We carried out bone marrow transplantation (BMT) in 50 patients with myelodysplastic syndrome (MDS) who were 55.3 to 66.2 years of age (median, 58.8 years). According to the criteria of the French-American-British (FAB) classification, 13 patients had refractory anemia (RA), 19 had RA with excess blasts (RAEB), 16 had RAEB in transformation or acute myelogenous leukemia (RAEB-T/AML), and 2 had chronic myelomonocytic leukemia (CMML). According to the recently established International Prognostic Scoring System (IPSS), available for 45 patients, 2 patients were considered low risk; 14, intermediate 1 risk; 19, intermediate 2 risk; and 10, high risk. Conditioning regimens were cyclophosphamide (CY) (120 mg/kg of body weight) plus 12-Gy fractionated total-body irradiation (FTBI) (n = 15), CY plus FTBI with lung and liver shielding (n = 4), busulfan (7 mg/kg) plus FTBI (n = 4), or busulfan (16 mg/kg) plus CY (n = 27). The busulfan-plus-CY group included 16 patients in whom busulfan was targeted to plasma levels of 600 to 900 ng/mL. In these 16 patients, steady-state levels of busulfan actually achieved were 714 to 961 ng/mL (mean ± SD, 845 ± 64 ng/mL; median, 838 ng/mL). The donors were HLA-identical siblings for 34 patients, HLA-nonidentical family members for 4, identical twins for 4, and unrelated volunteers for 6. All 46 patients surviving > 21 days had engraftment, and 22 patients (44%) are surviving 9 to 80 months after BMT. Specifically, among 13 patients with RA, 1 had relapse (cumulative incidence [CI] at 3 years, 8%) and 8 are surviving, for a Kaplan-Meier (KM) estimate of survival at 3 years of 59% (disease-free survival [DSF], 53%). Among 19 patients with RAEB, 3 had relapse (CI at 3 years, 16%), and 8 are surviving disease free (KM estimate at 3 years, 46%). Among 18 patients with RAEB-T/AML or CMML, 6 had relapse (CI at 3 years, 28%), and the KM estimate of DSF at 3 years is 33%. Relapse-free survival had an inverse correlation with cytogenetic risk classification and with the risk score according to the IPSS. Survival in all FAB categories was highest among patients enrolled in a protocol in which busulfan plasma levels were targeted to 600 to 900 ng/mL. These data indicate that BMT can be carried out successfully in patients with MDS who are older than 55 years of age.
Myelodysplastic syndrome (MDS) comprises a spectrum of diagnoses that have in common single-lineage or multilineage cytopenias and a propensity to transform into acute leukemia. Approximately half of the patients have clonal cytogenetic abnormalities that have been classified into 3 risk groups and, along with the proportion of blasts in the marrow and the number of cytopenias in peripheral blood, have been incorporated into the International Prognostic Scoring System (IPSS).1 The higher the IPSS score, the higher the risk of leukemic transformation and the shorter the life expectancy. For patients with high-risk cytogenetic abnormalities, high marrow blast counts, and multi-lineage cytopenias, the median life expectancy is several months. It is important to recognize, however, that life expectancy is determined not only by transformation of MDS into frank leukemia. In fact, 40% to 45% of patients with MDS die from infections or hemorrhagic complications without ever developing leukemia.2 Therefore, the mainstay of treatment, particularly in older patients, has generally been supportive care, although the morbidity and cost of this approach are considerable.
Transplantation of marrow or peripheral blood stem cells from a healthy donor is the only curative therapy currently available for MDS.2 However, the median age at the time of diagnosis of MDS is in the seventh decade of life,3 and studies have generally shown an increase in the frequency and severity of transplantation-related complications with increasing age.4-6 Even among younger patients, regimen-related mortality has been reported to be as high as 30% to 35%.7-10 Furthermore, the incidence of both acute and chronic graft-versus-host disease (GVHD) increases with age.11,12 These considerations are particularly important in patients with MDS who lack high-risk features and who may have a median life expectancy of 5 to 6 years with conservative management.1,2 Therefore, allogeneic transplantation has been explored only cautiously in older patients.13-16
At the Fred Hutchinson Cancer Research Center (FHCRC), we have chosen MDS as one of the diseases for which to develop transplantation regimens that can be applied successfully in patients older than 55 years. Here we describe our experience in 50 patients 55 to 66 years of age, with MDS ranging from refractory anemia (RA) to transformation into acute myelogenous leukemia (AML), who underwent transplantation with marrow from an allogeneic or syngeneic donor and in whom radiation-containing and chemotherapy-only conditioning regimens were used.
