Abstract
The transcription factor STAT5 is constitutively tyrosine phosphorylated and activated after transformation of hematopoietic cells by p210Bcr/Abl. A truncated form of STAT5B (▵STAT5; aa, 1-683) that lacks tyrosine 699 and the transcriptional activation domain was introduced into Ba/F3p210 cells under the control of a tetracycline-inducible promoter. Treatment of these cells with doxycycline, a tetracycline analogue, induced expression of ▵STAT5 and inhibited STAT5-dependent transcription. ▵STAT5 coprecipitated with STAT5 and decreased Bcr/Abl-dependent tyrosine phosphorylation of endogenous STAT5. Induction of ▵STAT5 inhibited growth of Ba/F3p210 cells (26%-52% of control levels at 4 days) but did not cause cell-cycle arrest. ▵STAT5 reduced viability of Ba/F3p210 cells and increased sensitivity of the cells to the cytotoxic drugs hydroxyurea and cytarabine. These results indicate that high-level expression of ▵STAT5, as achieved here by using a tetracycline-inducible promoter, inhibits STAT5 activity, reduces the growth rate of Ba/F3p210 cells by inhibiting viability, and results in increased sensitivity to chemotherapeutic drugs. It is therefore likely that STAT5 activation plays a role in the transformation of hematopoietic cell lines by p210Bcr/Abl.
Chronic myelogenous leukemia (CML) is a chronic myeloproliferative disorder caused by a translocation of chromosomes 9 and 22 [t(9;22)] that fuses the BCR gene from chromosome 22 to the ABL gene from chromosome 9.1 Transcription of this hybrid gene generates a 210-kd fusion protein (p210Bcr/Abl), which has enhanced tyrosine kinase activity.2 Bcr/Abl transforms primary murine hematopoietic cells and converts interleukin 3 (IL-3)–dependent cell lines to growth-factor independence. Several studies have identified signaling pathways activated by Bcr/Abl, including activation of p21Ras3,4 PI3-kinase,5c-jun, and c-myc.6 Also, we and others showed that STAT5 is phosphorylated in Bcr/Abl-transformed cells,7 8 although the importance of STAT5 tyrosine phosphorylation has remained unclear.
Two closely related forms of STAT5 exist, STAT5A and STAT5B, which are encoded by 2 different genes.9 Both are latent transcription factors, which are coordinately tyrosine phosphorylated in normal hematopoietic cells in response to several cytokines, including erythropoietin, IL-3, and granulocyte-macrophage colony stimulating factor (GM-CSF).9 Also, several oncogenes have been shown to be associated with constitutive phosphorylation of STAT5,10,11 and STAT5 has been reported to be phosphorylated in a variety of malignant disorders other than CML. Tyrosine phosphorylation of STATs is believed to induce dimerization,12 translocation to the nucleus, and binding of STAT5 dimers to specific DNA motifs. Only a small number of genes have so far been shown to be regulated by STAT5, includingβ-casein,13CIS,14OSM,15 andBclX.16 The role of STAT5 in hematopoiesis of adult mice is not clear, since mice with a targeted disruption of the STAT5A and STAT5B genes have largely normal blood counts.17 However, these mice had reduced myeloid progenitor counts, suggesting that STAT5 might be important for maintenance of the stem-cell compartment.
STAT5 has several important structural domains, including a DNA-binding sequence, an SH2-domain, and a transactivation domain. Phosphorylation of tyrosine 694 of STAT5A and tyrosine 699 of STAT5B creates a binding site for the SH2 domain of another STAT5 molecule. Deletions of various domains within the STAT5 molecule have been found to modify STAT5 function. In particular, expression of a STAT5B mutant truncated at position 683 was found to exert a dominant negative effect on endogenous STAT5.18
In the current study, a STAT5 complementary DNA encoding STAT5B truncated at aa 683 was introduced into Ba/F3p210 cells under the control of an inducible promotor. Induction of the truncated STAT5 gene (ΔSTAT5) was found to inhibit STAT5 activity and STAT5-induced gene expression. Using this system, we analyzed the role of STAT5 in transformation by p210Bcr/Abl.
Materials and methods
Cell lines and cell culture
Ba/F3 is an IL-3–dependent murine hematopoietic precursor cell line. Ba/F3 cells were maintained in RPMI 1640 medium (Mediatech Cellgro, Herndon, VA) supplemented with 10% (vol/vol) fetal calf serum and 10% (vol/vol) WEHI-3B–conditioned medium as a source of IL-3. Ba/F3 cells containing the reverse Tet transactivator pUHD172-1 (Ton.BaF.119) and Ton B.210.1 cells,19 in which p210Bcr/Abl can be induced by the addition of doxycycline, were grown in the same medium. Transformed Ba/F3p210 cells are growth-factor independent and were maintained without WEHI-3B supernatant unless otherwise stated. All cell lines were grown in a humidified incubator at 37°C (5% carbon dioxide).
Introduction of a truncated STAT5 mutant into Ba/F3p210 cells
A truncated form of the murine STAT5B (ΔSTAT5; aa, 1-683, in plasmid pBABE) was obtained from A. L. Mui18 (DNAX Research Institute, Palo Alto, CA). The construct was cloned into pTRE plasmid (Clontech, Palo Alto, CA) by using EcoRI and XbaI sites. The pTRE plasmid containing the truncated STAT5 construct was then cotransfected with a p210Bcr/Abl plasmid (pGD21020) into Ton.BaF.1 cells containing the reverse Tet transactivator.19 Cell lines were then selected for growth-factor independence in the absence of doxycycline. Factor-independent sublines were analyzed for doxycycline-inducible ΔSTAT5 expression by Western blotting, as well as for expression of p210Bcr/Abl.
STAT5 reporter gene assay
STAT5-dependent transcription was measured by using a reporter gene construct (GAS-luciferase) containing 4 tandem β-casein–like GAS elements from the β-globin locus control region cloned into the pGL2 luciferase vector (from A. D'Andrea, Boston, MA).21 This construct (20 μg) was transiently transfected into Ba/F3p210 cells. Twelve hours later, transfected cells were divided into 2 equal cultures and then maintained in either the presence or absence of doxycycline (1 μg/mL) for the next 24 hours. After being washed twice with phosphate-buffered saline (PBS) at 4°C, cells were resuspended in lysis buffer E397A (Promega, Madison, WI). The lysates (20 μL) were then incubated with 300 μL of luciferase assay buffer (25 mmol/L of glycylglycine [pH 7.8], 15 mmol/L of potassium phosphate [pH 7.8], 15 mmol/L of magnesium sulfate, 4 mmol/L of ethylene glycol tetraacetic acid, 2 mmol/L of adenosine triphosphate, and 1 mmol/L of dithiothreitol) and 100 μL of D-luciferin (0.3 mg/mL; Pharmingen, San Diego, CA). Luciferase activity was assessed with an automated liminometer (Lumat LB 9507; EG&G Berthold, Gaithersburg, MD). The plasmid CMVβ-GAL (20 μg; Invitrogen, San Diego, CA) was used as a reporter for transfection efficiency, and β-GAL activity was measured with a β-GAL assay kit (Invitrogen, Carlsbad, CA). When STAT5 reporter gene activity in 2 different cell lines was compared, luciferase activity was reported as a ratio of luciferase activity to βGAL activity.
Preparation of cell lysates and Western blotting
Cells were washed twice in Dulbecco's PBS at 4°C and resuspended in lysis buffer (1 mL/108 cells) consisting of 50 mmol/L of Tris (pH 8.0), 150 mmol/L of sodium chloride (NaCl), 1% (vol/vol) NP-40, 0.5% (wt/vol) deoxycholic acid, 0.1% (wt/vol) sodium dodecyl sulfate (SDS), 100 mmol/L of sodium fluoride, 1 mmol/L of phenylmethylsulfonyl fluoride, 20 μg/mL of aprotinin, 40 μg/mL of leupeptin, and 1 mmol/L of sodium orthovanadate. Lysates were then incubated on ice for 30 minutes (resuspended vigorously every 5 minutes), centrifuged for 15 minutes (12 000g) to remove insoluble particles, and either subjected to immunoprecipitation or analyzed directly by immunoblotting. For immunoprecipitation, lysates from 2 × 107 cells were incubated with an anti-STAT5 antibody (Ab) (C-17; Santa Cruz Biotechnology, Santa Cruz, CA) and protein G Sepharose beads (Pharmacia, Uppsala, Sweden) in lysis buffer for 3 hours at 4°C and washed 3 times with 1% (vol/vol) NP-40 at 4°C. Lysates and immunoprecipitates were then separated under reducing conditions by 7.5% SDS-polyacrylamide gel electrophoresis and electrophoretically transferred to a nitrocellulose membrane (Protran; Schleicher & Schuell, Keene, NH) in buffer containing 25 mmol/L of Tris, 192 mmol/L of glycine, and 20% (vol/vol) methanol at 4°C. The membrane was blocked for 1 hour in 5% (wt/vol) notfat dry milk powder in Tris-buffered saline (10 mmol/L of Tris-hydrochloric acid [pH 8.0] and 150 mmol/L of NaCl). The membrane was incubated sequentially with the primary and the secondary horseradish peroxidase (HRP)-coupled Ab (1:5000 dilution in Tris-buffered saline; Amersham, Piscataway, NJ) for 1 hour. HRP activity was detected by using HRP substrates (Renaissance; NEN, Boston, MA) and X-Omat film (Kodak, Rochester, NY).
