In an excellent review on the complications of peripheral blood stem cell harvesting from healthy donors,1 Anderlini et al pointed out the lack of data on late side effects. Lymphocytopenia (primarily T-lymphocytopenia) is a recognized short-term side effect of the harvest,2,3 possibly due to the large number of lymphocytes coharvested with the stem cells.2,4,5 The duration of the postharvest lymphocytopenia is unknown. Total white blood cell counts late after harvest appear normal.6 7 We have evaluated lymphocyte subset counts and monocyte counts prior to and at about 1 year after harvest in order to determine whether T-lymphocytopenia might last for more than 1 year after harvest.
Nine healthy donors (6 males and 3 females) who agreed to have blood drawn at 1 year after harvest were studied at 391-468 days (median, 408) following harvest of filgrastim-mobilized blood stem cells. Donor age was 17-63 years (median, 49). Seven of the donors were also studied at baseline (premobilization). Filgrastim (16 μg/kg/d) was given on 5 consecutive days. The donors underwent 1 or 2 12-liter aphereses, using Cobe Spectra (Lakewood, CO). For the enumeration of mononuclear cell (MNC) subsets, blood cells were separated on a Ficoll density gradient and MNCs were stained with fluorochrome-conjugated monoclonal antibodies and analyzed by 3-color flow cytometry as described.8,9 B cells were defined as MNCs expressing CD19 or CD20 and not expressing CD3, CD13, CD14, CD16, CD56, CD10 or CD34. Total CD4 T cells were defined as CD3+CD4+CD8− MNCs. Total CD8 T cells were defined as CD3+CD4−CD8+ MNCs. Naive T cells (CD45RAhigh CD4 T cells10,11 and CD11alow CD8 T cells12,13) were specifically studied, as reconstitution of these cells may be extremely slow in adults.11 Naive CD4 T cells were defined as MNCs expressing CD4, brightly expressing CD45RA, and not expressing CD8, CD13, CD14, or CD16. Naive CD8 T cells were defined as MNCs brightly expressing CD8, dimly expressing CD11a, and not expressing CD4, CD13, CD14, CD16, or CD19. NK cells were defined as MNCs expressing CD16 or CD56 and not expressing CD3 or CD14, and monocytes were defined as CD14+MNCs. Absolute MNC subset counts were calculated as the absolute MNC count multiplied by the percent of the subset divided by 100. Absolute MNC count was the sum of absolute lymphocyte count and absolute monocyte count determined by a clinical hematology laboratory. Normal values of the MNC subset counts were established using blood from 103 adult healthy volunteers.
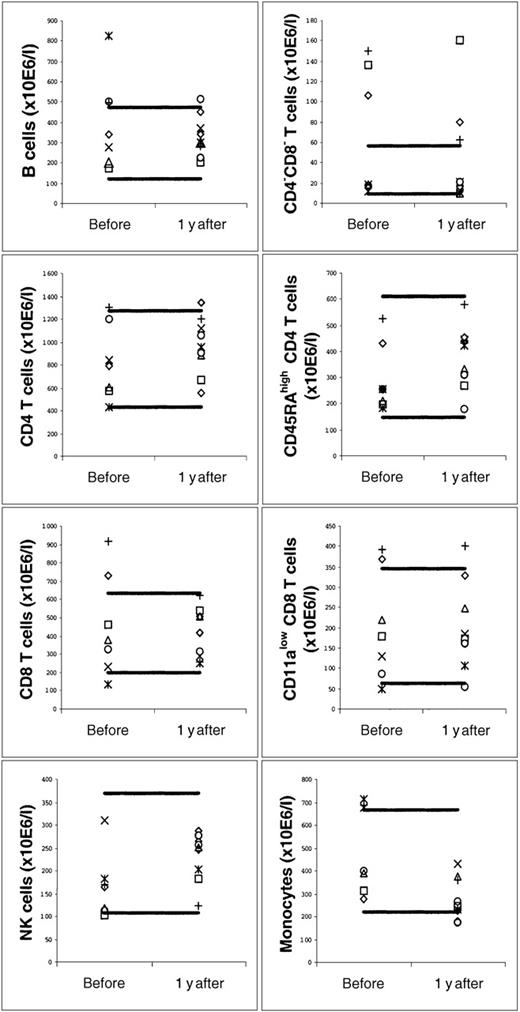
As shown in the Figure, at about 1 year after harvest, B cell, CD4−CD8− T cell, CD4 T cell (both total and naive), CD8 T cell (both total and naive), and NK cell counts were not lower than in the same donors at baseline and not lower than in the 103 normal adult volunteers. However, monocyte counts were lower at about 1 year, compared either to the same donors at baseline (P = .02, paired Wilcoxon signed rank test) or to the normal adult volunteers (P = .01, Mann-Whitney rank sum test). We have not documented that the count of each MNC subset was low in our donors early postharvest. However, consistent with the published data,2 3 our donors' MNC subset counts were probably low early after harvest because their median MNC count at 7-14 days after harvest (1.5 × 109/L) was significantly lower than the median MNC count of the same donors at baseline (2.2 × 109/L, P = .047, paired Wilcoxon signed rank test) and significantly lower than the median MNC count of the normal adult volunteers (2.2 × 109/L, P = .006, Mann-Whitney rank sum test). It was also significantly lower than the median MNC count of the donors at about 1 year after harvest (2.2 × 109/L, P = .03, paired Wilcoxon signed rank test). We cannot exclude that our donors' lymphocyte subset counts returned to normal before 1 year after harvest.
Mononuclear cell subset counts in the blood of blood stem cell donors before and at about 1 year after harvest, showing that B, T, and NK cell counts are not low but that monocyte counts may be low at about 1 year after harvest.
Each patient is represented by a specific symbol. Two of the 9 donors were studied only at about 1 year and not before harvest (gray diamond and gray circle). The thick horizontal lines denote the normal “range” (10th-90th percentile of 103 adult healthy volunteers).
Mononuclear cell subset counts in the blood of blood stem cell donors before and at about 1 year after harvest, showing that B, T, and NK cell counts are not low but that monocyte counts may be low at about 1 year after harvest.
Each patient is represented by a specific symbol. Two of the 9 donors were studied only at about 1 year and not before harvest (gray diamond and gray circle). The thick horizontal lines denote the normal “range” (10th-90th percentile of 103 adult healthy volunteers).
Our data suggest that quantitative deficiency of B, T, and NK cells is not present late after harvest while mild monocytopenia of unclear clinical significance may occur.
Supported by National Institutes of Health (USA) grants no. CA68496 and AI46108.