Patients and methods
Patients
Patient and transplantation characteristics are summarized in Table1. Some of these patients were included in previous reports.7,9,17 Fifty patients with MDS who were 55.3 to 66.2 years of age were referred to the FHCRC for marrow transplantation. Two patients, treated in 1978 and 1983, respectively, underwent transplantation according to leukemia protocols used at the time the procedure was done. All remaining patients underwent transplantation according to disease-specific protocols between November 22, 1989, and January 16, 1998. Fifteen patients were women; 35 were men. According to the French-American-British (FAB) classification,18,19 13 patients had RA or RA with ringed sideroblasts; 19 had RA with excess blasts (RAEB); 16 patients had advanced beyond RAEB (ie, the marrow contained > 20% blasts) to RAEB in transformation (RAEB-T) or AML; and 2 patients had chronic myelomonocytic leukemia (CMML). Cytogenetic analysis of marrow cells was successful in 46 patients. Among these, 19 had a normal karyotype and 5 had −y, 5q−, or 20q− (low risk, group 1); 15 had monosomy 7 or complex rearrangements (high risk, group 3); and 7 had other simple or double abnormalities (intermediate risk, group 2).1,20,21 The disease duration was 2 to 108 months (median, 9 months). In 42 patients, MDS had developed de novo (primary disease). In 8 patients, the disease occurred after chemotherapy, radiotherapy, or both, for non-Hodgkin lymphoma (n = 3), multiple myeloma (n = 1), bladder cancer (n = 1), multiple cancers (n = 2), or cardiac transplantation (n = 1) and was considered secondary MDS.22 23
Previous therapy
Among the 42 patients with primary MDS, pretransplantation therapy included transfusion with red blood cells, platelets, or both (n = 21); hemopoietic growth factors (n = 9); steroids or androgens (n = 12); or induction chemotherapy (n = 3), given either alone or in combination. Ten patients had not received previous therapy.
Conditioning regimens
Several conditioning regimens were used during the study interval, depending on the procedures in ongoing trials, which often included younger patients (Table 1). Patients were given a combination of total-body irradiation (TBI) and busulfan (n = 4),24 or TBI and cyclophosphamide (CY) (n = 19, with lung and liver shielding [total marrow irradiation] in 4),8,25 or a combination of busulfan and CY (n = 27).8,15,26-28 In the 16 patients who most recently underwent transplantation and received busulfan and CY, the busulfan was targeted to plasma levels of 600 to 900 ng/mL by increasing or decreasing the originally prescribed dose of 1 mg/kg of body weight for 16 doses.29 Levels were determined successively; the reported values represent the means of levels after doses 5 and 9. Phenytoin was administered to all patients who received busulfan plus CY in accordance with our standard protocol, which includes a loading dose of 15 mg/kg divided among 3 doses and given before busulfan administration and a maintenance dosage of 300 mg orally per day continued until 24 hours after the last dose of busulfan.29
Pharmacokinetic studies of busulfan
The chemical (gas chromatography and mass spectrometry) and pharmacokinetic analyses of busulfan were previously described.29-31 To provide accurate assessments of busulfan exposure during conditioning, blood samples were collected 0, 1, 2, 4, and 6 hours after any 2 of the morning doses on days 2, 3, or 4 of busulfan administration (ie, the fifth, ninth, or 13th doses) in patients who were to receive a dose of 16 mg/kg. The busulfan plasma levels numerically represent the ratio of busulfan area under the curve over the dosage interval to the time interval between doses (6 hours). The busulfan plasma values reported are the means of the values observed in each patient on those 3 days.
