Induction of mitochondrial permeability transition (MPT) and cytosolic translocation of cytochrome C are considered essential components of the apoptotic pathway. Hence, there is the realization that mitochondrial-specific drugs could have potential for use as chemotherapeutic agents to trigger apoptosis in tumor cells. Recently, we showed that photoproducts of merocyanine 540 (pMC540) induced tumor cell apoptosis. In this study, we focused on identifying mitochondrial-specific compounds from pMC540 and studied their apoptotic potential. One purified fraction, C5, induced a drop in mitochondrial transmembrane potential and cytosolic translocation of cytochrome C in HL60 human leukemia cells. Moreover, the addition of C5 to purified rat liver mitochondria induced MPT as indicated by mitochondrial matrix swelling, which was completely inhibited by cyclosporin A, an inhibitor of the inner-membrane pore. Supernatant of C5-treated mitochondria showed a dose-dependent increase in cytochrome C, which was also inhibited in the presence of cyclosporin A, strongly indicating a direct effect on the inner-membrane pore. Despite the strong mitochondrial reactivity, C5 elicited minimal cytotoxicity (less than 25%) against HL60 leukemia and M14 melanoma cells because of inefficient caspase activation. However, prior exposure to C5 significantly enhanced the apoptotic response to etoposide or the CD95 receptor. Thus, we demonstrate that MPT induction and cytochrome C release by the novel compound C5, in the absence of effective caspase activation, is insufficient for triggering efficient apoptosis in tumor cells. However, when used in combination with known apoptosis inducers, such compounds could enhance the sensitivity of tumor cells to apoptosis.
There is convincing evidence to implicate mitochondria in the execution of the apoptotic pathway. At least 3 mitochondrial-specific events have been well defined in cells undergoing apoptosis, namely, loss of mitochondrial transmembrane potential (Δψm), induction of mitochondrial permeability transition (MPT), and cytosolic translocation of apoptogenic factors, such as cytochrome C (Cyt C), and apoptosis-inducing factor.1-10 The reduction in Δψm is thought to be mediated by the opening of the MPT pore, a dynamic multiprotein complex located at the contact site between the inner and the outer mitochondrial membranes11-17 that in some forms of apoptosis precedes the release of Cyt C from the mitochondria. Once in the cytosol, Cyt C can activate downstream events, such as processing of caspase proteases,4 and it helps to amplify the apoptotic process. Thus, it is only logical to hypothesize that if mitochondria decide the fate of the cell during apoptosis, they may constitute useful molecular targets for novel drug design. In this regard, compounds with a predilection for mitochondrial membrane structures, including the MPT pore complex, and the ability to induce cytosolic translocation of Cyt C could prove effective as anticancer agents. By targeting the effector component of the apoptotic pathway, the need for “private” signaling mechanisms could be bypassed, which could significantly improve the response of tumor cells to chemotherapy. We are interested in identifying purified fractions derived from photoactivation of merocyanine 540(pMC540) for their ability to induce mitochondrial permeability transition, to trigger Cyt C release in tumor cells, and to study the correlation between mitochondrial-specific action and antitumor activity. We have previously shown that pMC540 triggers caspase-dependent apoptosis in human leukemia and melanoma cells.18 Here we report the purification of a mitochondrial-specific fraction, named C5, from pMC540, and we demonstrate that although C5 is a potent inducer of MPT and cytosolic translocation of Cyt C in HL60 leukemia cells, it does not trigger effective apoptosis in HL60 and M14 melanoma cells. We also show that the weak antitumor activity of C5 is attributed to its inability to induce efficient caspase activation in tumor cells. Thus, we demonstrate for the first time that compounds that selectively target the mitochondria and trigger the release of Cyt C may not be as effective as thought if they are unable to induce efficient caspase activation in tumor cells.
Materials and methods
Tumor cell lines
The human promyelocytic leukemia cell line HL60 was obtained from ATCC (Rockville, MD) and maintained in culture in RPMI 1640 supplemented with 10% fetal bovine serum (FBS; Gibco-BRL, Gaithersburg, MD). M14 human melanoma cell line, a generous gift from Dr Armando Bartolazzi (Oncologia Clinica e Sperimentale, Rome, Italy), was cultured in DMEM/5% FBS. M14TF4 clone, a generous gift from Dr M.-V. Clément (Oncology Research Institute, NUMI, Singapore), was maintained in DME/5% FBS supplemented with 1 g/mL G418 (Gibco-BRL).
Purification of photo-oxidation products from pMC540
Photoactivation of MC540 was performed as described previously.18 Briefly, 500 mg MC540 at 1mg/mL in 70% aqueous ethanol was photoactivated by exposure to a bank of fluorescent lamps (GE Cool White; 40 W; Cleveland, OH) for 18 hours. After photoactivation, ethanol was removed by freeze drying, and the mixture of photo-oxidation product was analyzed by thin-layer chromatography (TLC) on aluminium sheets coated with fluorescent silica gel 60F254 (Merck, Darmstadt, Germany) by using the following solvent systems: (1) ethyl acetate/hexane 8:2 and (2) CHCl3/methanol (6:4). Ultraviolet/VIS absorption spectra were obtained using a spectrophotometer (Biospec 1601; Shimadzu, Japan). Each fraction was then subjected to mass spectrometry (MS) and to proton (H1)- and carbon (C13)-NMR (Bioscience Centre, National University of Singapore). Collected fractions were then resuspended in dimethyl sulfoxide at 100 mg/mL and stored at −80°C protected from light.
Determination of mitochondrial ▵ψm by flow cytometry
Potentially sensitive probe 3, 3′ dihexyloxacarbocyanine iodide (DiOC6), was used to measure mitochondrial Δψm as described elsewhere.11 HL60 cells (1 × 106) were exposed to 50 to 150 μg/mL C5 for 8 hours; this was followed by the loading of cells with DiOC6(40 nmol/L for 15 minutes. at 37°C). As a positive control, cells were incubated at the same time with uncoupling agent CICCP (100 μmol/L), a protonophore that disrupts the Δψm. Cells were then washed twice with 1 × phosphate-buffered saline (PBS) and immediately analyzed in an Epics Profile (Coulter, Hialeah, FL) flow cytometer with the excitation set at 488 nm. At least 10 000 events were collected per sample, and data were analyzed by the WINMDI software (University of Massachusetts, Amherst, MA). In a separate set of experiments, 50 μg purified rat liver mitochondria were exposed to 100 μg/mL C5 for 1 hour at 25°C, followed by incubation for 15 minutes at 37°C with 40 nmol/L DiOC6. After 2 gentle washes with 1 × PBS, mitochondria were analyzed by flow cytometry as above.
