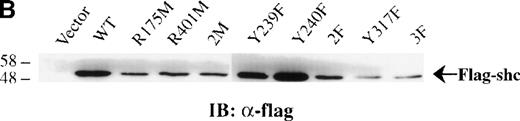
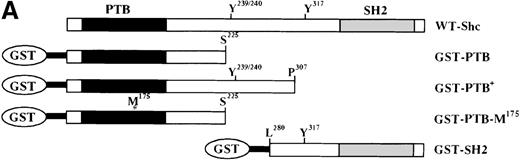
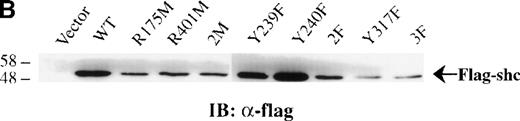
Previous studies showed that Shc becomes Tyr phosphorylated in vitro and in vivo after stimulation with either IL-3 or epidermal growth factor (EGF) at 3 major Tyr phosphorylation sites—Y239, Y240, and Y317.12,16,46 47 We examined the in vivo roles of the Shc PTB and SH2 domains, as well as the functions of each Shc phosphorylation site, in IL-3 signaling. For this purpose, IC2 mast cells were infected with retroviral vectors encoding either wild-type or mutant forms of p52 Shc in which either the PTB or SH2 modular domains or the Tyr phosphorylation sites had been inactivated (Figure3A). These proteins were all tagged with a FLAG epitope at their amino-terminals. Stable cell lines were established from pools of infected cells, and the expression levels of the different FLAG-tagged Shc proteins were assessed by immunoblotting with anti-FLAG antiserum (Figure 3B).
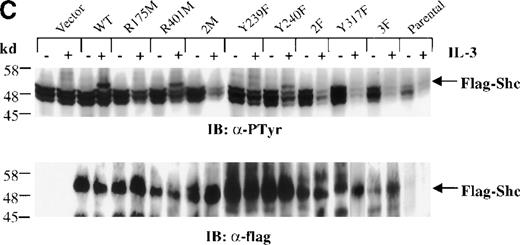
Inhibition of IL-3–stimulated tyrosine phosphorylation of Shc by different Shc mutant forms.
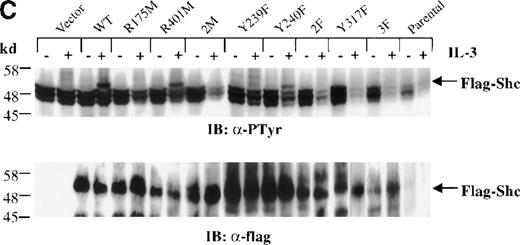
(A) Schematic representation of Shc mutations expressed as FLAG-tagged proteins. (B) IC2 cell lysates from each FLAG-tagged Shc infection were subjected to Western blotting with anti-FLAG antibodies to confirm expression of the different FLAG-tagged Shc proteins. (C) Total cell lysates from cells expressing different Shc mutant forms were unstimulated (−) or stimulated (+) with IL-3 for 5 minutes and analyzed by immunoblotting with anti-pTyr (upper panel) or anti-FLAG antibodies (lower panel). WT indicates wild type; 2M, R175/401M; 2F, Y239/240F; and 3F, Y239/240/317F.
Inhibition of IL-3–stimulated tyrosine phosphorylation of Shc by different Shc mutant forms.
(A) Schematic representation of Shc mutations expressed as FLAG-tagged proteins. (B) IC2 cell lysates from each FLAG-tagged Shc infection were subjected to Western blotting with anti-FLAG antibodies to confirm expression of the different FLAG-tagged Shc proteins. (C) Total cell lysates from cells expressing different Shc mutant forms were unstimulated (−) or stimulated (+) with IL-3 for 5 minutes and analyzed by immunoblotting with anti-pTyr (upper panel) or anti-FLAG antibodies (lower panel). WT indicates wild type; 2M, R175/401M; 2F, Y239/240F; and 3F, Y239/240/317F.