The following Abs were used: the anti-STAT5 Ab, Ab 89 (Transduction Laboratories, Lexington, KY), and C-17 (Santa Cruz Biotechnology); anti-myc Ab A-14 (Santa Cruz Biotechnology); antiphosphotyrosine Ab 4G10 (Brian Druker, Oregon Health Sciences University, Portland, OR); anti-CIS Ab (Santa Cruz Biotechnology); anti-BclX Ab 2H12 (Transduction Laboratories); and anti-Bcl2 Ab C-21 (Santa Cruz Biotechnology). The anti-Abl monoclonal Ab 3F12 was obtained from Ravi Salgia, Dana Farber Cancer Institute, Boston, MA.
Cell-cycle analysis
For each sample, 0.5 × 106 cells were washed in Dulbecco's PBS at 4°C and resuspended in 500 μL of staining solution containing 50 μg/mL of propidium iodide (PI), 0.1% (vol/vol) NP-40, and 0.1% (wt/vol) sodium citrate. Cells were incubated at 4°C in the dark for 15 minutes and then analyzed by flow cytometry (Epics XL flow cytometer; Coulter Corp, Miami, FL).
Combined annexin V/PI staining procedure
Viability was assessed by using an annexin V staining kit (FLUOS; Boehringer Mannheim, Indianapolis, IN) according to the manufacturer's recommendations. Binding of fluorescein-conjugated annexin V and PI was measured by fluorescence-activated cell separation. In some experiments, cells were exposed to cytarabine for 36 hours (Bedford Laboratories, Bedford, OH) or hydroxyurea (Sigma, St Louis, MO) for 5 hours before annexin V/PI staining.
Results
Generation of stable Ba/F3p210 cells inducibly expressing ▵STAT5
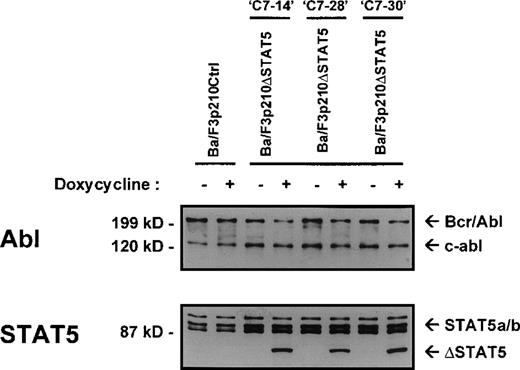
The Ba/F3 cell line is a nonleukemic hematopoietic cell line that requires murine IL-3 for growth and viability. Transfection of Ba/F3 cells with p210Bcr/Abl makes these cells leukemic and growth-factor independent.22 To investigate the contribution of STAT5 activation to Bcr/Abl transformation, a series of cell lines was created in which a truncated, dominant negative, mutant form of STAT518 (ΔSTAT5, a C-terminal deletion that retains aa 1-683 of STAT5B) was expressed under the control of a doxycycline-inducible promotor. Ba/F3 cells stably expressing the reverse Tet transactivator pUHD172-1 (Ton.BF.119) were cotransfected with a ΔSTAT5 construct (pTRE-ΔSTAT5) and p210Bcr/Abl (pGD210) and selected for growth-factor independence. These cells were designated Ba/F3p210ΔSTAT5 cells. As a control, TonBaF.1 cells were transfected with pGD210 and the empty pTRE plasmid (designated Ba/F3p210Ctrl cells). Eight subclones of Ba/F3p210ΔSTAT5 cells were obtained by single-cell cloning. On induction with doxycycline (1μg/mL), the truncated form of STAT5 (ΔSTAT5 [78 kd]) was induced in 3 of 8 clones tested. Inducible ΔSTAT5 expression in these 3 subclones (C7.14, C7.28, and C7.30) is shown in Figure1 (lower panel). Doxycycline did not induce any form of STAT5 protein in Ba/F3p210Ctrl cells. In the absence of doxycycline, ΔSTAT5 expression in Ba/F3p210ΔSTAT5 cell clones was minimal but detectable, indicating a small degree of leakiness in this system. Each clone expressed endogenous (full-length) STAT5A and STAT5B (Figure 1, lower panel), p210Bcr/Abl, and c-abl (upper panel). Growth-factor independence was due specifically to Bcr/Abl, since each of these clones died rapidly in the presence of the Bcr/Abl kinase inhibitor STI57123 (1μmol/L). The expression levels of c-abl and Bcr/Abl in the 3 Ba/F3p210ΔSTAT5 cell clones and in Ba/F3p210Ctrl cells were approximately equivalent.
Expression of ▵STAT5 and p210Bcr/Abl in Ba/F3-p210 cells.
Ba/F3 cells transfected with p210Bcr/Abl and either the empty pTRE plasmid (Ba/F3p210Ctrl) or pTRE ΔSTAT5 (Ba/F3p210ΔSTAT5) were maintained in either the presence or absence of doxycycline (1 μg/mL) for 18 hours and then subjected to Western blot analysis. Although all cell lines were found to express both forms of STAT5 (STAT5A and STAT5B), incubation with doxycycline induced the truncated form of STAT5 (estimated molecular weight, 78 kd) in 3 clones of Ba/F3p210ΔSTAT5 (lower panel). No induction of ΔSTAT5 protein was found in Ba/F3p210Ctrl cells. Probing with an Abl antibody revealed expression of c-abl (145 kd) and of p210Bcr/Abl (210 kd) (upper panel).
Expression of ▵STAT5 and p210Bcr/Abl in Ba/F3-p210 cells.
Ba/F3 cells transfected with p210Bcr/Abl and either the empty pTRE plasmid (Ba/F3p210Ctrl) or pTRE ΔSTAT5 (Ba/F3p210ΔSTAT5) were maintained in either the presence or absence of doxycycline (1 μg/mL) for 18 hours and then subjected to Western blot analysis. Although all cell lines were found to express both forms of STAT5 (STAT5A and STAT5B), incubation with doxycycline induced the truncated form of STAT5 (estimated molecular weight, 78 kd) in 3 clones of Ba/F3p210ΔSTAT5 (lower panel). No induction of ΔSTAT5 protein was found in Ba/F3p210Ctrl cells. Probing with an Abl antibody revealed expression of c-abl (145 kd) and of p210Bcr/Abl (210 kd) (upper panel).
Interference with tyrosine phosphorylation of endogenous STAT5 by expression of ▵STAT5
Expression of truncated forms of STAT5 was previously shown to inhibit STAT5 activity.13,18 ΔSTAT5 was also shown to inhibit tyrosine phosphorylation of STAT5 on stimulation with IL-3 receptor.19 Therefore, in Ba/F3p210ΔSTAT5 cells, tyrosine phosphorylation of STAT5 was measured before and after induction of ΔSTAT5 with doxycycline for 24 hours. Immunoprecipitation of STAT5 by using a STAT5 Ab directed against the C-terminal of STAT5 precipitated full-length STAT5A and STAT5B (Figure 2, middle panel). When probed with antiphosphotyrosine Ab 4G10, STAT5 was found to be phosphorylated in unstimulated Ba/F3p210ΔSTAT5 cells as well as in Ba/F3p210Ctrl cells (Figure 2, upper panel). Tyrosine phosphorylation of STAT5 was reduced in Ba/F3p210ΔSTAT5 cells when ΔSTAT5 was induced by incubation with doxycycline for 24 hours. In Ba/F3p210Ctrl cells, addition of doxycycline had no effect on tyrosine phosphorylation of STAT5.
Reduction of phosphorylation of endogenous STAT5 by ▵STAT5 expression.
Lysates from Ba/F3p210ΔSTAT5 cells (maintained in either the presence or absence doxycycline [1 μg/mL] for 24 hours) were incubated with anti-STAT5 antibody C17 and protein G beads for 3 hours and then subjected to 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting. Probing with an anti-STAT5 antibody raised against the SH2 and SH3 domains of STAT5 revealed equal amounts of STAT5A and STAT5B (middle panel). When probed with antiphosphotyrosine antibody 4G10, STAT5 was found to be constitutively phosphorylated in both Ba/F3p210Ctrl cells and Ba/F3p210ΔSTAT5 cells (upper panel). Induction of ΔSTAT5 for 24 hours was associated with a reduction of tyrosine phosphorylation of STAT5. A longer exposure of the blot revealed that ΔSTAT5 coprecipitated with endogenous STAT5 in Ba/F3p210ΔSTAT5 cells (lower panel). Neither endogenous STAT5 nor ΔSTAT5 coprecipitated when lysates were incubated with normal rabbit serum and beads.
Reduction of phosphorylation of endogenous STAT5 by ▵STAT5 expression.