Detection of cytosolic C
For the determination of Cyt C, HL60 cells (30 × 106) were treated with 50 to 150 μg/mL C5 for 18 hours and cytosolic fractions were obtained. Briefly, cells were washed twice with ice-cold PBS, pH 7.4, which was followed by centrifugation at 200g for 5 minutes. The cell pellet was then resuspended in 600 μL extraction buffer containing 200 mmol/L mannitol, 68 mmol/L sucrose, 50 mmol/L PIPES-KOH, pH 7.4, 50 mmol/L KCl, 5 mmol/L EGTA, 2 mmol/L MgCl2, and 1 mmol/L dithiothreitol and protease inhibitors (Complete Cocktail; Boehringer Mannheim, Mannheim, Germany). After 30 minutes' incubation on ice, cells were homogenized with a dounce homogenizer, and the homogenate was spun at 14 000g for 15 minutes. Supernatants were removed and stored at −80°C until analysis by gel electrophoresis for Cyt C. In a separate set of experiments, M14 cells (2 × 103) were grown on coverslips, exposed to 150 μg/mL C5, fixed with methanol:acetone (1:1 vol/vol), and incubated for 2 hours at 37°C with 1:150 dilution (in 3% bovine serum albumin) of monoclonal anti-Cyt C antibody (clone 7H8.2C12; Pharmingen, San Diego, CA) as described previously.19 After 3 washes with 1 × PBS + 1% FBS, cells were exposed to a 1:20 dilution of antimouse fluorescein isothiocyanate-conjugated IgG (Pharmingen, San Diego, CA) for 1 hour, washed twice, and analyzed by confocal microscopy (NUMI Core Facility, NUS, Singapore). Cytosolic C was defined as diffuse cytoplasmic staining compared with punctate mitochondrial Cyt C staining obtained with nontreated control cells.
Preparation of rat liver mitochondria
Mitochondria were isolated from rat liver (albino rats, Wistar strain) as described elsewhere.20 Briefly, liver cells were homogenized in 10 mL buffer A (0.3 mol/L sucrose, 5 mmol/L TES, 0.2 mmol/L EGTA, pH 7.2 with KOH), and centrifuged at 2000g for 10 minutes at 4°C. The supernatant (S1) was removed and the pellet was resuspended in 10 mL buffer A and centrifuged at 2000g for10 minutes at 4°C. The supernatant obtained (S2) was then mixed with S1 and centrifuged at 8000gfor 10 minutes at 4°C. The pellet was then resuspended in 1 mL buffer A and loaded on top of a Percoll gradient (60%, 30%, 18%) prepared in buffer A and centrifuged at 8000g for 10 minutes at 4°C. Mitochondria were then separated from nonmitochondrial membranes and nonfunctional organelles, collected at the 30%/60% interface, and washed with 10 vol buffer A at 8000g for 10 minutes at 4°C to wash off the Percoll. Mitochondria were then resuspended in 2 mL buffer A and kept at 4°C with gentle stirring. All experiments with isolated mitochondria were performed within 4 hours of the preparation.
Mitochondrial swelling and release of Cyt C
Large-amplitude mitochondrial swelling was determined spectroscopically by the loss of absorbance at 540 nm as described elsewhere.15 To induce mitochondrial swelling 200 μmol/L diethyl pyrocarbonate (DEPC; Sigma, St. Louis, MO) was used as a positive control.13 Where indicated, 10 μmol/L cyclosporin A (CsA; Sigma, St. Louis, MO) was added to the mitochondria before the addition of DEPC or C5.
In a separate set of experiments, 0.5 mg mitochondria was incubated with 150 μg C5 for 1 hour at 30°C in the presence and absence of 10 μmol/L CsA, followed by centrifugation at 4000g for 5 minutes. Control samples received equal volumes of the carrier solvent. The resultant supernatants and mitochondrial pellets were analyzed by Western blot to detect Cyt C as described below.
Western blotting for Cyt C
Fifty micrograms cytosolic protein extracts or supernatants from mitochondria treated with C5 or 0.2% Triton-X treated mitochondrial pellets were subjected to 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride membranes using a Trans-blot SD semidry system (Bio-Rad Laboratories, Hercules, CA). Membranes were then blocked overnight with 5% dry milk in TBST (50 mmol/L Tris/HCl, pH 7.4, 150 mmol/L NaCl, 0.1% Tween 20). Exposure of blocked membranes to primary anti-Cyt C antibody 7H8.2C12 was accomplished at room temperature for 1 hour using an antibody dilution of 1:5000 made in TBS + 0.05% Tween 20 and 1% bovine serum albumin. After 3 washes with TBST, the membranes were exposed to 1:5000 dilution of goat antimouse IgG–horseradish peroxidase (HRP) conjugate supplied as 0.8 mg/ml (Pierce, Rockford, IL) for 1 hour and washed 3 times with TBST. Chemiluminescence was detected using the SuperSignal Substrate Western Blotting Kit (Pierce, Rockford, IL).
Determination of caspases 3, 8, 2, and 9 activity
Activation of intracellular caspases 3 and 8 was assayed by the ApoAlert Fluorescent Assay Kits (Clontech, Palo Alto, CA), whereas activity of caspases 2 and 9 was measured using AFC-conjugated substrates supplied by Bio-Rad Laboratories (Hercules, CA). HL60 cells (1 × 106 cells/mL) were exposed to C5 (50-150 μg/mL), washed twice with 1 × PBS, resuspended in 50 μL chilled cell lysis buffer (provided by the supplier), and incubated on ice for 10 minutes. Fifty microliters 2 × reaction buffer containing 10 mmol/L dithiothreitol and 6 μL fluorogenic caspase-specific substrate (DEVD-AFC for caspase 3, IETD-AFC for caspase 8, VDVAD-AFC for caspase 2, and LEHD-AFC for caspase 9) was added to each sample, and the samples were incubated at 37°C for 30 minutes. As a comparison, HL60 cells were exposed to 100 μg/mL of the potent caspase-inducing photoproduct C2, which has been described in a recent report,19 and caspase assays were performed as below. In a separate set of experiments, HL60 and M14 cells (1 × 106/mL) were treated with 40 μmol/L etoposide or 0.5 μmol/L staurosporine for 8 hours, and lysates were analyzed for caspase 3 activity in the presence or absence of 1 μmol/L caspase 3-specific inhibitor DEVD-CHO. Protease activity was determined by measuring the relative fluorescence intensity at 505 nm after excitation at 400 nm using a spectrofluorometer (Luminescence Spectrometer LS50B; Perkin Elmer, Buckinghamshire, UK). Results are shown as fold increase in activity relative to untreated control cells. Cell lysates obtained from C5- or C2-treated HL60 cells were also analyzed for cleavage of poly-adenosine diphosphate ribose polymerase (PARP) by 10% SDS-PAGE and Western blotting using a monoclonal anti-PARP antibody (clone C2-10 from Pharmingen, San Diego, CA). Detection of the 116-kd or the cleaved 85-kd bands, or both, was carried out using HRP-conjugated anti-mouse IgG as described in “Western Blotting for Cyt C.”