Donors
In 44 cases, the marrow donor was a relative (an HLA-identical sibling in 36, a relative who differed for at least 1 HLA-A, HLA-B, or HLA-DR antigen in 4, and an identical twin in 4). In 6 patients without a suitably matched related donor, a search for an HLA-matched unrelated volunteer was initiated.17 32 Although our policy was to transplant marrow from unrelated donors only in patients < 56 years of age, the search in these 6 patients had been initiated when they were < 56 years old, but they had passed this anniversary by the time they had transplantation. All 50 patients were given transplants of bone marrow; this was unmanipulated in all patients but one, to whom column-enriched CD34+ cells were given.
GVHD prophylaxis
Prophylaxis for acute GVHD consisted of methotrexate (MTX) and cyclosporine (CSP) in 38 patients and CSP alone or combined with glucocorticoids in 8.33,34 Four patients given transplants from a syngeneic donor received no GVHD prophylaxis. MTX was given intravenously at a dose of 15 mg/m2 of body surface area on day 1 and 10 mg/m2 on days 3, 6, and 11. CSP was administered intravenously at a dose of 3 mg/kg per day in 2 divided doses, starting on the day before marrow infusion. Oral CSP (12.5 mg/kg per day) was substituted for intravenous administration when tolerated. Starting on day 50, the CSP dose was tapered by 5% weekly and discontinued on day 180 whenever possible. Doses of CSP were adjusted downward if necessary (usually because of renal or hepatic dysfunction) or occasionally upward (if evidence of GVHD developed). MTX administration was adjusted downward for severe mucositis, extravascular fluid accumulation, or impaired renal function. Methylprednisolone was started on day 7 and administered at gradually reduced doses through day 72 as previously described.34Acute and chronic GVHD were diagnosed and graded by using established criteria.34 Acute GVHD was treated with prednisone, antithymocyte globulin, or monoclonal antibodies.35 Chronic GVHD was treated with prednisone alone or combined with CSP.
Regimen-related toxicity
Regimen-related toxicity was assessed by using the criteria described by Bearman et al.36 Any patient with a maximum grade of at least 2 for the bladder, renal, pulmonary, hepatic, central nervous system, mucosal, or gastrointestinal organ systems during the first 28 days after transplantation was considered to have important regimen-related toxicity.
Engraftment and rejection
Evidence of graft rejection was sought in patients who had relapse. When the donor and patient were of different sexes, in situ Y chromosome hybridization37 was performed on bone marrow and peripheral blood mononuclear cells (PBMC) stimulated with phytohemagglutinin. When the patient and donor were of the same sex, DNA from bone marrow and PBMC was amplified for several variable-number tandem repeat loci. The amplified fragments were examined to identify informative host and donor markers.38
Relapse
All patients were scheduled to have marrow samples examined morphologically and by cytogenetic and flow cytometric analyses on days 28 and 84 after transplantation and then annually or as clinically indicated. Relapse was defined as the detection of metaphases in the marrow that showed the same clonal marker or markers identified before transplantation, or the reemergence of dysplastic cells or aberrant precursors defined with use of flow cytometry.8 39
Infection
Blood samples were examined weekly for evidence of cytomegalovirus (CMV) either by culture or the presence of CMV antigenemia. Interstitial pneumonia was diagnosed with use of culture, histologic or histochemical analysis of bronchoalveolar lavage, open lung biopsy, or autopsy. Various strategies to prevent infectious diseases were employed during this study, including the prophylactic use of systemic antibiotics, including fluconazole, acyclovir, and ganciclovir. All CMV-seronegative patients received either screened (CMV-negative) or filtered blood products. Acyclovir was given for prophylaxis throughout the study period to all patients who were seropositive for herpes simplex virus. Ganciclovir was given to all CMV-seropositive recipients at engraftment or at the first documentation of antigenemia.40
Causes of death
Deaths that occurred after posttransplantation relapse were categorized as due to relapse irrespective of the proximate cause; deaths in the absence of relapse were categorized as nonrelapse mortality. Infection was considered the cause of death when a bacterial, viral, or fungal infection other than interstitial pneumonia was the proximate cause of death in patients who had not had relapse. Infections were further categorized according to whether or not they were associated with GVHD and with organ failure. Multiorgan failure was considered the cause of death if decompensation occurred in at least 2 organ systems (eg, liver and kidneys or liver and lungs) and could not be attributed to GVHD or infection alone.