Lysates from Ba/F3p210ΔSTAT5 cells (maintained in either the presence or absence doxycycline [1 μg/mL] for 24 hours) were incubated with anti-STAT5 antibody C17 and protein G beads for 3 hours and then subjected to 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting. Probing with an anti-STAT5 antibody raised against the SH2 and SH3 domains of STAT5 revealed equal amounts of STAT5A and STAT5B (middle panel). When probed with antiphosphotyrosine antibody 4G10, STAT5 was found to be constitutively phosphorylated in both Ba/F3p210Ctrl cells and Ba/F3p210ΔSTAT5 cells (upper panel). Induction of ΔSTAT5 for 24 hours was associated with a reduction of tyrosine phosphorylation of STAT5. A longer exposure of the blot revealed that ΔSTAT5 coprecipitated with endogenous STAT5 in Ba/F3p210ΔSTAT5 cells (lower panel). Neither endogenous STAT5 nor ΔSTAT5 coprecipitated when lysates were incubated with normal rabbit serum and beads.
Because STAT5 is believed to dimerize after tyrosine phosphorylation, the possible formation of ΔSTAT5/STAT5 dimers was investigated. Immunoprecipitation was performed with an anti-STAT5 C-terminal Ab (this epitope is missing in ΔSTAT5). This was followed by immunoblotting with a different STAT5 Ab that recognizes endogenous STAT5 as well as ΔSTAT5. A small amount of ΔSTAT5 coprecipitated with endogenous (full-length) STAT5 (Figure 2, lower panel), suggesting that ΔSTAT5 can form heterodimers with wtSTAT5.
Inhibition by ▵STAT5 of Bcr/Abl-dependent activation of a STAT5 reporter gene
Phosphorylation of STAT5 leads to dimerization and translocation into the nucleus, where STAT5 dimers then activate transcription through binding to specific DNA sequences. The consensus STAT5 binding site is TTCNNNGAA.24 Genes known to have STAT5 binding sites in their promotors include β-casein, IRF1, CIS, andBclX.24 The STAT5 binding site of theβ-casein gene has previously been used for STAT5 reporter gene constructs.13 18
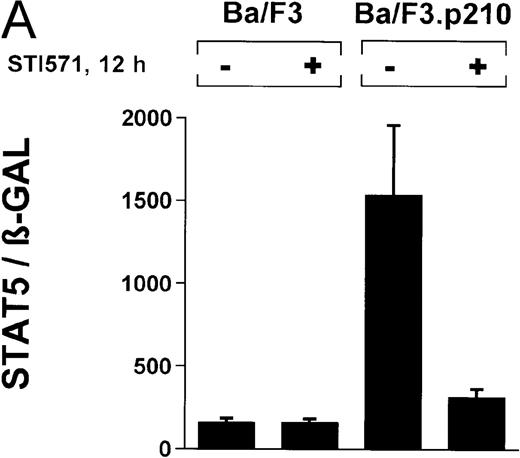
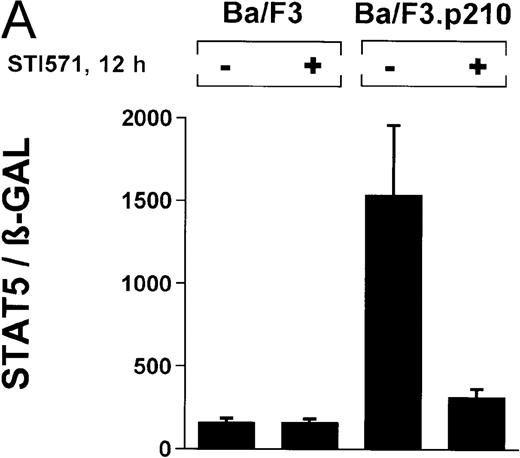
In the current study, we used a reporter gene construct (GAS-luciferase) in which a tandem repeat of 4 STAT binding motifs was cloned upstream of the luciferase gene in the pGL2 promotor vector. Using this reporter gene construct, we analyzed p210Bcr/Abl-transformed cells for STAT5 reporter gene activity and compared the results with findings in nontransformed Ba/F3 cells. A β-GAL reporter gene construct was used as a transfection control. STAT5 reporter gene activity was considerably higher in Ba/F3p210 cells (Figure3A), (ratio of STAT5 to β-GAL, 1533 ± 423) compared with untransformed Ba/F3 cells (ratio of STAT5 to β-GAL, 157 ± 30). Increased STAT5 reporter gene activity could directly be attributed to Bcr/Abl, since incubation with STI571 (1 μmol/L for 12 hours) inhibited STAT5 activity (ratio of STAT5 to β-GAL, 312 ± 51) in Ba/F3p210 cells but had no effect in untransformed Ba/F3 cells (Figure 3A), (ratio of STAT5 to β-GAL, 157 ± 28).
Induction of STAT5 reporter gene activity with Bcr/Abl.
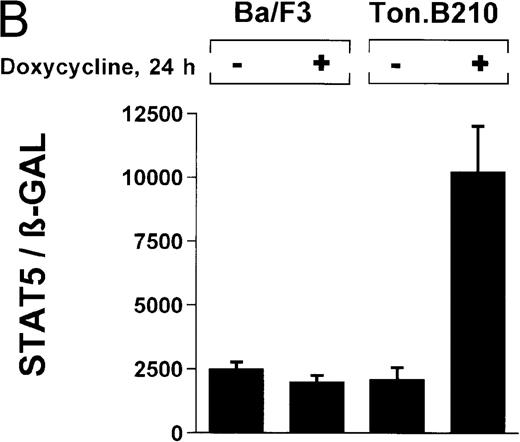
(A) Ba/F3p210 cells and untransformed Ba/F3 cells in RPMI plus WEHI (10%) were transfected with the β-casein luciferase reporter gene (together with the β-GAL construct). Twelve hours after transfection, cells were split and maintained in either the presence or absence of Bcr/Abl kinase inhibitor STI571 (1 μmol/L). Cells were then harvested, and lysates were assayed for luciferase and β-GAL activities, expressed as a ratio of luciferase activity to β-GAL-activity. Ba/F3p210 cells expressed significantly more STAT5 reporter gene activity than did Ba/F3 cells, and the increased STAT5 activity was due directly to Bcr/Abl, since it could be abrogated by Bcr/Abl kinase inhibitor STI571. (B) Untransformed Ba/F3 cells and TonB.210 cells were transfected with the β-casein luciferase reporter gene (together with the β-GAL construct). Twelve hours after transfection, cells were split, maintained in either the presence or absence of doxycycline (1 μg/mL), and assayed for luciferase and βGAL activities. Induction of Bcr/Abl in Ton B.210.1 cells by doxycycline increased STAT5 reporter gene activity. Doxycycline had no effect on STAT5 reporter gene activity in untransformed Ba/F3 cells.
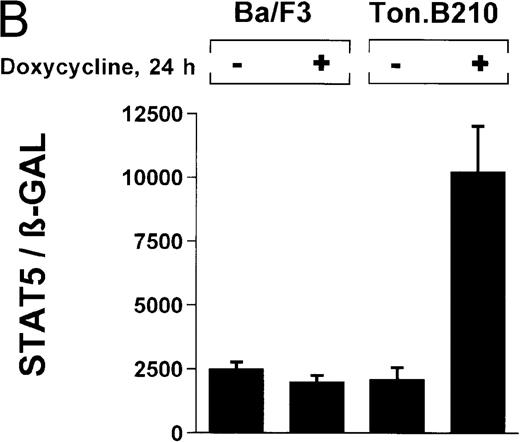
Induction of STAT5 reporter gene activity with Bcr/Abl.
(A) Ba/F3p210 cells and untransformed Ba/F3 cells in RPMI plus WEHI (10%) were transfected with the β-casein luciferase reporter gene (together with the β-GAL construct). Twelve hours after transfection, cells were split and maintained in either the presence or absence of Bcr/Abl kinase inhibitor STI571 (1 μmol/L). Cells were then harvested, and lysates were assayed for luciferase and β-GAL activities, expressed as a ratio of luciferase activity to β-GAL-activity. Ba/F3p210 cells expressed significantly more STAT5 reporter gene activity than did Ba/F3 cells, and the increased STAT5 activity was due directly to Bcr/Abl, since it could be abrogated by Bcr/Abl kinase inhibitor STI571. (B) Untransformed Ba/F3 cells and TonB.210 cells were transfected with the β-casein luciferase reporter gene (together with the β-GAL construct). Twelve hours after transfection, cells were split, maintained in either the presence or absence of doxycycline (1 μg/mL), and assayed for luciferase and βGAL activities. Induction of Bcr/Abl in Ton B.210.1 cells by doxycycline increased STAT5 reporter gene activity. Doxycycline had no effect on STAT5 reporter gene activity in untransformed Ba/F3 cells.
The ability of Bcr/Abl to induce STAT5-dependent gene transcription was confirmed by using a cell line (TonB.210) in which p210Bcr/Abl can be induced by the addition of doxycycline.19 Doxycycline (1 μg/mL for 24 hours) significantly increased STAT5 reporter gene activity (Figure 3B) (ratio of STAT5 to β-GAL, 2089 ± 468 for the control and 10 216 ± 1805 for doxycycline-treated cells). Addition of doxycycline had no effect on untransformed Ba/F3 cells (Figure 3B) (ratio of STAT5 to β-GAL, 2476 ± 285 for the control and 1982 ± 276 for doxycycline-treated cells).