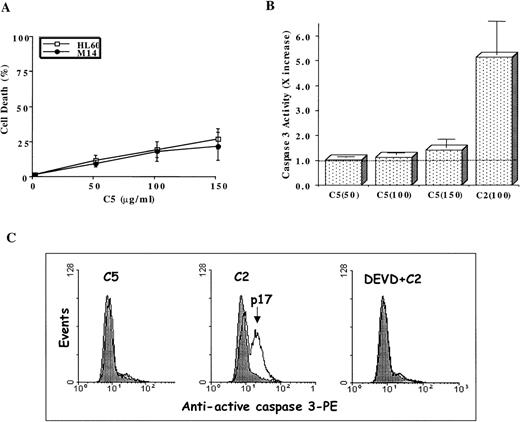
Detection of processed caspase 3
Processing of procaspase 3 was assessed by either flow cytometry using a phycoerythrin-conjugated rabbit polyclonal antiactive caspase 3 antibody (Pharmingen) or by Western blot analysis of processed caspase 3 (17 kd) using 1:750 dilution of a polyclonal rabbit anticaspase 3 antibody (Pharmingen). The HRP-conjugated goat antirabbit IgG (DAKO, Carpenteria, CA) was used at 1:2000 dilution as the secondary antibody, and chemiluminescence was detected using the SuperSignal Substrate Western Blotting Kit (Pierce) as described above. For flow cytometry, HL60 cells (1 × 106) were treated with 150 μg/mL C5 or 100 μg/mL C2 in the presence or absence of caspase 3-specific inhibitor DEVD-CHO (1 μmol/L) for 12 hours, washed with PBS, fixed, and permeabilized as described elsewhere.21 The cell pellets were then resuspended in 20 μL antiactive caspase 3 antibody for 20 minutes at 25 °C, washed twice with PBS + 1% FBS, and at least 10 000 events were analyzed by flow cytometry with the detection wavelength set at 585 nm.
Determination of antitumor activity
HL60 and M14 cells were treated with increasing concentrations of C5 (50-150 μg/mL) for 18 hours. HL60 cells were plated at 1 × 105/well in a 96-well plate, and viability was determined by the MTT assay. Ten microliters of 5 mg/mL MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) was then added to each well of cells and incubated for 4 hours at 37°C. Elution of the precipitate was performed with 100 μL dimethyl sulfoxide + 10 μL Tris-glycine buffer. Cell viability was calculated from the absorption values obtained at 570 nm using an automated ELISA reader. M14 cells were plated at 2 × 104/well in RPMI/5%FBS and allowed to attach overnight. Medium was then removed and replaced with fresh medium containing C5. After an 18-hour incubation, wells were aspirated and 50 μL 0.75% crystal violet solution containing 50% ethanol, 0.25% NaCl, and 1.75% formaldehyde was added to each well for 10 minutes. Cells were then washed with water and air dried, and the dye was eluted with PBS/1% SDS. Cell viability was measured by dye absorbance at 590 nm on an automated ELISA reader. To evaluate the ability of C5 to sensitize tumor cells to apoptosis triggered by either etoposide or the cell surface receptor CD95 (Fas/Apo1), M14 cells or M14TF4 cells expressing a chimeric receptor consisting of the extracellular portion of the tumor necrosis factor (TNF)-α receptor p55 and the entire cytoplasmic region of the CD95 receptor22 (generous gift from Dr Clément) were exposed to 40 μmol/L etoposide or 10 ng/mL rTNF-α for 8 hours in the presence or absence of 150 μg/mL C5, and caspase 3 activity was determined as described above. To ascertain whether the measured activity was caspase 3-specific, cells were exposed to 1 μmol/L DEVD-CHO before the addition of the apoptotic triggers, and lysates were analyzed for caspase 3 activity.
Cell-free system of apoptosis
To generate a cell-free system of apoptosis, cytosolic extracts from HL60 cells (50 × 106) were prepared as described above. 150 μg of the cytosolic extracts were then incubated for 6 hours at 30°C with supernatants obtained from C5 or C2-treated mitochondria (30 μL 1 mg/mL mitochondria) or 1-10 μmol/L purified Cyt C (Sigma, St. Louis, MO) in the presence of 1 mmol/L dATP (Sigma). Samples were then analyzed for caspase 3 activation by enzyme activity assay and by Western blotting of processed caspase 3 as described above.
Results and discussion
Purification of C5 from pMC540
In a previous study, we showed that tumor cells undergo caspase-mediated cell death after exposure to a mixture of photoproducts generated by the photoactivation of MC540.18Because pMC540 is a mixture of photoproducts, and for it to achieve potential as a clinical chemotherapeutic agent, the biologically active component(s) in the mixture have to be identified. The conditions used for photoactivation of MC540 were identical to those described in our in vitro studies demonstrating caspase-dependent antitumor activity of pMC540.18 After photoactivation in ethanol, the solvent was evaporated and the mixture of photo-oxidation product was analyzed by TLC on aluminum sheets coated with fluorescent silica gel. TLC solvent system 1 yielded 2 fractions that have been described elsewhere,19 and solvent system 2 yielded a product at Rf 0.25, termed C5, a highly photosensitive intermediate product with 2 absorbance peaks at 230 nm and 270 nm in methanol, which accounted for approximately 60% of the photoactivated mixture.