Cells expressing the different Shc mutants were treated with IL-3 for 5 minutes, and total cell lysates were subjected to Western analysis with anti-pTyr antibodies. As shown in Figure 3C (upper panel), Tyr phosphorylation of the FLAG-tagged Shc protein with substitution of Tyr239 (Y239F) was markedly decreased compared with that of wild-type, R401M (SH2 inactive), or Y240F mutant Shc proteins. Moreover, Shc proteins containing an inactive PTB domain unable to recognize NXXpY motifs (R175M and 2M [R175/401M]), substitutions of Tyr317 (Y317F), both Tyr239 and 240 (2F [Y239/240F]), or of all Tyr phosphorylation sites (3F [Y239/240/317F]) were not detectably Tyr phosphorylated after IL-3 stimulation. Reprobing this blot with anti-FLAG antiserum confirmed the presence of the transfected Shc proteins (Figure 3C, lower panel). These results indicate that the PTB domain is necessary for IL-3–induced Shc phosphorylation, supporting a model in which binding of Shc to the receptor βc chain precedes phosphorylation of Shc. In addition, Y239 and Y317 appear to be important Shc phosphorylation sites in mast cells stimulated with IL-3.
The Shc PTB domain and Tyr residues Y239 and Y317 are required for complex formation of Shc with SHIP and Grb2 in mast cells
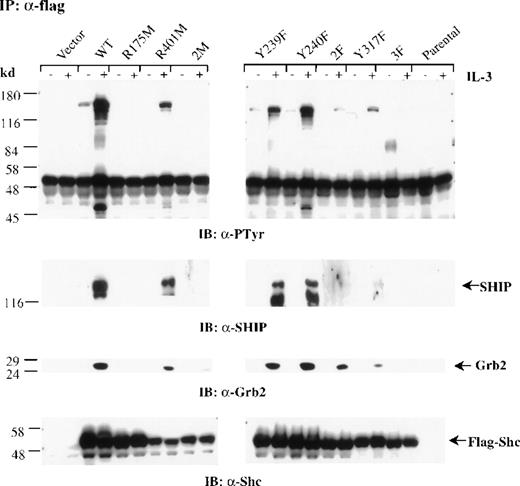
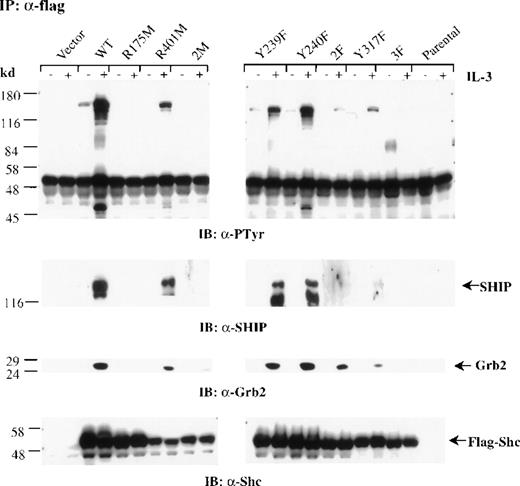
To examine whether the mutant forms of Shc can associate with SHIP, Grb2, or both, after IL-3 stimulation, we treated mast cells expressing the different Shc mutant proteins with IL-3. Cells were then lysed, immunoprecipitated with anti-FLAG antibodies, and immunoblotted with either anti-pTyr or anti-Grb2 antibodies. Densitometric analysis was performed on all blots (Table 1). Wild-type Shc coprecipitated with both SHIP and Grb2 after IL-3 stimulation. Substitution of either Y239 or Y240 had only a small effect on association with Grb2 and SHIP, whereas replacement of both Y239 and 240 had a more significant effect on association with SHIP than with Grb2 (Figure 4 and Table 1). Shc mutants possessing either an inactive PTB domain (R175M Shc and 2M Shc) or lacking all 3 major phosphorylation sites (3F Shc) were unable to associate with SHIP or Grb2. Furthermore, substitution of Y317 resulted in a markedly impaired association with SHIP and Grb2, although residual binding to both proteins was detected (Figure 4 and Table 1). Reprobing this blot with anti-Shc antibodies confirmed similar expression levels of the FLAG-tagged Shc proteins (Figure4). Thus, these results indicate that in mast cells, an active PTB domain as well as IL-3–induced phosphorylation of Shc, is required for high-affinity interactions with SHIP and Grb2.
The PTB domain, Y239 and Y317 of Shc are required for SHIP and Grb2 association.
Factor-deprived IC2 stable transfectants (4 × 107cells/sample) were stimulated with 100 ng/mL IL-3 for 5 minutes. Cells were lysed and immunoprecipitated with anti-FLAG antibodies. Protein samples were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and subjected to Western blot analysis with anti-pTyr, anti-SHIP, anti-Grb2, or anti-Shc antibodies.
The PTB domain, Y239 and Y317 of Shc are required for SHIP and Grb2 association.