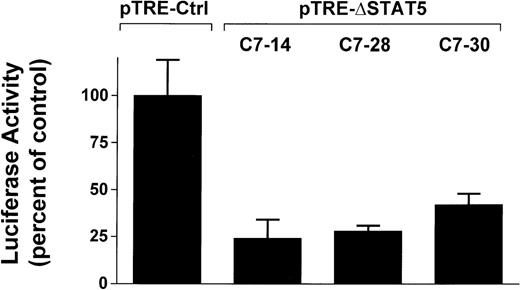
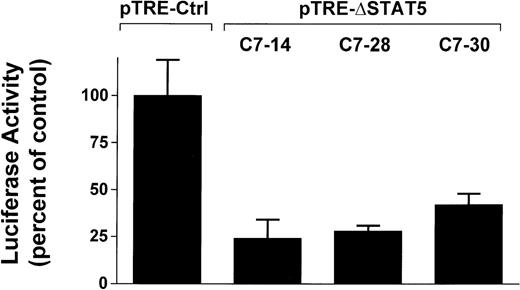
To determine whether induction of ΔSTAT5 in Bcr/Abl-transformed cells would inhibit STAT5 reporter gene activity, the Ba/F3p210ΔSTAT5 clones were transfected with the β-casein reporter gene construct. Twelve hours after transfection, cells were split and then maintained in either the presence or absence of doxycycline (1μg/mL) for 24 hours. ΔSTAT5 expression significantly reduced luciferase reporter gene activity in each of the 3 clones tested (Figure4), (C7.14, 23.9% ± 9.5% of unstimulated control; C7.28, 28.2% ± 3.5% of unstimulated control; and C7.30, 42.0% ± 5.8% of unstimulated control). No reduction of STAT5 reporter gene activity was found in the Ba/F3p210Ctrl cells (99.5% ± 18.6% of unstimulated control). Therefore, doxycycline-induced expression of ΔSTAT5 in Ba/F3p210ΔSTAT5 cells inhibited STAT5 reporter gene activity.
Inhibition of STAT5 reporter gene activity by δSTAT5 expression.
Cells from 3 clones of Ba/F3p210ΔSTAT5 cells were transfected with the β-casein luciferase construct. Twelve hours after transfection, cells were split and maintained in either the presence or absence of doxycycline (1 μg/mL) for 24 hours. Cells were then assayed for luciferase activity, and results are expressed as the percentage of activity in unstimulated controls. Induction of ΔSTAT5 in Ba/F3p210ΔSTAT5 cells by doxycycline significantly inhibited STAT5 reporter gene activity in all clones tested. No inhibition of STAT5 activity was observed when Ba/F3p210Ctrl cells were exposed to doxycycline.
Inhibition of STAT5 reporter gene activity by δSTAT5 expression.
Cells from 3 clones of Ba/F3p210ΔSTAT5 cells were transfected with the β-casein luciferase construct. Twelve hours after transfection, cells were split and maintained in either the presence or absence of doxycycline (1 μg/mL) for 24 hours. Cells were then assayed for luciferase activity, and results are expressed as the percentage of activity in unstimulated controls. Induction of ΔSTAT5 in Ba/F3p210ΔSTAT5 cells by doxycycline significantly inhibited STAT5 reporter gene activity in all clones tested. No inhibition of STAT5 activity was observed when Ba/F3p210Ctrl cells were exposed to doxycycline.
Inhibition of CIS protein expression but not of BclXprotein expression in Ba/F3p210 cells by induction of ▵STAT5
CIS is a protein involved in the negative regulation of JAK/STAT signaling.25 Binding of CIS protein to the cytoplasmic domain of a cytokine receptor is associated with inhibition of STAT phosphorylation, thereby creating a negative feedback loop that limits cytokine-induced STAT activation. The CIS gene, like theβ-casein gene, has STAT5 binding sites upstream of its initiation site,24,26 and STAT5 activation leads to CIS expression.26 In the current study, CIS expression was constitutively expressed in Ba/F3p210 cells (Figure5A), and induction of ΔSTAT5 with doxycycline for 24 hours reduced CIS protein expression (Figure 5A). Two forms of CIS, a 37-kd form and a 45-kd form, were detected by immunoblotting. The 45-kd form of CIS is likely to represent a ubiquitinated form of CIS.27 These results show that expression of ΔSTAT5 is associated with reduced transcription of a known STAT5-inducible gene, which is consistent with the idea that ΔSTAT5 functions as a dominant negative mutant.
Differential regulation of STAT5-induced genes,CIS and BclX, in Bcr/Abl transformed cells.
(A) Inhibition of CIS protein expression by ΔSTAT5 expression. Lysates from Ba/F3p210ΔSTAT5 cells (after treatment with or without doxycycline [1 μg/mL] for 24 hours) were incubated with a CIS antibody and protein G beads. Immunoprecipitates were then subjected to SDS-PAGE (9%). Probing with anti-CIS antibody revealed constitutive expression of CIS protein (37 kd) in Bcr/Abl-transformed cells, and expression of ΔSTAT5 inhibited CIS protein expression. Addition of doxycycline had no effect on CIS protein expression in Ba/F3p210Ctrl cells. A larger, presumably ubiquitinated form of CIS protein (∼ 45 kd) was also expressed in Ba/F3p210 cells. (B) Lack of effect on BclX protein expression of induction of ΔSTAT5. Lysates from Ba/F3 and Ba/F3p210 cells (maintained in the presence of WEHI-CM) were subjected to SDS-PAGE. Ba/F3p210 cells expressed more BclXLthan untransformed Ba/F3 cells (upper left-hand panel). Enhanced BclXL expression could be attributed directly to Bcr/Abl, since incubation with Bcr/Abl kinase inhibitor STI571 (1 μmol/L for 24 hours) reversed the enhanced BclXL expression in Ba/F3p210 cells. However, induction of ΔSTAT5 with doxycycline (1 μg/mL for 3 days) had no effect on BclXL expression in Ba/F3p210ΔSTAT5 cells (upper right-hand panel). Induction of ΔSTAT5 in Ba/F3p210ΔSTAT5 cells is shown as a control (lower panel).
Differential regulation of STAT5-induced genes,CIS and BclX, in Bcr/Abl transformed cells.
(A) Inhibition of CIS protein expression by ΔSTAT5 expression. Lysates from Ba/F3p210ΔSTAT5 cells (after treatment with or without doxycycline [1 μg/mL] for 24 hours) were incubated with a CIS antibody and protein G beads. Immunoprecipitates were then subjected to SDS-PAGE (9%). Probing with anti-CIS antibody revealed constitutive expression of CIS protein (37 kd) in Bcr/Abl-transformed cells, and expression of ΔSTAT5 inhibited CIS protein expression. Addition of doxycycline had no effect on CIS protein expression in Ba/F3p210Ctrl cells. A larger, presumably ubiquitinated form of CIS protein (∼ 45 kd) was also expressed in Ba/F3p210 cells. (B) Lack of effect on BclX protein expression of induction of ΔSTAT5. Lysates from Ba/F3 and Ba/F3p210 cells (maintained in the presence of WEHI-CM) were subjected to SDS-PAGE. Ba/F3p210 cells expressed more BclXLthan untransformed Ba/F3 cells (upper left-hand panel). Enhanced BclXL expression could be attributed directly to Bcr/Abl, since incubation with Bcr/Abl kinase inhibitor STI571 (1 μmol/L for 24 hours) reversed the enhanced BclXL expression in Ba/F3p210 cells. However, induction of ΔSTAT5 with doxycycline (1 μg/mL for 3 days) had no effect on BclXL expression in Ba/F3p210ΔSTAT5 cells (upper right-hand panel). Induction of ΔSTAT5 in Ba/F3p210ΔSTAT5 cells is shown as a control (lower panel).
It was previously reported that the BclXgene, like the CIS gene, contains STAT5 binding sites,24 and induction of a constitutive active STAT5 mutant was found to enhance BclX levels.28Bcr/Abl also induces BclX (Figure 5B, upper left panel), suggesting the possibility that BclX induction might be due to STAT5 activation by Bcr/Abl. However, when ΔSTAT5 was induced in Ba/F3p210ΔSTAT5 cells (using 1 μg/mL of doxycycline for 3 days), no effect on BclX protein expression was found (Figure 5B, upper right panel). These results suggest that, in Bcr/Abl-transformed cells, induction of CIS depends on STAT5 activity, whereas expression of BclX does not.