C5 induces a drop in mitochondrial ▵ψm of HL60 cells and triggers cytosolic translocation of Cyt C
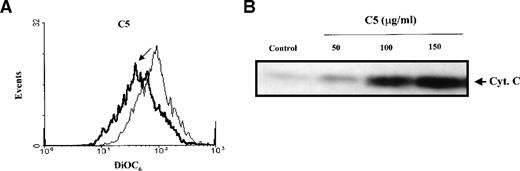
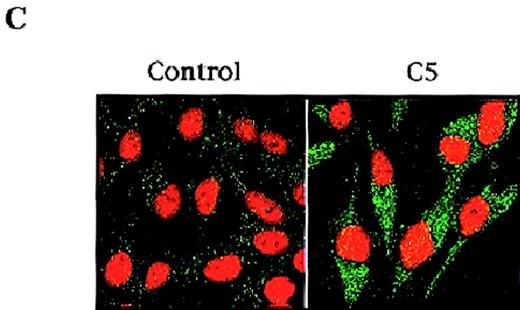
Given the crucial role that mitochondria play in the apoptotic process, we are interested in evaluating potential anticancer agents for their ability to trigger mitochondria events of apoptosis. One of the mitochondrial events associated with apoptosis is a drop in Δψm secondary to the induction of mitochondrial permeability transition.9 Therefore, we set out to identify the fraction(s) purified from pMC540, which triggered changes in Δψm of tumor cells and induced cytosolic translocation of Cyt C. Our results showed that purified fraction C5 induced a significant drop in the mean Δψm of HL60 cells as shown in Figure 1A. Recent observations suggest that mitochondrial release of pro-apoptotic factors, such as Cyt C, is dependent on a drop in Δψm secondary to opening of the inner membrane MPT pore. Having shown that C5 induced a drop in mitochondrial Δψm, we next investigated the ability of C5 to trigger the release of apoptogenic factors from mitochondria of tumor cells. One of the factors released from the mitochondria during apoptosis is Cyt C, which can then activate downstream caspases and amplify the apoptotic signal. Thus, we tested the potential of C5 to induce Cyt C translocation to the cytosol of HL60 and M14 cells. Cytosolic extracts prepared from C5-treated HL60 cells were subjected to SDS-PAGE and Western blot analysis for Cyt C. Indeed, our results showed that whereas no detectable Cyt C was found in the cytosolic extract of untreated cells, C5 triggered the translocation of Cyt C to the cytosols of HL60 cells, as shown in Figure 1B. To alleviate concerns that the process of homogenization could have resulted in artifactual “release” of Cyt C in HL60 cells, we assessed the translocation of Cyt C in intact cells after exposure to C5. To do so, M14 cells (2 × 103) were grown on coverslips, exposed to 150 μg/mL C5, fixed with methanol:acetone (1:1 vol/vol), incubated with monoclonal anti-Cyt C antibody, and analyzed by confocal microscopy as described in “Materials and methods.” Analysis of M14 cells by confocal microscopy showed a punctate pattern of staining for Cyt C in untreated control cells, suggesting mitochondrial localization. However, cells exposed to C5 exhibited bright green, diffuse cytoplasmic staining, indicating the translocation of Cyt C to the cytosol (Figure 1C). These data clearly suggested a mitochondrial-specific activity for C5 and stimulated us to focus on elucidating the mechanism of action of C5 and its potential as an antitumor agent.
(A) C5 induces a drop in ▵ψm of HL60 cells.
HL60 (1 × 106 cells/mL) were exposed to 150 μg/mL C5 for 8 hours and loaded with membrane-sensitive probe (40 nmol/L) at 37°C for 30 minutes, washed, and analyzed by flow cytometry, as described in “Materials and methods.” (B) C5 triggered cytosolic translocation of mitochondrial Cyt C in HL60 cells. HL60 (30 × 106 cells) were treated with 50-150 μg/mL C5 for 18 hours, and cytosolic fractions were subjected to SDS-PAGE electrophoresis, transferred to polyvinylidene difluoride membrane, and subjected to Western blot analysis for Cyt C, as described in “Materials and methods.” (C) M14 cells (1 × 103) were grown on coverslips and treated with 150 μg/mL C5 for 12 hours, and Cyt C localization was determined by confocal microscopy using anti-Cyt C, as described in “Materials and methods.”
(A) C5 induces a drop in ▵ψm of HL60 cells.
HL60 (1 × 106 cells/mL) were exposed to 150 μg/mL C5 for 8 hours and loaded with membrane-sensitive probe (40 nmol/L) at 37°C for 30 minutes, washed, and analyzed by flow cytometry, as described in “Materials and methods.” (B) C5 triggered cytosolic translocation of mitochondrial Cyt C in HL60 cells. HL60 (30 × 106 cells) were treated with 50-150 μg/mL C5 for 18 hours, and cytosolic fractions were subjected to SDS-PAGE electrophoresis, transferred to polyvinylidene difluoride membrane, and subjected to Western blot analysis for Cyt C, as described in “Materials and methods.” (C) M14 cells (1 × 103) were grown on coverslips and treated with 150 μg/mL C5 for 12 hours, and Cyt C localization was determined by confocal microscopy using anti-Cyt C, as described in “Materials and methods.”