Factor-deprived IC2 stable transfectants (4 × 107cells/sample) were stimulated with 100 ng/mL IL-3 for 5 minutes. Cells were lysed and immunoprecipitated with anti-FLAG antibodies. Protein samples were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and subjected to Western blot analysis with anti-pTyr, anti-SHIP, anti-Grb2, or anti-Shc antibodies.
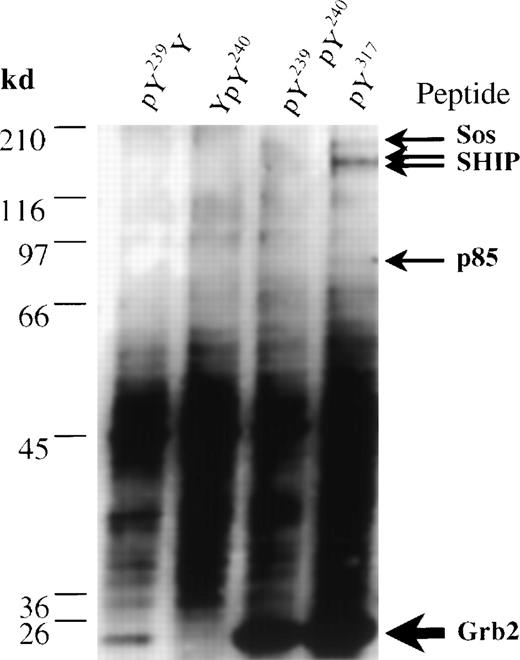
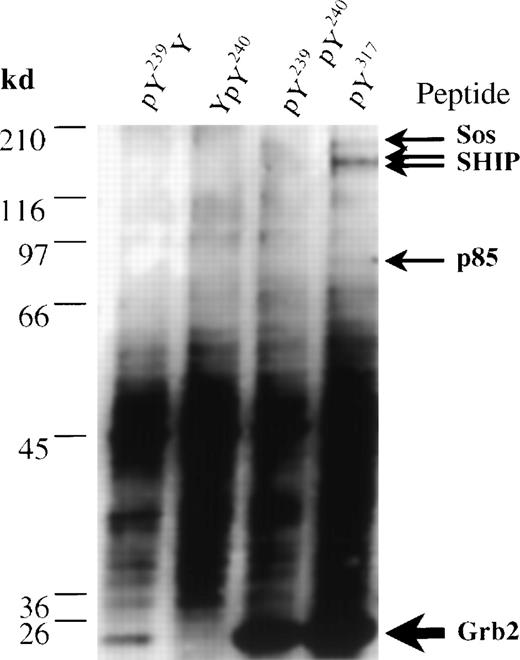
To assess only the ability of the Tyr residues of Shc to precipitate proteins such as Grb2 and SHIP from lysates of IC2 mast cells, we generated synthetic peptides corresponding to the phosphorylated Y239/240 and Y317 Shc sites. Immobilized peptides were used to isolate binding partners from an IC2 cell lysate, which were separated by SDS-PAGE, analyzed by silver staining, and identified by cleavage with trypsin and MS. The phosphorylated Y317 peptide bound a major polypeptide (relative molecular weight, 25 kd) that was identified by trypsin digestion and subsequent MS as Grb2 (Figure5, lane 4, and Table2). In contrast, phosphorylated peptides modeled on the Y239/240 Shc site displayed a differential ability to precipitate Grb2. A Y240 phosphopeptide was unable to associate with Grb2, whereas a peptide phosphorylated only at Y239 bound Grb2 with a relatively low affinity (Figure 5, lane 1, 2). However, the diphosphorylated Y239/240 peptide was able to bind Grb2 at levels similar to those observed with the Y317 phosphopeptide (Figure 5, lane 3). These data are consistent with our previous results indicating that phosphorylation of Y240 markedly enhances the ability of a phosphorylated Y239 site to bind Grb2.12
Affinity isolation and identification by mass spectrometry of proteins from IC2 cells that associate with synthetic Shc phosphopeptides.
Immobilized biotinylated phosphopeptides pY239Y (lane 1), YpY240 (lane 2), pY239pY240 (lane 3), and pY317 (lane 4) were incubated with IC2 cell lysates. Bound proteins were eluted by phenyl phosphate and analyzed in a silver-stained gel. Bands identified as Grb2 (accession number Q60631), Sos1 (accession number Q62245), the p85 subunit of phosphatidylinositol 3′ kinase (accession number P26450), and SHIP (accession number U39203) are marked.
Affinity isolation and identification by mass spectrometry of proteins from IC2 cells that associate with synthetic Shc phosphopeptides.