Statistical analysis
Overall survival and relapse-free survival estimates were obtained by using the method of Kaplan and Meier.41 Relapse and nonrelapse mortality were summarized by using cumulative incidence estimates.42 For the endpoint of relapse, death without relapse was regarded as a competing risk, and relapse was considered a competing risk for the endpoint of nonrelapse mortality. No formal statistical comparisons are made between groups; rather, the data are presented in a descriptive fashion. The data analyzed were current through November 11, 1998.
Results
Engraftment
Four patients died before day 21 after transplantation—on days 2, 13, 14 and 16, respectively—and were considered unevaluable for engraftment. The remaining 46 patients all had sustained engraftment. A neutrophil count of 0.5 × 109/L was reached at 12 to 29 days (median, 20 days), and a platelet count of 20 × 109/L at 7 to 87 days (median, 19.5 days).
GVHD
Among 46 patients with engraftment, 43 survived beyond 28 days and were evaluated for the development of acute GVHD. Thirty-three (77%) had grades II-IV and 7 (16%) had grades III-IV GVHD (Table2). Chronic GVHD occurred in 20 of 32 patients at risk (62%).
Relapse
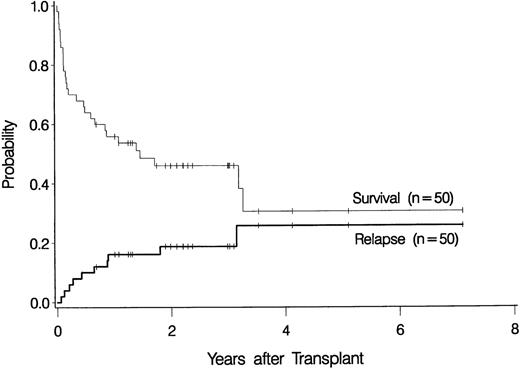
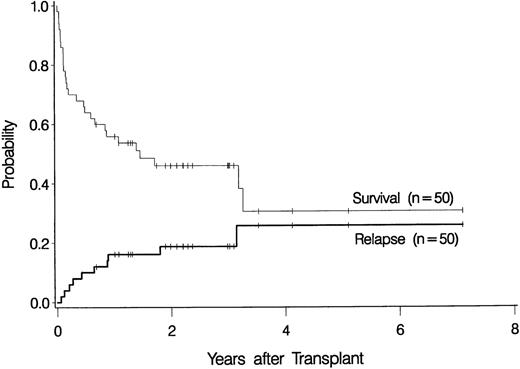
Ten patients relapsed at 23 days to 38 months after transplantation (Table 2), for a cumulative incidence of 19% at 3 years (Figure1). Seven of these recurrences were observed in the 42 patients with primary MDS (17%), and 3 were among the 8 patients with secondary MDS (38%) (P = NS).
Overall probability of survival and relapse in patients more than 55 years of age with myelodysplastic syndrome who underwent bone marrow transplantation.
Overall probability of survival and relapse in patients more than 55 years of age with myelodysplastic syndrome who underwent bone marrow transplantation.
Overall survival
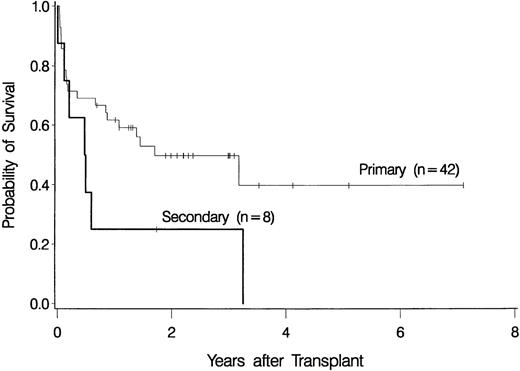
The overall probability of survival is shown in Figure 1. Currently, 22 patients (44%) are surviving 0.7 to 7.1 years (median, 2.2 years) after transplantation, for a Kaplan-Meier estimate of survival of 46% (relapse-free survival, 42%). Among 4 patients given a transplant from an HLA-nonidentical related donor, none are surviving. Survival was comparable for the other subgroups of patients: 2 of 4 patients (57.6-65 years of age) given marrow from a syngeneic donor are surviving 3.2 and 3.3 years, respectively, after transplantation, 3 of 6 patients (55.5-56.4 years of age) given marrow from an unrelated donor are surviving 1 to 3 years after transplantation, and 17 of 36 patients (55.3-66.2 years of age) given transplants from an HLA-identical sibling are surviving 0.7 to 7.1 years after transplantation. As illustrated in Figure2, the survival probability appeared to be higher in patients with de novo (primary) MDS than in those in whom MDS developed after therapy for another disease (secondary MDS). At the time of last contact, the Karnofsky performance scores of the surviving patients were 100 in 11 patients, 90 to 95 in 6 patients, and < 90 in 5 patients.