C5 induces mitochondrial permeability transition and opening of CsA-sensitive MPT pore
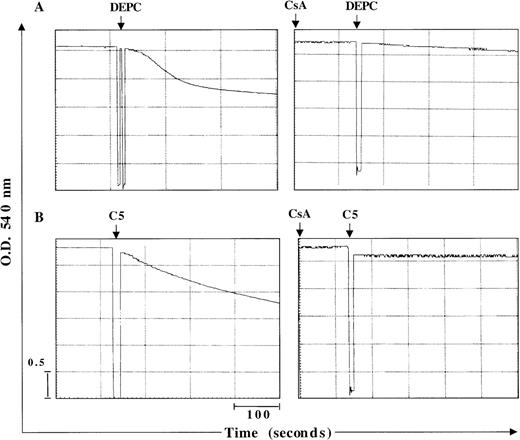
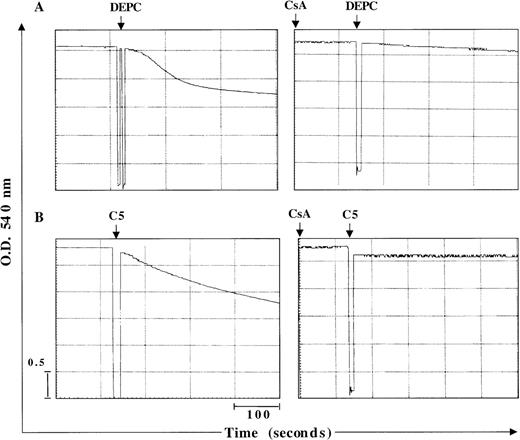
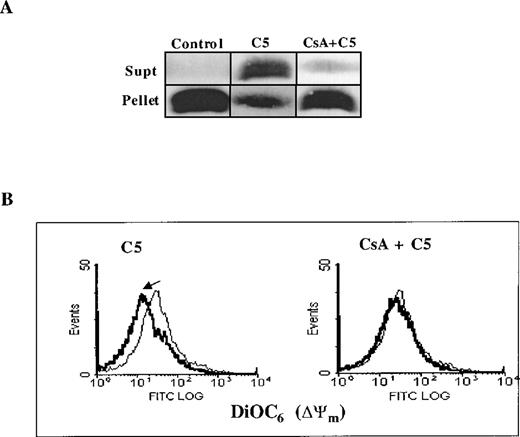
Two major hypotheses have been experimentally suggested for the mechanism of Cyt C release from the mitochondria during apoptosis. One is dependent on a drop in Δψm secondary to opening of the inner-membrane MPT pore,23 and the other contends that Cyt C release is independent of a decrease in Δψm.2 MPT pore opening results in volume dysregulation of the mitochondria from the hyperosmolality of the matrix, causing the matrix space to expand. Inhibitors of MPT pore opening, such as CsA and bongkrekic acid, appear to block apoptosis in some systems,10,24,25 implying a general role. However, some studies have provided evidence that Cyt C release can occur independent of any detectable loss in mitochondrial Δψm.2 Encouraged by our findings that C5 triggered the release of mitochondrial Cyt C in HL60 cells, we set out to investigate whether this cytosolic translocation of Cyt C resulted from a direct effect on mitochondrial membrane structures, specifically the MPT pore. To accomplish that, we purified rat liver mitochondria and asked whether C5 induced mitochondrial swelling secondary to MPT pore opening and whether Cyt C release was dependent on opening of the pore. Freshly isolated mitochondria (0.5 mg) were exposed to 150 μg C5 under conditions previously shown to be conducive for swelling induced by activators of MPT, such as Ca++ and DEPC.13 As expected, the addition of DEPC (0.2 mmol/L) to isolated mitochondria resulted in mitochondrial matrix swelling, measured by a decrease in absorbance at 540 nm using a double-beam spectrophotometer, which was completely inhibited by prior addition of CsA (10 μmol/L), an MPT pore inhibitor (Figure2A). As with DEPC, the addition of C5 to fresh mitochondria resulted in a rapid loss of absorbance, which was completely inhibited by the earlier addition of CsA (Figure 2B). Furthermore, incubation of purified mitochondria with C5 (100 μg) for 1 hour resulted in alkalinization of the matrix milieu from pH 7.5 to more than 8.5 (data not shown); matrix alkalinization has been shown to facilitate the opening of the MPT pore, whereas acidification impedes pore opening.26 Thus, a possible mechanism by which C5 triggers opening of the MPT pore could be by alkalinization of the matrix environment. To provide further impetus to these findings, we directly assessed the ability of C5 to induce Cyt C release from isolated mitochondria and to determine whether it was dependent on opening of the MPT pore and a drop in mitochondrial Δψm. Purified mitochondria (0.5 mg) was incubated with 150 μg/mL C5 in the presence or absence of MPT pore inhibitor CsA (10 μmol/L). Mitochondria were then gently pelleted, and supernatants were used for Western blot analysis of Cyt C. Our results showed that C5 triggered the release of Cyt C from purified mitochondria, which was completely inhibited by CsA (Figure 3A). Moreover, similar to our observations with tumor cells, incubation of purified mitochondria for 30 minutes with C5 induced a drop in mitochondrial Δψm, which was completely inhibited by CsA, as shown in Figure 3B. These results demonstrate that C5 directly targets the MPT pore and induces mitochondrial swelling in a CsA-inhibitable manner, supporting a mitochondrial-specific mode of action for this purified fraction.
C5 induces mitochondrial swelling by induction of the MPT pore.
Large-amplitude mitochondrial swelling was determined spectroscopically by monitoring the loss of absorbance at 540 nm, as described in “Materials and methods.” Mitochondria (0.5 mg) were treated with (A) 200 μmol/L DEPC as a positive control to induce mitochondrial swelling or (B) C5 (150 μg) in the absence or presence of MPT pore inhibitor CsA (10 μmol/L).
C5 induces mitochondrial swelling by induction of the MPT pore.
Large-amplitude mitochondrial swelling was determined spectroscopically by monitoring the loss of absorbance at 540 nm, as described in “Materials and methods.” Mitochondria (0.5 mg) were treated with (A) 200 μmol/L DEPC as a positive control to induce mitochondrial swelling or (B) C5 (150 μg) in the absence or presence of MPT pore inhibitor CsA (10 μmol/L).
C5-triggered Cyt C release and drop in mitochondrial ▵ψm is dependent on opening of the MPT pore.
(A) 0.5 mg purified rat liver mitochondria was exposed to 150 μg/mL C5 in the presence or absence of CsA (10 μmol/L) for 30 minutes at 30°C. Mitochondria were then pelleted, and supernatants were subjected to SDS-PAGE and Western blot analysis for Cyt C, as described in “Materials and methods.” (B) Mitochondria were treated with C5 in the presence or absence of CsA as above and stained with membrane potential-sensitive dye DiOC6 (40 nmol/L) at 37°C for 30 minutes, washed, and analyzed by flow cytometry for Δψm.
C5-triggered Cyt C release and drop in mitochondrial ▵ψm is dependent on opening of the MPT pore.
(A) 0.5 mg purified rat liver mitochondria was exposed to 150 μg/mL C5 in the presence or absence of CsA (10 μmol/L) for 30 minutes at 30°C. Mitochondria were then pelleted, and supernatants were subjected to SDS-PAGE and Western blot analysis for Cyt C, as described in “Materials and methods.” (B) Mitochondria were treated with C5 in the presence or absence of CsA as above and stained with membrane potential-sensitive dye DiOC6 (40 nmol/L) at 37°C for 30 minutes, washed, and analyzed by flow cytometry for Δψm.