Immobilized biotinylated phosphopeptides pY239Y (lane 1), YpY240 (lane 2), pY239pY240 (lane 3), and pY317 (lane 4) were incubated with IC2 cell lysates. Bound proteins were eluted by phenyl phosphate and analyzed in a silver-stained gel. Bands identified as Grb2 (accession number Q60631), Sos1 (accession number Q62245), the p85 subunit of phosphatidylinositol 3′ kinase (accession number P26450), and SHIP (accession number U39203) are marked.
In addition to Grb2, a small amount of SHIP was precipitated with the Y317 phosphopeptide (Figure 5 and Table 2). Interestingly, the pY317 peptide also bound 2 other minor proteins that were identified using MS of tryptic digests as Sos1 and the p85α subunit of phosphatidylinositol 3′ kinase (p85-PI3′K), (Figure 5, lane 4; Table 2). Taken together, these data provide direct evidence that Grb2 is the most prominent pY317-binding protein in IC2 mast cells and that additional proteins can interact either directly or indirectly with this motif.
Discussion
The aim of the current study was to analyze the function of the modular domains and Tyr-phosphorylation sites of Shc in IL-3 signaling and its effects in IL-3 cellular responses. Data obtained using coprecipitation experiments and in vitro binding to GST-fusion proteins indicated that 2 of the main phosphoproteins interacting with Shc after stimulation of IC2 mast cells with IL-3 were the receptor βc chain and SHIP. These interactions were mediated primarily by the Shc PTB domain and not the SH2 domain. Because Shc possesses only 1 PTB domain, it most likely interacts with these proteins separately. Indeed, preclearing experiments using either anti-SHIP or anti-βc antibodies indicated that Shc-SHIP and Shc-βc exist as separate complexes (data not shown). Moreover, the interaction between Shc and the βc subunit seems to be transient and of a lower affinity than the association of Shc with SHIP. This could explain the low amount of Shc coprecipitating with the receptor, as well as the absence of the βc subunit in a Shc immunoprecipitate.
Previous studies showed that the Shc PTB domain binds to a pTyr residue in the consensus sequence NXXpY found in a variety of Tyr kinase receptors and cytoplasmic proteins.9-11,48,49 Y577 of the βc chain is located in such an NXXY motif (NGPpY577) and is important not only for association with the Shc PTB domain (as shown in this study and previously45) but also because it is essential for phosphorylation of Shc after stimulation with GM-CSF.35 An analogous situation occurs with IL-2 and thrombopoietin signaling, in which Shc interacts with phosphorylated IL-2Rβ or c-Mpl receptors through its PTB domain.50,51 In both cases, the sequence surrounding the pTyr of the receptor involved in the association is similar to the consensus Shc PTB-binding motif, NQGpY338 and NHSpY599 for IL-2Rβ and c-Mpl receptors, respectively. Thus, it appears that Shc interacts with cytokine receptors mainly through its PTB domain. We cannot exclude the possibility of a role for the SH2 domain in the association of Shc with other cellular targets and in mediating different cytokine-dependent biologic responses. Indeed, previous work26 47 suggests that the Shc SH2 domain can bind to the phosphorylated βc chain. However, in the system described here, the sole interaction appeared to be between the βc chain and the Shc PTB domain.
To investigate the factors that control Shc phosphorylation and its ability to form protein complexes in activated mast cells, we stably expressed wild-type and mutant forms of Shc in IC2 cells. The resulting data showed that the Shc PTB domain is necessary for the phosphorylation of Shc induced by IL-3 in vivo. This conclusion is supported by the observation that PTB-inactive Shc proteins (R175M Shc and 2M Shc) failed to become phosphorylated on Tyr after IL-3 treatment. Thus, our data strongly suggest a model in which stimulation with IL-3 leads to receptor phosphorylation, recruitment of Shc to the phosphorylated βc chain through the Shc PTB domain, and subsequent phosphorylation of Shc by Tyr kinases in the receptor complex.
On stimulation with either IL-3 or EGF, Shc was observed to become phosphorylated at Tyr residues 239, 240, and 317.12,16 47Consistent with these data were our findings that no residual Tyr phosphorylation was detectable in the triple Tyr mutant (3F Shc) after IL-3 stimulation of transfected cells. Interestingly, cells expressing Y239F or Y317F Shc showed almost undetectable levels of Shc phosphorylation compared with wild-type Shc transfectants, suggesting that Y239 and Y317 are the main IL-3–induced phosphorylation sites in these cells.