Survival among patients with de novo (primary) myelodysplastic syndrome (n = 42) and among those with secondary myelodysplastic syndrome (n = 8).
Survival among patients with de novo (primary) myelodysplastic syndrome (n = 42) and among those with secondary myelodysplastic syndrome (n = 8).
Survival according to disease variables
Results are summarized in Table 3. The probabilities of relapse-free survival at 3 years in patients with RA, RAEB, RAEB-T/AML, and CMML were 0.53, 0.46, 0.38, and 0.0, respectively. In addition to FAB classification as a prognostic risk factor, recent studies have examined the impact of the type of cytogenetic abnormalities, and a scoring system (the IPSS) has been developed that considers the effect of proportion of blasts in the marrow, the number of cytopenias, and cytogenetic abnormalities on disease course in patients who have not undergone transplantation.1 The current study in older patients undergoing transplantation suggests that those risk factors are also relevant for the posttransplantation outcome: patients with low-risk or intermediate-risk cytogenetic findings did better than those with a high-risk karyotype, and patients with a score that was low according to the IPSS fared better than those with a higher score. Specifically, patients with high-risk cytogenetic abnormalities and those with high-risk disease according to the IPSS tended to do worse than the remaining patients.
Survival according to conditioning regimen
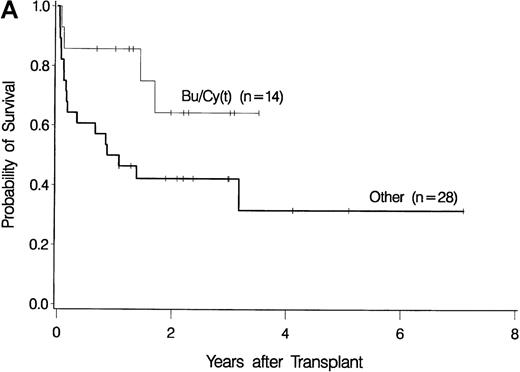
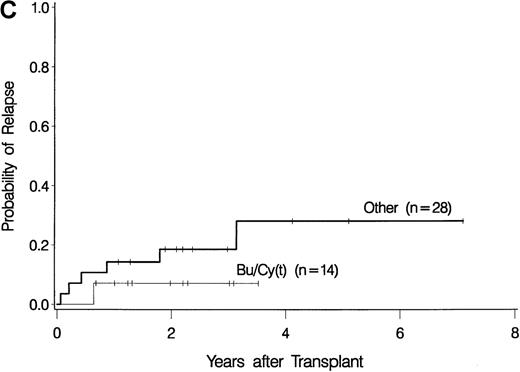
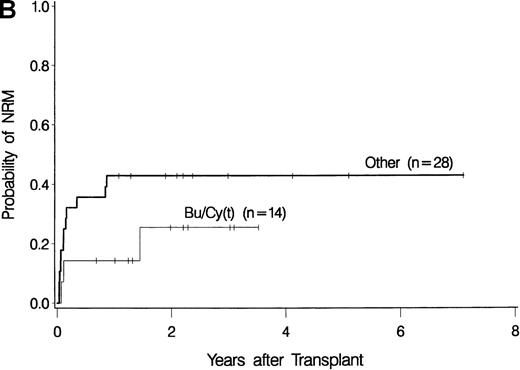
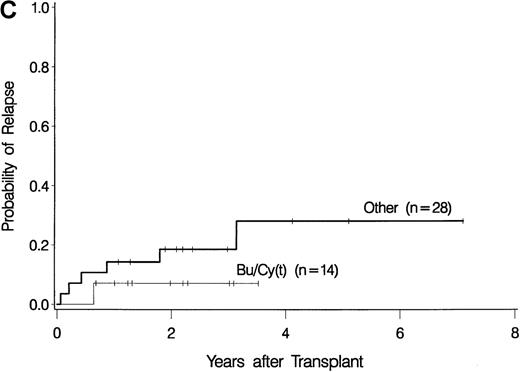
The development of transplantation conditioning regimens for patients with MDS has been driven by concerns about disease recurrence and regimen-related toxicity, particularly in older patients.7,8,26,28 Previous observations in patients with chronic myelogenous leukemia (CML) given transplants from a sibling donor suggested that “optimization” of busulfan dosage to achieve a certain plasma level (targeted busulfan) would reduce toxicity (due to excessive levels) and disease recurrence (due to low levels).29 43 Therefore, the MDS protocol was changed so that patients ≥ 55 years of age at transplantation would be conditioned with targeted busulfan. Thus, the 16 patients in this study who most recently underwent transplantation were given oral busulfan with the aim of achieving busulfan plasma levels of 600 to 900 ng/mL. As illustrated in Figure 3, patients in this group had the best survival (A) and the lowest nonrelapse mortality (B) of all patients in this study, and the incidence of relapse did not appear to be increased (C). For the “best case” scenario, that is, among 6 patients in cytogenetic-risk group 1 who were conditioned with busulfan (targeted) and CY, 5 are surviving, compared with only 1 among 6 patients in cytogenetic-risk group 3 who were conditioned with other regimens.
Results achieved with a conditioning regimen of targeted busulfan combined with cyclophosphamide compared with other regimens in patients with primary myelodysplastic syndrome.