C5 lacks antitumor activity because of inefficient caspase activation
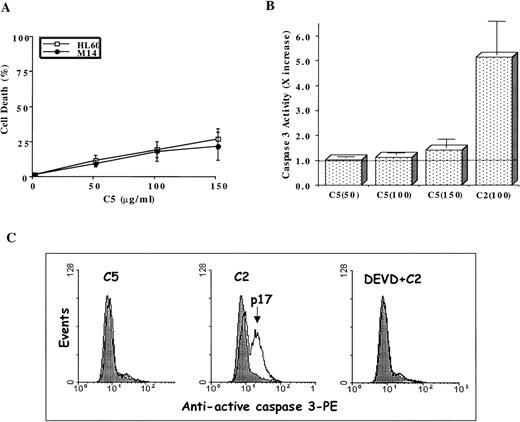
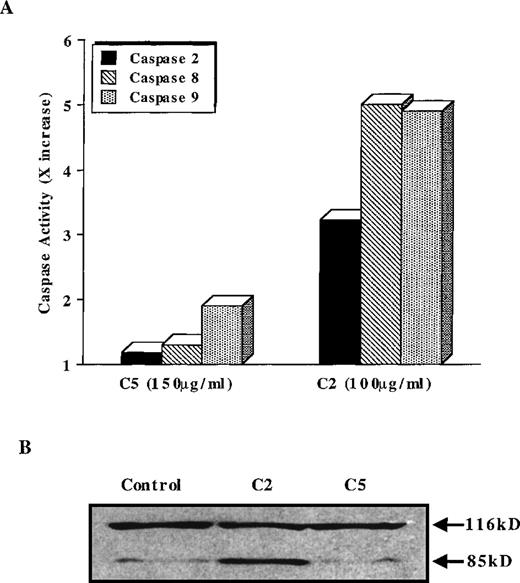
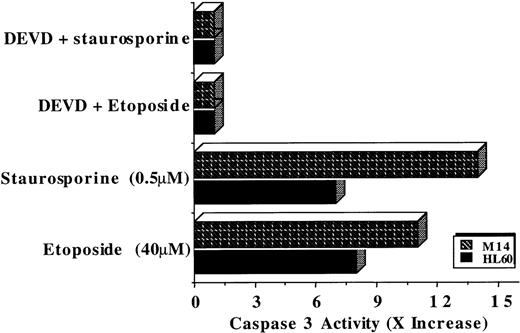
Stimulated by the findings that C5 effectively targeted the mitochondrial pore and induced translocation of Cyt C, we next assessed the antitumor potential of C5. If mitochondrial events were directly involved in triggering the execution of drug-induced apoptosis, then C5 should make an excellent antitumor agent. Hence, we investigated the ability of C5 to trigger cell death in HL60 leukemia and M14 melanoma cells. Our results showed that exposure of both cell lines to C5 did not trigger significant cell death (less than 25%) as shown in Figure4A, which did not increase significantly on longer incubation for 48 hours (data not shown). We were intrigued by the findings that despite its ability to induce mitochondrial permeability transition and cytosolic translocation of Cyt C, C5 was an inefficient inducer of cell death in both tumor cell lines. Because of the central role of caspase proteases in the execution of the apoptotic program, we argued that this low antitumor activity could be caused by the inability of C5 to trigger caspase activation efficiently. Therefore, we assayed HL60 cells after exposure to C5 (50-150 μg/mL) for caspase 3 activity, 1 of the executioner caspases extensively studied in drug-induced apoptosis. Indeed, lysates obtained from C5-treated HL60 cells showed minimal activation of caspase 3 compared to the potent caspase-inducing photoproduct C2 (Figure 4B), the antitumor activity of which has been well described in a recent communication.19 The lack of caspase 3 activity was further reinforced by the absence of the processed form (17 kd) of caspase 3 in HL60 cells treated with C5, unlike cells treated with C2 (Figure4C). Prior exposure of cells to DEVD-CHO completely inhibited the processing of caspase 3 in C2-treated HL60 cells, as shown in Figure4C. Furthermore, exposure of HL60 cells to C5 resulted in insufficient activation of other upstream caspases, such as caspase 8, 2, and 9, compared to C2 (Figure 5A). The inability of C5 to activate intracellular caspases was also evidenced by the absence of PARP cleavage in lysates of C5-treated HL60 cells, a hallmark of apoptotic cells (Figure 5B). To ensure that the absence of executioner caspase 3 activity in response to C5 was not the result of low expression of the caspase in HL60 and M14 cells, we used 2 commonly used apoptotic stimuli, etoposide and staurosporine, to trigger apoptosis in tumor cells. Both apoptotic stimuli induced efficient activation of caspase 3 in both cell lines, which were completely inhibited by prior incubation of cells with DEVD-CHO as shown in Figure6. We also ruled out the possibility of C5 as a caspase inhibitor by exposing HL60 cells to etoposide for 8 hours and then by caspase 3 activity assay in the presence of either the specific inhibitor DEVD or C5. Although DEVD completely inhibited caspase 3 activity, C5 had no effect on etoposide-induced caspase 3 activity (data not shown). These data clearly indicate that despite triggering MPT pore opening and release of Cyt C, the low antitumor activity of C5 could be attributed to an inefficient activation of the caspase cascade. Thus, compounds that can target mitochondria and at the same time induce activation of caspases (upstream or downstream) could efficiently activate the apoptotic pathway in tumor cells. These findings highlight the importance of drug-induced caspase activation in the effectiveness of antitumor agents.
Antitumor activity of C5 against HL60 and M14 cell lines.
1 × 106 cells/mL were exposed to increasing concentrations (25-150 μg/mL) C5 for 18 hours, and cell death was determined by the MTT assay (HL60) or crystal violet assay (M14), as described in “Materials and methods.” Data shown are mean ± SD of 3 independent experiments performed in triplicate. (B) Determination of caspase 3 activation by a fluorometric assay designed to detect cleavage of tetrapeptide substrate DEVD-AFC. HL60 cells (1 × 106) were treated with 50-150 μg/mL C5 for 18 hours, and lysates were analyzed for DEVDase (caspase 3) activity, as described in “Materials and methods.” Data shown are mean ± SD of 3 independent experiments and are expressed asx increase in activity over the untreated HL60 cells. C2 (100 μg/mL), a potent inducer of caspase 3 activity, was used for the sake of comparison. The dotted line represents the activity obtained in lysates from untreated HL60 cells. (C) HL60 cells were exposed to C5 (150 μg/mL) or C2 (100 μg/mL) for 12 hours in the presence or absence of DEVD (1 μmol/L), permeabilized, incubated with phycoerythrin-conjugated antiactive caspase 3 antibody, and analyzed by flow cytometry, as described in “Materials and methods.” At least 10 000 events were analyzed for caspase 3 processing.
Antitumor activity of C5 against HL60 and M14 cell lines.