Effects of ▵STAT5 expression on the growth and cell cycle of Ba/F3p210 cells
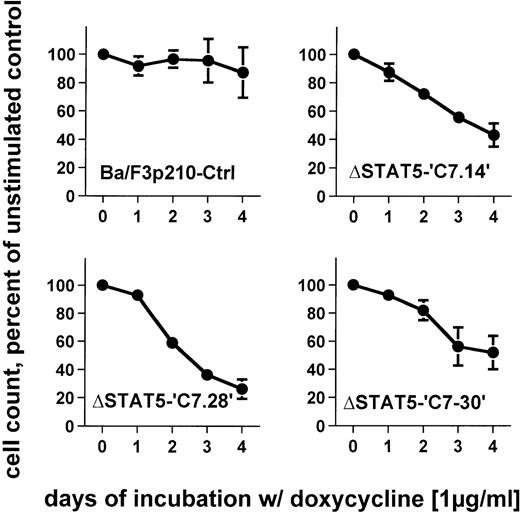
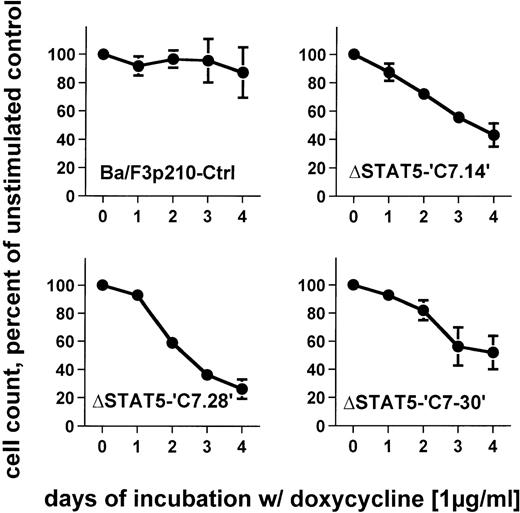
To determine the effects of ΔSTAT5 induction on the growth of Ba/F3p210 cells, Ba/F3p210ΔSTAT5 cells were maintained in either the presence or absence of doxycycline (1 μg/mL), and daily cell counts were obtained. Cells expressing ΔSTAT5 were found to grow more slowly than untreated cells. After 4 days of culture, Ba/F3p210ΔSTAT5 cells induced with doxycycline (1 μg/ml) grew at rates that were 26% to 52% of the growth rates of untreated cells (Figure6). The degree of growth inhibition was similar in each of the 3 clones tested. On day 4, the values were as follows: C7.14, 43.2% ± 8.3% of control; C7.28, 26.2% ± 6.8% of control; and C7.30, 51.9% ± 11.8% of control. In contrast, doxycycline had no significant effect on growth of Ba/F3p210Ctrl cells; on day 4, the value was 87.1% ± 17.8% of control.
Effects of ▵STAT5 expression on growth of Ba/F3p210 cells.
Ba/F3p210ΔSTAT5 cells were maintained in either the presence or absence of doxycycline (1 μg/mL), and daily cell counts were obtained. Cell counts from cells maintained in the presence of doxycycline are expressed as a percentage of the cell counts obtained from cells maintained in the absence of doxycycline. Expression of ΔSTAT5 significantly inhibited cell growth; however, the cells were still growing although at a slower rate. No significant effect of doxycycline was observed in Ba/F3p210Ctrl cells.
Effects of ▵STAT5 expression on growth of Ba/F3p210 cells.
Ba/F3p210ΔSTAT5 cells were maintained in either the presence or absence of doxycycline (1 μg/mL), and daily cell counts were obtained. Cell counts from cells maintained in the presence of doxycycline are expressed as a percentage of the cell counts obtained from cells maintained in the absence of doxycycline. Expression of ΔSTAT5 significantly inhibited cell growth; however, the cells were still growing although at a slower rate. No significant effect of doxycycline was observed in Ba/F3p210Ctrl cells.
To determine whether ΔSTAT5 induced cell-cycle arrest, cell-cycle analysis was performed by using a standard PI staining protocol and flow cytometry. ΔSTAT5 expression did not result in G1 cell-cycle arrest or other alterations in the distribution of cells in other phases of the cell cycle (not shown).
Reduction of viability of Ba/F3p210 cells by ▵STAT5 expression
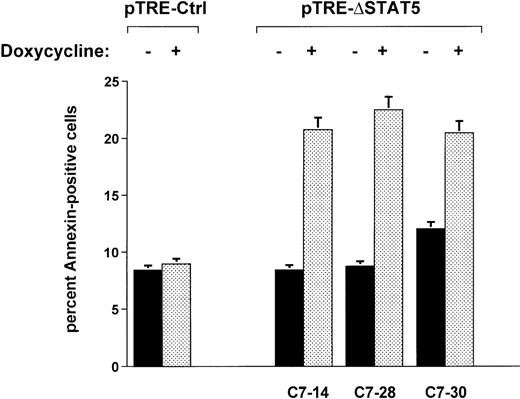
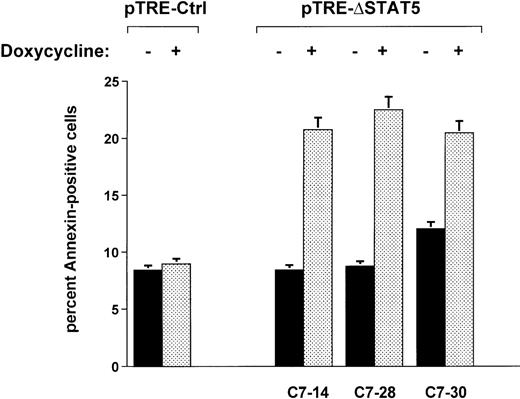
When Ba/F3p210ΔSTAT5 cells were maintained in the presence of doxycycline (1 μg/mL) for 2 days, a small but significant increase in annexin V–positive (apoptotic) cells was observed in all clones tested (Figure 7). The addition of doxycycline to clone C7.14 increased annexin V–positive cells from 8.4% ± 1.1% to 20.8% ± 4.9%; from 8.7% ± 0.5% to 22.5% ± 3.3% in clone C7.28; and from 12.0% ± 1.3% to 20.5% ± 3.2% in clone C7.30 (Figure 7). In contrast, doxycycline (1 μg/mL) had no significant effect on viability of Ba/F3p210Ctrl cells (control, 8.4% ± 0.8% annexin V-positive cells; and doxycycline-treated cells, 9.0% ± 1.8% annexin V-positive cells). The reduced viability associated with ΔSTAT5 is likely to have accounted for some or all of the reduced growth rate of Ba/F3p210ΔSTAT5 cells.
Effects of ▵STAT5 on viability of Ba/F3p210 cells.
Cells from 3 clones of Ba/F3p210ΔSTAT5 cells were maintained in either the presence or absence of doxycycline (1 μg/mL) for 2 days, and viability was determined by using a combined annexin V/PI staining protocol.29 30 Compared with cells not subjected to induction (black bars), ΔSTAT5 expression induced by doxycycline (stippled bars) increased the percentage of annexin V-positive cells (apoptotic cells) in all clones tested. Doxycycline had no effect on the viability of Ba/F3p210Ctrl cells.
Effects of ▵STAT5 on viability of Ba/F3p210 cells.
Cells from 3 clones of Ba/F3p210ΔSTAT5 cells were maintained in either the presence or absence of doxycycline (1 μg/mL) for 2 days, and viability was determined by using a combined annexin V/PI staining protocol.29 30 Compared with cells not subjected to induction (black bars), ΔSTAT5 expression induced by doxycycline (stippled bars) increased the percentage of annexin V-positive cells (apoptotic cells) in all clones tested. Doxycycline had no effect on the viability of Ba/F3p210Ctrl cells.