Outcomes shown are survival (A), nonrelapse mortality (B), and relapse (C).
Results achieved with a conditioning regimen of targeted busulfan combined with cyclophosphamide compared with other regimens in patients with primary myelodysplastic syndrome.
Outcomes shown are survival (A), nonrelapse mortality (B), and relapse (C).
Causes of death
As shown in Table 4, 8 patients died with relapse and 20 died from other causes, for overall and nonrelapse mortality rates at 2 years of 55% and 39%, respectively. When the 42 patients with primary MDS were considered separately, the 2-year overall mortality and nonrelapse mortality rates were 51% and 37%, respectively. The most frequent cause of death was multiorgan failure, either alone or combined with infection, which accounted for half of all deaths in patients who did not relapse. GVHD was expected to be a problem in this older patient population and, indeed, was the primary cause of death in 5 patients. All but one of the deaths associated with infections were due to fungal (Aspergillus or Candida) organisms. Other causes of death included hemorrhage in one patient and late development of a colon carcinoma in another patient.
Discussion
Because studies in younger patients have shown that MDS can be cured by hemopoietic stem-cell transplantation,8,9,21,26,28 we investigated whether this treatment can also be applied successfully to older patients. Our results indicate that transplantation in patients 55 to 66 years of age is feasible and offers an excellent outlook for certain subgroups of patients. As previously observed in younger patients, advanced disease stage and high-cytogenetic-risk category were associated with higher relapse probability and lower survival. Outcome was better for primary than for secondary MDS, and patients given transplants from an HLA-matched donor fared better than did recipients of an HLA-nonidentical transplant. However, because of the small numbers of patients in each of these subgroups, differences did not reach statistical significance. The Kaplan-Meier estimates for relapse-free survival at 3 years for patients with primary RA, RAEB, and RAEB-T/AML were 57%, 52%, and 45%, respectively. For patients with low-risk, intermediate-risk, and high-risk cytogenetic features, the probabilities of survival were 49%, 83%, and 30%, respectively. If the IPSS criteria are used, survival estimates at 3 years were 100%, 54%, 44%, and 30% for low-risk, intermediate-1 risk, intermediate-2 risk, and high-risk groups, respectively. The IPSS was based on data from patients who did not undergo transplantation. However, recent reports suggest that the variables considered in this scoring system also affect posttransplantation outcome.21 44 The results of our study are consistent with such an assessment, although it is not a priori clear why multiple cytopenias, which are corrected by transplantation, should affect posttransplantation outcome. It is possible that problems due to pretransplantation transfusion support or infections acquired because of neutropenia affect the posttransplantation course.