Tyr phosphorylation of Shc is important for its association with SH2 domain signaling proteins such as Grb2, the Grb2-related protein Gads, SHIP, and possibly other, still unidentified targets12,52(L. Velazquez, G.D. Gish, and T. Pawson, unpublished data). The sequences surrounding Y239 and Y317 fit the consensus motif for Grb2-SH2–domain recognition (ie, pYXNX), and indeed both Tyrs can potentially function as Grb2-binding sites.12,18 47 We found that both the Y239 and Y317 sites could form complexes with Grb2 after IL-3 stimulation. However, the Y317F Shc mutant only bound a low level of Grb2, whereas the 2F Shc mutation had less of an effect on Grb2 binding. Although the phosphorylation levels of Y317F and 2F Shc were remarkably reduced, the remaining Grb2 binding detected with these mutants is likely explained by the association of Grb2 with the pY239 and pY317 sites, respectively. On the basis of this result, we conclude that although both Y239 and Y317 can associate with Grb2, the latter is the principal site for Grb2 binding in IL-3–stimulated IC2 cells.
On the other hand, the association of SHIP with Shc is a more complex interaction. It requires both the Shc PTB domain and Tyr residue 317. The idea that Y317 is involved is supported by the observations that mutation of the Shc Y317 site significantly decreased coprecipitation of SHIP with Shc and that a pY317 phosphopeptide selectively bound SHIP in an IC2 cell lysate. Indeed, previous studies found that IL-3–dependent binding of SHIP and Shc can be inhibited by a phosphopeptide corresponding to the Y317 sequence in Shc.25,30 Therefore, these data suggest that Grb2 and SHIP may compete in vivo for binding to Y317 in IL-3–stimulated cells. An alternative mechanism proposed in studies with B cells stimulated through the B-cell antigen receptor suggests the association of SHIP with Grb2 and the existence of a ternary complex between Shc, Grb2, and SHIP.53 We cannot exclude this possibility, although we found a significantly lower amount of SHIP associated with the 2F Shc mutant (Y239/240F), whereas the amount of bound Grb2 was not strongly affected. Although this may suggest that the SH2 domain of SHIP can also interact directly with the doubly phosphorylated Y239/240 site, the absence of a detectable level of phosphorylation of this mutant and the lack of SHIP binding to a pY239/240 phosphopeptide constitute evidence against this idea.
The use of immobilized phosphopeptides, coupled with trypsin digestion and MS, to analyze binding partners for the Y239/240 and Y317 sites provides a possible explanation for these findings. Although the phosphorylated Y317 peptide precipitated Grb2, SHIP, and p85-PI3′K from an IC2 lysate, the phosphorylated Y239 peptide effectively bound to Grb2 only when Y240 was also phosphorylated. Thus, phosphorylation of Y240 may act as a switch to modulate the set of proteins bound to the Y239 site. In this regard, it is of particular interest that both the pY317 and 239 Shc sites can potentially interact with proteins involved in both Ras signaling and phosphoinositide metabolism. This suggests an important role of these Tyr residues in coordinating different IL-3–dependent cell functions.
Preliminary biologic analysis has suggested that the Y239 Shc phosphorylation site may be important for optimal proliferation of mast cells in response to IL-3 (L. Velazquez and T. Pawson, unpublished data). Because the Y239 mutant retains the Y317 site, which is the preferential site for Grb2 binding, it is possible that the Y239 site activates an unidentified pathway important for proliferation.
Together, the data reported here indicate that Shc phosphorylation in IL-3–stimulated mast cells depends on binding of the PTB domain to the IL-3 receptor and that Shc pTyr sites subsequently bind SH2 proteins involved in different signaling pathways. Identification of the complete set of molecules that bind to the Shc Tyr residues and other Shc motifs should contribute to a better understanding of the role of Shc in cytokine signaling and, in particular, the mechanisms mediating the different biologic responses of IL-3.
Acknowledgments
We thank Dr G. Caruana and Dr T. Kubiseski for discussions and critical review of this manuscript.
Supported by grants from the Medical Research Council of Canada (MRC), the National Cancer Institute of Canada (NCIC), and MDS-Sciex. L.V. was supported by an MRC fellowship and P.v.d.G. was a postdoctoral fellow of the NCIC. T.P. is a distinguished MRC professor.
Reprints:Tony Pawson, Programme in Molecular Biology and Cancer, Samuel Lunenfeld Research Institute, Mount Sinai Hospital, 600 University Ave, Toronto, ON M5G 1X5, Canada; e-mail:pawson@mshri.on.ca.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.