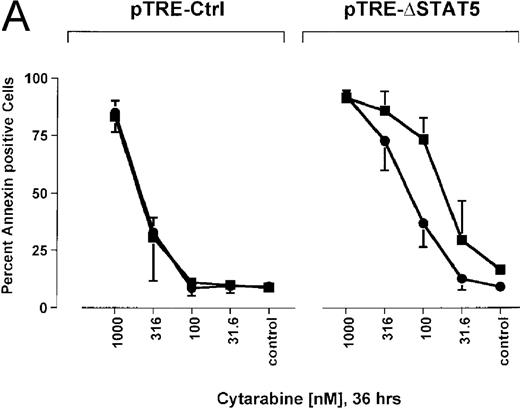
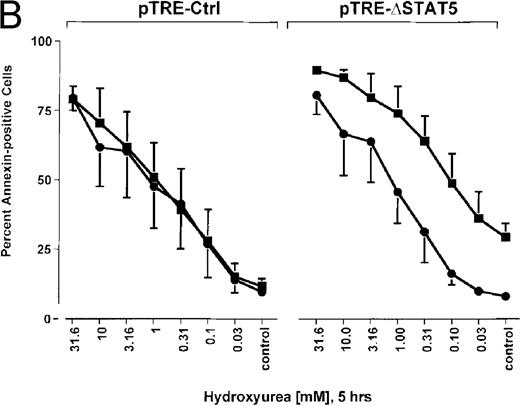
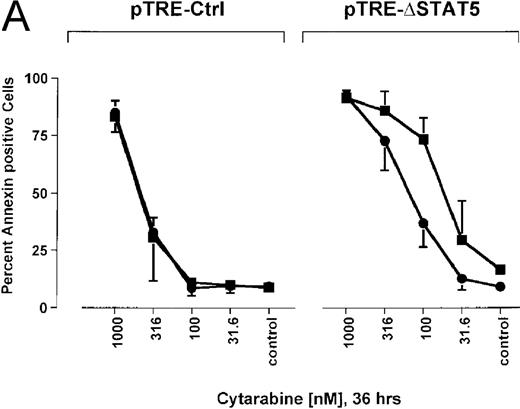
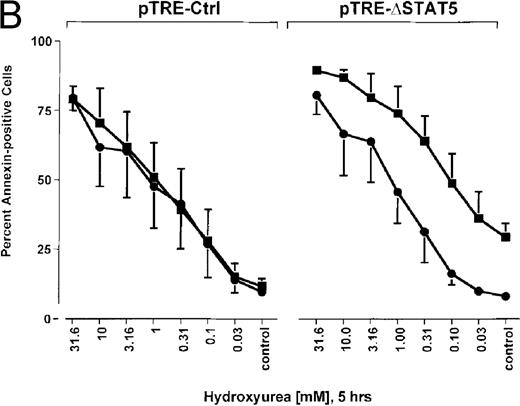
Because many cytotoxic drugs inhibit growth of cancer cells by inducing apoptosis, we wondered whether the presence of ΔSTAT5 would alter the sensitivity of Bcr/Abl-transformed cells to chemotherapeutic drugs used to treat CML. Ba/F3p210ΔSTAT5 cells were incubated with cytarabine in either the presence or absence of doxycycline (1 μg/mL). Thirty-six hours later, cell viability was analyzed by using annexin V/PI staining. Incubation of uninduced Ba/F3p210ΔSTAT5 cells with cytarabine resulted in a dose-dependent increase in apoptotic cells, as expected (Figure 8A). Induction of ΔSTAT5 by doxycycline enhanced the sensitivity of these cells to cytarabine and produced a moderate but reproducible shift in the dose-response curve. Doxycycline had no effect on the sensitivity of Ba/F3p210Ctrl cells to cytarabine. However, Ba/F3p210Ctrl cells were less sensitive to cytarabine than were uninduced Ba/F3p210ΔSTAT5 cells. This may represent clonal variation, or it could reflect low-level expression of ΔSTAT5 due to leakiness of the inducible vector. Similar results were obtained when Ba/F3p210ΔSTAT5 cells were incubated with various concentrations of hydroxyurea for 5 hours. The percentage of apoptotic cells was higher after ΔSTAT5 induction than in cells not subjected to induction (Figure 8B). Viability in response to hydroxyurea did not change when Ba/F3p210Ctrl cells were incubated with doxycycline. These results show that STAT5 activation contributes to viability in Ba/F3p210 and that withdrawal of STAT5 activity synergizes with the cytotoxic effects of cytarabine or hydroxyurea.
Effects of ▵STAT5 expression and coincubation with cytarabine and hydroxyurea on viability of Ba/F3p210 cells.
(A) The effects of ΔSTAT5 expression on viability were tested after cells were incubated with various concentrations of cytarabine (1000-31.6 nmol/L) for 36 hours. When coincubated with doxycycline (1 μg/mL) (▪), Ba/F3p210ΔSTAT5 cells were more susceptible to killing induced by cytarabine than were cells maintained in the absence of doxycycline (•). No significant effect on viability was observed when Ba/F3p210Ctrl cells were coincubated with doxycycline and cytarabine. (B) Ba/F3p210ΔSTAT5 cells were coincubated in either the presence (▪) or absence (•) of doxycycline (1 μg/mL) with various doses of hydroxyurea for 5 hours. After 36 hours, they were analyzed for viability. Coincubation with doxycycline enhanced killing induced by various doses of hydroxyurea. No effect of doxycycline on viability was observed when Ba/F3p210Ctrl cells were coincubated with hydroxyurea.
Effects of ▵STAT5 expression and coincubation with cytarabine and hydroxyurea on viability of Ba/F3p210 cells.
(A) The effects of ΔSTAT5 expression on viability were tested after cells were incubated with various concentrations of cytarabine (1000-31.6 nmol/L) for 36 hours. When coincubated with doxycycline (1 μg/mL) (▪), Ba/F3p210ΔSTAT5 cells were more susceptible to killing induced by cytarabine than were cells maintained in the absence of doxycycline (•). No significant effect on viability was observed when Ba/F3p210Ctrl cells were coincubated with doxycycline and cytarabine. (B) Ba/F3p210ΔSTAT5 cells were coincubated in either the presence (▪) or absence (•) of doxycycline (1 μg/mL) with various doses of hydroxyurea for 5 hours. After 36 hours, they were analyzed for viability. Coincubation with doxycycline enhanced killing induced by various doses of hydroxyurea. No effect of doxycycline on viability was observed when Ba/F3p210Ctrl cells were coincubated with hydroxyurea.
Discussion
CML is a malignant disorder of hematopoietic stem cells caused by the Bcr/Abl oncogene. The disease is characterized by an abnormal expansion of hematopoietic progenitor cells and accumulation of mature myeloid cells in the blood, spleen, and bone marrow. Bcr/Abl has been shown to constitutively activate a number of different signaling pathways that under normal circumstances are tightly regulated by such cytokines as IL-3 or GM-CSF. The result is enhanced proliferation, prolonged viability, altered adhesion, and increased mobility.
It was previously shown that STAT5 is constitutively phosphorylated in p210Bcr/Abl-transformed cells and binds to DNA in gel-shift assays.7 Our current study extends these observations by showing that Bcr/Abl was associated with increased STAT5-dependent gene transcription with use of a STAT5 reporter gene assay and constitutive expression of a STAT5-regulated gene, CIS. These activities were due directly to Bcr/Abl, since they could be inhibited by treatment of cells with the Abl tyrosine kinase inhibitor STI571. Furthermore, we found that STAT5 activity is higher in Ba/F3 cells transformed with p210Bcr/Abl than in untransformed Ba/F3 cells, even when the latter cells were maintained in the presence of IL-3.
A tetracycline-inducible promoter was used to regulate expression of a truncated form of STAT5B in Ba/F3p210 cells. This mutant was truncated at aa 683 and lacked the transcriptional activation domain and the major tyrosine phosphorylation site of STAT5B at aa 699. ΔSTAT5 functioned as a dominant negative inhibitor of wild-type STAT5 function, since it inhibited GAS-luciferase activity and also reduced expression of CIS, a STAT5-regulated gene. Induction of ΔSTAT5 resulted in a slower growth rate but did not induce cell-cycle arrest. This finding suggested that ΔSTAT5 might reduce viability, and when viability was analyzed by the sensitive technique of annexin V staining, expression of ΔSTAT5 resulted in a reduction in viability sufficient to account for the slower growth rate. Moreover, induction of ΔSTAT5 activity was associated with increased sensitivity to the apoptotic effects of hydroxyurea and cytarabine, 2 chemotherapeutic drugs commonly used to treat CML. A reduction in viability with ΔSTAT5 expression in Bcr/Abl-transformed 32Dcl3 cells was observed by Nieborowska-Skorska et al.31 However, in contrast to what we observed in Ba/F3p210 cells, ΔSTAT5 induction in the 32Dcl3p210 cells in their study led to inhibition of cell-cycle progression. The different results in the 2 studies could possibly be attributed to phenotypic differences between the cell lines used.
The Bcr/Abl oncogene exerts several biologic effects on hematopoietic cells that are partly dependent on the model system being studied. In cell-line models and in vivo studies in mice, Bcr/Abl is mitogenic, induces factor independence,22 inhibits apoptosis,32 and alters integrin-mediated adhesion and motility.33 In primary CML cells, the major defects associated with Bcr/Abl are aberrant regulation of adhesion33 and possibly enhanced motility34 and viability,32 although not all investigators agree on the latter.35,36 Studies have suggested, however, that very immature primary myeloid cells from patients in the stable phase of CML are more likely to show enhanced viability and growth-factor independence.37 Despite the identification of many different signaling pathways activated by Bcr/Abl, it has been difficult to link any specific signaling event to a specific biologic effect. Thus, our results showing a link between constitutive activation of STAT5 and enhanced viability of Bcr/Abl-transformed cells are especially interesting.
Enhanced viability of myeloid lineage cells would be expected to result in an accumulation of cells with relatively normal function, in accordance with the known clinical phenotype of CML. In fact, available data suggest that Bcr/Abl activates several biochemical pathways that enhance viability. For example, Bcr/Abl is known to activate p21ras through binding of GRB2/SOS to tyrosine 177 of Bcr3 and probably also through SHP2, which is tyrosine phosphorylated by Bcr/Abl.38 Ras has been linked to viability signaling in a number of different cell types.39,40 Also, Bcr/Abl is known to activate PI3K, which initiates a pathway that enhances viability, possibly through activation of Akt and subsequent phosphorylation of Bad.5 Finally, Bcr/Abl induces expression of the antiapoptotic mitochondrial protein, BclX.41Because the BclX promotor has STAT5 binding sites24 and we and others28 have shown that a constitutive active mutant of STAT5 can induce BclX, we wondered whether Bcr/Abl induction of STAT5 was required for increased BclX expression. Although we found that increased CIS expression was dependent on STAT5, expression of BclXwas not.