1 × 106 cells/mL were exposed to increasing concentrations (25-150 μg/mL) C5 for 18 hours, and cell death was determined by the MTT assay (HL60) or crystal violet assay (M14), as described in “Materials and methods.” Data shown are mean ± SD of 3 independent experiments performed in triplicate. (B) Determination of caspase 3 activation by a fluorometric assay designed to detect cleavage of tetrapeptide substrate DEVD-AFC. HL60 cells (1 × 106) were treated with 50-150 μg/mL C5 for 18 hours, and lysates were analyzed for DEVDase (caspase 3) activity, as described in “Materials and methods.” Data shown are mean ± SD of 3 independent experiments and are expressed asx increase in activity over the untreated HL60 cells. C2 (100 μg/mL), a potent inducer of caspase 3 activity, was used for the sake of comparison. The dotted line represents the activity obtained in lysates from untreated HL60 cells. (C) HL60 cells were exposed to C5 (150 μg/mL) or C2 (100 μg/mL) for 12 hours in the presence or absence of DEVD (1 μmol/L), permeabilized, incubated with phycoerythrin-conjugated antiactive caspase 3 antibody, and analyzed by flow cytometry, as described in “Materials and methods.” At least 10 000 events were analyzed for caspase 3 processing.
C5 does not activate caspase 8, 2, or 9.
(A) HL60 cells were treated with 150 μg/mL C5 or 100 μg/mL C2 for 12 hours, and lysates were analyzed for caspase 8, 2, or 9 activation by fluorometric assays designed to detect cleavage of the AFC-conjugated specific substrate (IETD-AFC for caspase 8, VDVAD-AFC for caspase 2, and LEHD-AFC for caspase 9), as described in “Materials and methods.” Caspase activity is expressed as fold increase (x increase) over the activity obtained in untreated cells, shown here as 1 × . (B) Lysates obtained from C5- and C2-treated HL60 cells were also analyzed by SDS-PAGE and Western blotting for cleavage of PARP (85-kd fragment) using a monoclonal anti-PARP antibody.
C5 does not activate caspase 8, 2, or 9.
(A) HL60 cells were treated with 150 μg/mL C5 or 100 μg/mL C2 for 12 hours, and lysates were analyzed for caspase 8, 2, or 9 activation by fluorometric assays designed to detect cleavage of the AFC-conjugated specific substrate (IETD-AFC for caspase 8, VDVAD-AFC for caspase 2, and LEHD-AFC for caspase 9), as described in “Materials and methods.” Caspase activity is expressed as fold increase (x increase) over the activity obtained in untreated cells, shown here as 1 × . (B) Lysates obtained from C5- and C2-treated HL60 cells were also analyzed by SDS-PAGE and Western blotting for cleavage of PARP (85-kd fragment) using a monoclonal anti-PARP antibody.
Caspase 3 expression is not deficient in HL60 and M14 cells.
Caspase 3 activity was measured (as described above) in lysates prepared from 2 × 106 cells from each cell line after incubation for 8 hours with etoposide (40 μmol/L) or staurosporine (0.5 μmol/L) in the presence or absence of caspase 3-specific inhibitor DEVD-CH) (1 μmol/L). Data are shown as fold increase (x increase) in activity over the untreated cells and are representative of at least 3 independent experiments.
Caspase 3 expression is not deficient in HL60 and M14 cells.
Caspase 3 activity was measured (as described above) in lysates prepared from 2 × 106 cells from each cell line after incubation for 8 hours with etoposide (40 μmol/L) or staurosporine (0.5 μmol/L) in the presence or absence of caspase 3-specific inhibitor DEVD-CH) (1 μmol/L). Data are shown as fold increase (x increase) in activity over the untreated cells and are representative of at least 3 independent experiments.
C5-treated mitochondrial supernatants lack caspase 3 processing activity
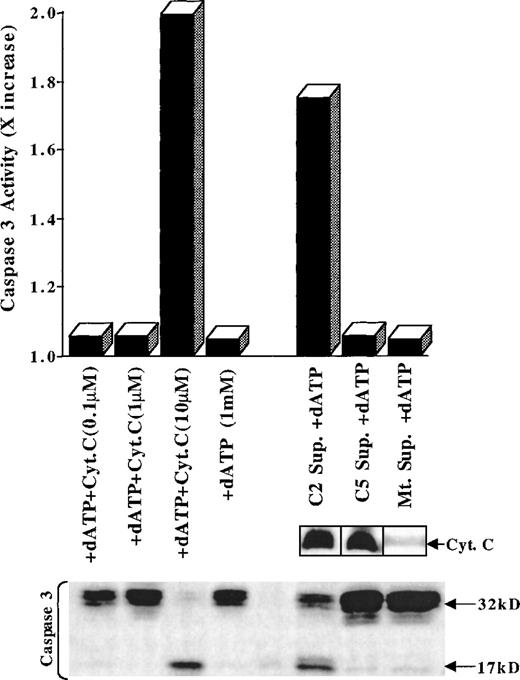
In light of our findings, compounds such as C5 that are potent inducers of mitochondrial permeability transition and Cyt C release may not prove effective if they do not sufficiently activate the caspase cascade. To substantiate our observations, we next asked whether supernatants obtained from C5-treated purified mitochondria could trigger activation and processing of caspase 3 in cytosolic extracts from HL60 cells in a cell-free system of apoptosis. Therefore, 150 μg cytosolic extracts were incubated with the supernatant from C5- or C2-treated mitochondria for 6 hours at 37°C in the presence of 1 mmol/L dATP. Moreover, to demonstrate that the cell-free system was functioning, HL60 cytosols were also incubated with increasing concentrations of purified Cyt C (0.1-10 μmol/L) under similar conditions. Interestingly, whereas the C5-treated mitochondrial supernatant was unable to activate caspase 3, the C2-treated supernatant induced processing/activation of caspase 3 in HL60 cytosols (Figure 7). Incubation of cytosols with relatively high concentrations of purified Cyt C (10 μmol/L) also triggered caspase 3 processing/activation, as shown in Figure 7, confirming the integrity of the apoptotic machinery in the cytosolic preparation. There are 2 reasons we think these observations are significant. First, as shown in our recently reported findings,19 purified photoproduct C2 also induces the release of Cyt C in the cytosol of tumor cells, albeit by mechanisms independent of the MPT pore opening. Indeed, analysis of supernatants derived from isolated mitochondria treated with C5 or C2 showed no significant difference in the amount of Cyt C released shown in Figure7. Therefore, the fact that the C2-treated mitochondrial supernatant was able to induce activation/processing of caspase 3 in HL60 cytosol, unlike C5, suggests the release of a factor “X” in addition to Cyt C from C2-treated mitochondria. Could it be the simultaneous release of Cyt C and apoptosis-inducing factor or another mitochondrial proapoptotic protein that determines the effectiveness of drug-induced caspase activation? Second, the concentration of exogenous Cyt C (10 μmol/L) required to activate caspase 3 in the cell-free system is perhaps many times higher than could be attained in vivo in the cytosol in response to a given anticancer agent. Hence, the ability of a drug to trigger cytosolic translocation of Cyt C may not independently induce effective caspase activation by itself, but it may help to amplify the response to other apoptotic stimuli.