As in other trials of transplantation for MDS, nonrelapse morbidity and mortality in our patients was high, and the incidence was similar for patients with good-risk disease characteristics and those with poor-risk characteristics. The high incidence was not readily explained by the older age of the patient population and was similar to that observed in younger patients.8 21 Although prolonged neutropenia, a feature of MDS that was present in some patients, is a risk factor for infectious complications, factors contributing to fatal organ failure require further study. Nevertheless, most patients who were prepared for transplantation with a regimen that combines cyclophosphamide and oral busulfan adjusted to achieve plasma levels of 600 to 900 ng/mL survived and recovered an excellent performance status, as indicated by their Karnofsky scores. The use of this conditioning regimen did not jeopardize the cure rate of the disease. In fact, only 3 of 16 patients so treated had disease recurrence and only 1 of 14 patients with primary MDS.
The plasma target range of busulfan was chosen on the basis of findings in patients with CML, in whom this approach appeared to ensure engraftment and reduce organ toxicity.29 Among patients with normal or low-risk cytogenetic features prepared with this regimen, relapse-free survival was > 80%, and even among primary MDS patients with intermediate-risk or high-risk cytogenetic features, 5 of 8 patients so treated are surviving in remission. Among patients who received transplants from an unrelated donor, 3 of 6 are surviving, a proportion that is higher than expected on the basis of recent results in patients in their 50s with CML who were conditioned with a regimen containing TBI.45 Considering the very encouraging results in patients > 55 years of age, it appears warranted to test a targeted busulfan regimen in younger patients, in whom it might allow improvements in results beyond those achieved with TBI-containing regimens. Whether results with a regimen using busulfan and cyclophosphamide can be further improved by, for example, narrowing the target range of busulfan remains to be determined. It will also be of interest to determine the impact of nonmyeloablative regimens on outcome in this patient population.
Age has generally been considered a high-risk factor for success after both allogeneic and autologous transplantation.2,6,7,14,23Several reports suggest, however, that this is not uniformly the case, although in most of the trials, any patient > 45 years of age or even > 40 years of age was considered among “older patients.”5,15,46,47 Some more recent studies and analyses involved patients > 50 or 55 years of age.13,14,46 Du and colleagues13 compared results in 59 patients > 50 years old with AML or MDS who received an allogeneic transplant with those in younger patients and observed that 1- and 2-year survival rates did not differ significantly. The authors emphasized that such success may be restricted to “selected” patients. The findings described here are in agreement with that study. Similar to those investigators, we cannot exclude a selection bias for, for example, highly motivated patients who pursued the transplantation option aggressively or for patients with a “superior” biology (survival was somewhat better in patients who had been given a diagnosis of MDS > 12 months before transplantation whereas, in other studies, shorter disease duration was generally associated with improved outcome8 44).
In conclusion, allogeneic marrow transplantation for MDS is associated with considerable clinical toxicity in patients of all ages. However, our results with hemopoietic stem-cell transplantation in patients 55 to 66 years of age are encouraging, particularly for patients with favorable-risk cytogenetic features and patients prepared for transplantation with a conditioning regimen that incorporates administration of plasma-level-targeted busulfan.
Acknowledgments
We thank Helen Crawford and Bonnie Larson for typing the manuscript and Elizabeth Soll and Deborah Monroe for assistance with data collection.
Supported in part by National Institutes of Health grants HL36444, CA15704 and CA18029; H.J.D. is also supported by a grant from the G. Rich Foundation. J.E.A. was supported by an American Cancer Society Career Development Award.
Reprints:H. Joachim Deeg, Fred Hutchinson Cancer Research Center, 1100 Fairview Avenue N, D1-100, PO Box 19024, Seattle, WA 98109-1024; e-mail: jdeeg@fhcrc.org.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.