These results suggest that the decreased viability induced by ΔSTAT5 is not due to decreased levels of BclX and that the increased expression of BclX induced by Bcr/Abl is not mediated only by STAT5. Overall, the finding of multiple signaling pathways to viability suggests that this is likely to be an important aspect of Bcr/Abl function and that inhibition of any single pathway is unlikely to eliminate the viability effect of this oncogene, as we showed here. This hypothesis can be tested more formally when mice with targeted disruption of genes involved in viability signaling pathways, including STAT5, are tested for sensitivity to Bcr/Abl transformation. Our data suggest that the defect will be small unless hematopoietic cells defective in multiple viability signaling pathways are used.
In normal hematopoiesis, the role of STAT5 remains unclear, since adult mice in which both alleles of STAT5A and STAT5B are inactivated by gene targeting have largely normal blood counts.17 However, STAT5 activation still seems to be required to support immature hematopoiesis, since the STAT5A and STAT5B double-knockout mice had a reduction in myeloid progenitor numbers in the bone marrow. Furthermore, a study has suggested that STAT5 is required for normal fetal erythropoiesis.16 Even if STAT5 turns out not to have a major role in postnatal hematopoiesis, constitutive activation of STAT5, as described here for CML cells, may confer an important phenotype. This idea is supported by a previous study showing that point mutations of the STAT5 molecule leading to a constitutively active form of STAT5 can support growth of Ba/F3 cells in the absence of murine IL-3.42 An analogous situation is that with IL-3 or GM-CSF: gene-targeting studies indicated that neither cytokine is required for normal hematopoiesis,43 but when the cytokines are expressed constitutively in transgenic mice, both cause a myeloproliferative disorder.44-46
The mechanism whereby ΔSTAT5 functions as a dominant negative inhibitor of STAT5 function in Bcr/Abl-transformed cells is of interest. Mui and colleagues18 showed that ΔSTAT5 inhibits tyrosine phosphorylation of wild-type STAT5, and our current findings confirm those results. The mechanism of reduced tyrosine phosphorylation is unclear, but it is possible that ΔSTAT5 binds to the kinase (Bcr/Abl may or may not be the kinase in this case) and then fails to disengage because it lacks the major tyrosine phosphorylation site and is therefore not phosphorylated. ΔSTAT5 would thereby block binding and subsequent phosphorylation of wild-type STAT5. We also found here that a small amount of ΔSTAT5 can coprecipitate with STAT5, suggesting that heterodimers are formed either in vivo or in vitro. Because the transcriptional activation domain of ΔSTAT5 has been deleted, the resulting heterodimer would be expected to have reduced ability to transactivate STAT5-dependent genes. The formation of heterodimers presumably depends on the binding of the SH2 domain of ΔSTAT5 to phosphotyrosine SH2-binding sites on wild-type STAT5.
Overall, our data suggest a model in which STAT5 activation by Bcr/Abl helps maintain viability in hemopoietic cells, thereby contributing to myeloproliferative disease. It will be of interest to determine the exact mechanisms involved, particularly the role ofBclX and other viability genes that may be regulated by STAT5, and to use dominant negative mutants of STAT5 in the transformation of primary fetal and adult primary cells by activated Abl oncogenes. Such studies should complement studies in STAT5 knockout mice and provide a better understanding of the role of this pathway in CML. Finally, it will be important to determine the contribution of the STAT5 pathway relative to that of other viability signaling pathways, including p21ras and PI3K/Akt.
Supported by grants J1458-MED and J1707-MED from the Austrian Fonds zur Förderung der wissenschaftlichen Forschung inÖsterreich (C.S.), fellowship grant FIJC-95/INT from the José Carreras International Leukemia Foundation (M.S.), the Association pour la Recherche sur le Cancer (F.G.), and grants CA36167, CA66996, and DK50654 from the National Institutes of Health (J.D.G.).
Reprints:James D. Griffin, Dana Farber Cancer Institute, Department of Adult Oncology, 44 Binney Street, Boston, MA 02115; email: james_griffin@dfci.harvard.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


![Fig. 2. Reduction of phosphorylation of endogenous STAT5 by ▵STAT5 expression. / Lysates from Ba/F3p210ΔSTAT5 cells (maintained in either the presence or absence doxycycline [1 μg/mL] for 24 hours) were incubated with anti-STAT5 antibody C17 and protein G beads for 3 hours and then subjected to 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting. Probing with an anti-STAT5 antibody raised against the SH2 and SH3 domains of STAT5 revealed equal amounts of STAT5A and STAT5B (middle panel). When probed with antiphosphotyrosine antibody 4G10, STAT5 was found to be constitutively phosphorylated in both Ba/F3p210Ctrl cells and Ba/F3p210ΔSTAT5 cells (upper panel). Induction of ΔSTAT5 for 24 hours was associated with a reduction of tyrosine phosphorylation of STAT5. A longer exposure of the blot revealed that ΔSTAT5 coprecipitated with endogenous STAT5 in Ba/F3p210ΔSTAT5 cells (lower panel). Neither endogenous STAT5 nor ΔSTAT5 coprecipitated when lysates were incubated with normal rabbit serum and beads.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/6/10.1182_blood.v95.6.2118/6/m_bloo00605002w.jpeg?Expires=1765895150&Signature=n1CqQn1T99ZvRTI-va~4hSz8yW4ieZxOyGhBo2cxHFnMj8a0vKRh3vjIqPaCAy0c4uxi3V9ql8HinfJ9kUUVv5FE8zlTF2GZ34i5nYGi9ZVQ9RcX4tsYxhzLHg6RAZX1Ao6Px2KA6~rxBh05ILNio-LF7lEx4ppPe-OPYIoHK2JiyU6i8MCSqTJfg6bwCzeKMboma3LVXzv8Vm~muRV4fDaMXYoz4RRH3AaEgGu4K-Ivjxv0Kw4a~iIdNP8-9~rqv0oY-itwYWtp1WGPDRbB~WThY2gSqdCzTJYNykSyWbMllVOq603gb9t3Zw2wT2w7xsMnCEv5iAE21QrWmq~t8Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Differential regulation of STAT5-induced genes,CIS and BclX, in Bcr/Abl transformed cells. / (A) Inhibition of CIS protein expression by ΔSTAT5 expression. Lysates from Ba/F3p210ΔSTAT5 cells (after treatment with or without doxycycline [1 μg/mL] for 24 hours) were incubated with a CIS antibody and protein G beads. Immunoprecipitates were then subjected to SDS-PAGE (9%). Probing with anti-CIS antibody revealed constitutive expression of CIS protein (37 kd) in Bcr/Abl-transformed cells, and expression of ΔSTAT5 inhibited CIS protein expression. Addition of doxycycline had no effect on CIS protein expression in Ba/F3p210Ctrl cells. A larger, presumably ubiquitinated form of CIS protein (∼ 45 kd) was also expressed in Ba/F3p210 cells. (B) Lack of effect on BclX protein expression of induction of ΔSTAT5. Lysates from Ba/F3 and Ba/F3p210 cells (maintained in the presence of WEHI-CM) were subjected to SDS-PAGE. Ba/F3p210 cells expressed more BclXLthan untransformed Ba/F3 cells (upper left-hand panel). Enhanced BclXL expression could be attributed directly to Bcr/Abl, since incubation with Bcr/Abl kinase inhibitor STI571 (1 μmol/L for 24 hours) reversed the enhanced BclXL expression in Ba/F3p210 cells. However, induction of ΔSTAT5 with doxycycline (1 μg/mL for 3 days) had no effect on BclXL expression in Ba/F3p210ΔSTAT5 cells (upper right-hand panel). Induction of ΔSTAT5 in Ba/F3p210ΔSTAT5 cells is shown as a control (lower panel).