C5-treated mitochondrial supernatant does not induce caspase 3 activation in cell-free system of apoptosis.
Cytosolic extract (150 μg) from HL60 cells were incubated for 6 hours at 30°C in the presence of 1 mmol/L dATP with supernatants derived from purified mitochondria after treatment with buffer (Mt Sup), 150 μg C5 (C5 Sup.), or 100 μg C2 (C2 Sup.). Alternatively, cytosolic extracts (150 μg) were incubated with 0.1 μmol/L to 10 μmol/L Cyt C. Caspase 3 activation was assessed in the cytosols by the fluorometric assay (x increase) and by Western blot analysis of active caspase 3 (17 kd), as described in “Materials and methods.” Supernatants from C5- and C2-treated mitochondria were also analyzed for the presence of Cyt C by Western blotting using a monoclonal anti-Cyt C antibody.
C5-treated mitochondrial supernatant does not induce caspase 3 activation in cell-free system of apoptosis.
Cytosolic extract (150 μg) from HL60 cells were incubated for 6 hours at 30°C in the presence of 1 mmol/L dATP with supernatants derived from purified mitochondria after treatment with buffer (Mt Sup), 150 μg C5 (C5 Sup.), or 100 μg C2 (C2 Sup.). Alternatively, cytosolic extracts (150 μg) were incubated with 0.1 μmol/L to 10 μmol/L Cyt C. Caspase 3 activation was assessed in the cytosols by the fluorometric assay (x increase) and by Western blot analysis of active caspase 3 (17 kd), as described in “Materials and methods.” Supernatants from C5- and C2-treated mitochondria were also analyzed for the presence of Cyt C by Western blotting using a monoclonal anti-Cyt C antibody.
Simultaneous exposure to C5 amplifies drug-induced apoptotic signaling
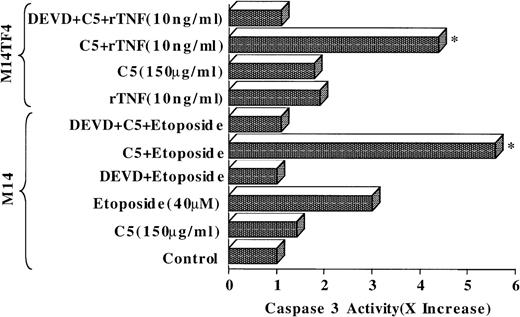
We have thus far demonstrated that in the absence of direct caspase activation, Cyt C translocation may not be able independently to activate the caspase pathway and to trigger effective apoptosis in tumor cells. However, the release of apoptogenic factors such as Cyt C from the mitochondria could certainly help to amplify the apoptotic process once the caspase(s) have been activated. Having shown the inability of C5 to activate intracellular caspases efficiently despite triggering cytosolic translocation of Cyt C in tumor cells, we questioned whether Cyt C release in C5-treated tumor cells could amplify the apoptotic signal in response to known inducers of apoptosis. Therefore, we investigated the usefulness of C5 as an adjuvant to etoposide and CD95 (Fas-Apo-1)-mediated apoptosis in tumor cells. Indeed, our results showed that prior exposure of M14 cells to 150 μg/mL C5 (2 hours) significantly enhanced caspase 3 activation in response to 8 hours of treatment with 40 μmol/L etoposide, which was completely inhibited in the presence of caspase 3 inhibitor DEVD-CHO (Figure 8). Similarly, M14TF4 cells expressing a chimeric receptor consisting of the extracellular portion of the TNF-α (p55) and the entire cytoplasmic region of the CD95 receptor showed significantly higher caspase 3 activation when incubated for 2 hours with C5 and then by 8-hour incubation with rTNF-α (10 ng/mL) than either of the drugs alone, as shown in Figure 8. These data strongly argue in support of our hypothesis that prior exposure to C5 serves to amplify significantly the apoptotic signal, perhaps because of its ability to the trigger cytosolic translocation of Cyt C. Hence, drugs similar to C5, despite having poor antitumor activity, could prove beneficial as part of a combination chemotherapy protocol to enhance tumor cell sensitivity to apoptosis by providing essential amplification factor(s) such as Cyt C.
C5 enhances tumor-cell sensitivity to apoptotic stimuli.
M14 or M14TF4 cells (1 × 106/mL) were exposed to 150 μg/mL C5 for 2 hours and then by 8-hour incubation with 40 μmol/L etoposide or 10 ng/mL rTNF-αb, respectively, in the presence or absence of DEVD (1 μmol/L). Lysates were then analyzed for caspase 3 activity, as described in “Materials and methods.” Data are shown as fold increase (x increase) in activity over the untreated control cells (1 × ).
C5 enhances tumor-cell sensitivity to apoptotic stimuli.
M14 or M14TF4 cells (1 × 106/mL) were exposed to 150 μg/mL C5 for 2 hours and then by 8-hour incubation with 40 μmol/L etoposide or 10 ng/mL rTNF-αb, respectively, in the presence or absence of DEVD (1 μmol/L). Lysates were then analyzed for caspase 3 activity, as described in “Materials and methods.” Data are shown as fold increase (x increase) in activity over the untreated control cells (1 × ).
In light of our findings, it seems more likely that an upstream caspase, such as caspase 8 activation, brought about by receptor ligation or anticancer drug treatment precedes mitochondrial events observed in cells undergoing apoptosis. Subsequently, mitochondrial-derived factors, such as Cyt C, can then amplify the processing of downstream caspases and trigger full-blown apoptosis. This hypothesis is supported by a recent observation demonstrating the presence of a caspase-activated factor that induces mitochondrial permeability transition and release of Cyt C from isolated mitochondria.27 Therefore, the ability of a given drug to activate intracellular caspases in tumor cells could be a much better measure of its antitumor potential than the mere induction of mitochondrial permeability transition. However, because mitochondrial-derived proapoptotic factors can facilitate processing of caspases, anticancer drugs similar to C5, though not effective by themselves, could be useful as adjuvants in combination chemotherapy by providing the amplification factor(s) and thereby enhancing the efficacy of drugs that effectively activate the caspase cascade.
Acknowledgment
The authors thank Dr Marie-Véronique Clément for useful discussions.
J.L.H. and M.A.S. contributed equally to this work.
Reprints:Shazib Pervaiz, Department of Physiology, National University of Singapore, 10 Kent Ridge Crescent, Singapore 119260; e-mail: phssp@leonis.nus.edu.sg.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.