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/6/10.1182_blood.v95.6.2118/6/m_bloo00605005aw.jpeg?Expires=1765895150&Signature=Geh2DEIBz67ma97mVKsVdBEhVV~HQJSW12vy3Tik7kGZk39OOD5~iMBOKqR417H6gAo-Ayz-LBjGc-3hGm5RNDUwmZDiL9cfMr0cMIB4Gc8xpthS96K6wliDHm1sMICIva2EE7k0oBefEQ1No~fc3xOOdYk6X9Jkydwi~65YNsFT9CIQIrVrlH9MKmAR0~WQQyOATlUSz-eg2IiCeslCT74BJGsf4ET7grI~e-3QrX0rmUEHgYzMcL1Y1vlsjvNXNSvbTFXtZDjWqLY9AEA0GrsEZChh3zzWcLKyFCoGCk52SmcLSLrp1xtkiaybRYPYVrJ9Hlm6wHyjL-iyXEJ78Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Differential regulation of STAT5-induced genes,CIS and BclX, in Bcr/Abl transformed cells. / (A) Inhibition of CIS protein expression by ΔSTAT5 expression. Lysates from Ba/F3p210ΔSTAT5 cells (after treatment with or without doxycycline [1 μg/mL] for 24 hours) were incubated with a CIS antibody and protein G beads. Immunoprecipitates were then subjected to SDS-PAGE (9%). Probing with anti-CIS antibody revealed constitutive expression of CIS protein (37 kd) in Bcr/Abl-transformed cells, and expression of ΔSTAT5 inhibited CIS protein expression. Addition of doxycycline had no effect on CIS protein expression in Ba/F3p210Ctrl cells. A larger, presumably ubiquitinated form of CIS protein (∼ 45 kd) was also expressed in Ba/F3p210 cells. (B) Lack of effect on BclX protein expression of induction of ΔSTAT5. Lysates from Ba/F3 and Ba/F3p210 cells (maintained in the presence of WEHI-CM) were subjected to SDS-PAGE. Ba/F3p210 cells expressed more BclXLthan untransformed Ba/F3 cells (upper left-hand panel). Enhanced BclXL expression could be attributed directly to Bcr/Abl, since incubation with Bcr/Abl kinase inhibitor STI571 (1 μmol/L for 24 hours) reversed the enhanced BclXL expression in Ba/F3p210 cells. However, induction of ΔSTAT5 with doxycycline (1 μg/mL for 3 days) had no effect on BclXL expression in Ba/F3p210ΔSTAT5 cells (upper right-hand panel). Induction of ΔSTAT5 in Ba/F3p210ΔSTAT5 cells is shown as a control (lower panel).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/6/10.1182_blood.v95.6.2118/6/m_bloo00605005bw.jpeg?Expires=1765895150&Signature=BKZTzaCjfyJXo~coACDEqJAKc3Nn-sQT32hmGZwh2BvVaMcYErcfXH57tCBtAOpa2wRD93TSZfrPnW2aNRUVvbxtKe1x3jaN7-7qxLu0k6IspRWchgaspIl6h9Ck~EmvcG4AJnc6iBgFx6LbJ4jr~nr3lZ5Sc27-RQwEASAMwtm4cpsS~OhdasCI~HVWUb5YCDIS3jQNNuOHC8KaJtfozLl1a8fACiw-xKHGn4O9Nhcr~JFjqfe7m56n0YI10hagUEMgfb0MXBbsBRy8fojQUzXS1DKcMUgnCERbA8J4HZ~pAjI8bsJJxJ8EzlLUaRLzfGiaFc6QEdwYlcFZr~nYLQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 2. Reduction of phosphorylation of endogenous STAT5 by ▵STAT5 expression. / Lysates from Ba/F3p210ΔSTAT5 cells (maintained in either the presence or absence doxycycline [1 μg/mL] for 24 hours) were incubated with anti-STAT5 antibody C17 and protein G beads for 3 hours and then subjected to 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting. Probing with an anti-STAT5 antibody raised against the SH2 and SH3 domains of STAT5 revealed equal amounts of STAT5A and STAT5B (middle panel). When probed with antiphosphotyrosine antibody 4G10, STAT5 was found to be constitutively phosphorylated in both Ba/F3p210Ctrl cells and Ba/F3p210ΔSTAT5 cells (upper panel). Induction of ΔSTAT5 for 24 hours was associated with a reduction of tyrosine phosphorylation of STAT5. A longer exposure of the blot revealed that ΔSTAT5 coprecipitated with endogenous STAT5 in Ba/F3p210ΔSTAT5 cells (lower panel). Neither endogenous STAT5 nor ΔSTAT5 coprecipitated when lysates were incubated with normal rabbit serum and beads.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/6/10.1182_blood.v95.6.2118/6/m_bloo00605002w.jpeg?Expires=1765945368&Signature=y2RIz0QTynXxxCRUcG6SfnJCmXkk8iVxI-0ODZjUXenPri~t5U4IdT8n-Sac4cgzHkZLdCKmQeME9cfAyai625zr2QEApEhxmHYm5cnrCEDF17dkGV822zSxtseW0rPCoRkH1-hHBsRZo0EFwUeIQYpyKzj86pyIL2SHlHwCq1W9Ps~c302OWMbR5woGRfR9VkxuEjI6MZHAkV4RNSbVl55WA8OAm2myD3PKgSFXx3Dj4SS7hO7PTCo2Pb1jhyd25wtN8~DkNApSdatNNQnI1hWWTMmU0mc1-0Sk290CRfwYe4gxzsnlnH2YpX1EiX8Bvts1GJdlacvlFl0MonVbhw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Differential regulation of STAT5-induced genes,CIS and BclX, in Bcr/Abl transformed cells. / (A) Inhibition of CIS protein expression by ΔSTAT5 expression. Lysates from Ba/F3p210ΔSTAT5 cells (after treatment with or without doxycycline [1 μg/mL] for 24 hours) were incubated with a CIS antibody and protein G beads. Immunoprecipitates were then subjected to SDS-PAGE (9%). Probing with anti-CIS antibody revealed constitutive expression of CIS protein (37 kd) in Bcr/Abl-transformed cells, and expression of ΔSTAT5 inhibited CIS protein expression. Addition of doxycycline had no effect on CIS protein expression in Ba/F3p210Ctrl cells. A larger, presumably ubiquitinated form of CIS protein (∼ 45 kd) was also expressed in Ba/F3p210 cells. (B) Lack of effect on BclX protein expression of induction of ΔSTAT5. Lysates from Ba/F3 and Ba/F3p210 cells (maintained in the presence of WEHI-CM) were subjected to SDS-PAGE. Ba/F3p210 cells expressed more BclXLthan untransformed Ba/F3 cells (upper left-hand panel). Enhanced BclXL expression could be attributed directly to Bcr/Abl, since incubation with Bcr/Abl kinase inhibitor STI571 (1 μmol/L for 24 hours) reversed the enhanced BclXL expression in Ba/F3p210 cells. However, induction of ΔSTAT5 with doxycycline (1 μg/mL for 3 days) had no effect on BclXL expression in Ba/F3p210ΔSTAT5 cells (upper right-hand panel). Induction of ΔSTAT5 in Ba/F3p210ΔSTAT5 cells is shown as a control (lower panel).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/6/10.1182_blood.v95.6.2118/6/m_bloo00605005aw.jpeg?Expires=1765945368&Signature=s0zlzvFI4geUIBCcm2xUZPYp4NtcO0JX82-VVoVkiRv~NxasUEpaaNv2rVfcdsl06IV0PQgbF--anIKh75RogLjtA1M~js4xXVvJMhubL1O7RWZP7XMOVj9TnZ~Dmb2k9J305hH78bA0kltBxnz5L2pxfd41eHGifIEVpYDaUugf4CYIa~uXPvB~y01LlNjAOpQFN8HWlJx8AjK-Odk2JJPBL1p48lmlorc3Lb6NxKnXg8EzOotMosQ94yofgQaxsBJtJlFXIs69~UfRyY8SJnLEIqzk-Fm2djAtLXntE09tsGHBmqgJY4ixkGF5KqK9eX2Y-9yOp8ZeRv0gaGBPcw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Differential regulation of STAT5-induced genes,CIS and BclX, in Bcr/Abl transformed cells. / (A) Inhibition of CIS protein expression by ΔSTAT5 expression. Lysates from Ba/F3p210ΔSTAT5 cells (after treatment with or without doxycycline [1 μg/mL] for 24 hours) were incubated with a CIS antibody and protein G beads. Immunoprecipitates were then subjected to SDS-PAGE (9%). Probing with anti-CIS antibody revealed constitutive expression of CIS protein (37 kd) in Bcr/Abl-transformed cells, and expression of ΔSTAT5 inhibited CIS protein expression. Addition of doxycycline had no effect on CIS protein expression in Ba/F3p210Ctrl cells. A larger, presumably ubiquitinated form of CIS protein (∼ 45 kd) was also expressed in Ba/F3p210 cells. (B) Lack of effect on BclX protein expression of induction of ΔSTAT5. Lysates from Ba/F3 and Ba/F3p210 cells (maintained in the presence of WEHI-CM) were subjected to SDS-PAGE. Ba/F3p210 cells expressed more BclXLthan untransformed Ba/F3 cells (upper left-hand panel). Enhanced BclXL expression could be attributed directly to Bcr/Abl, since incubation with Bcr/Abl kinase inhibitor STI571 (1 μmol/L for 24 hours) reversed the enhanced BclXL expression in Ba/F3p210 cells. However, induction of ΔSTAT5 with doxycycline (1 μg/mL for 3 days) had no effect on BclXL expression in Ba/F3p210ΔSTAT5 cells (upper right-hand panel). Induction of ΔSTAT5 in Ba/F3p210ΔSTAT5 cells is shown as a control (lower panel).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/6/10.1182_blood.v95.6.2118/6/m_bloo00605005bw.jpeg?Expires=1765945368&Signature=Q4vIG3842JuGigkv4kFAGVuFLLLTe81XNVD~Ruca0Y6qwBwD9iUNVexW2rJ2vN4xbCF-tn4NPjjtCDyCMAApHI5MvhkCLI31CkZZIHqce7VNHt47fMknjaJB7vhWlVtc9Mn~wit~rNyltFsw6fKObsz2dC3qP4F8hUdbMFDH5ZpWvSiDBsfGu~YwnlTHFwVrFM4IHvGkdNMUVl1ydFQH8Mbja7273jx~7VxHTDn027N7R95-jVkpcUammHTGiGK-~yBRQ1R-DcMc68bOoSFAkttv32jcO6unSRaHZQ4MRfZTm01SG~sgkkosME82lcAupozRMAUsHrAHv4ikaNF1rA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)