We recently reported that administration of recombinant 39 kd receptor–associated protein (RAP) to von Willebrand factor (vWf) knockout mice induced a significant, sustained rise in endogenous murine factor VIII levels comparable to that induced by the administration of recombinant vWf.1 RAP is an antagonist2 of low density lipoprotein receptor–related protein (LRP), a ubiquitously expressed multifunctional endocytic receptor.3 In vitro studies show that factor VIII binds to LRP and that, by specifically blocking LRP, RAP prevents the binding of factor VIII to LRP.4 Our in vivo data indicated that LRP facilitates the clearance of endogenous murine FVIII when vWf is absent. The report also included preliminary data suggesting that preadministration of RAP slows elimination of infused recombinant human FVIII (rFVIII) in vWf knockout mice, but the number of animals tested was too small to allow statistically relevant analysis of the data. We therefore extended the infusion study to further investigate the effect of LRP inhibition by RAP on recovery and half-life of infused human rFVIII in vWf-deficient knockout mice.
vWf knockout mice (with undetectable vWf and FVIII levels of about 20% due to secondary FVIII deficiency5) were treated with buffer, human rFVIII (Baxter Hyland Immuno, Thousand Oaks, CA), and RAP (made as a recombinant fusion protein with glutathione S-transferase), as described in our original study.1 Briefly, 3 groups were tested: (1) a control group received 40 mL/kg buffer followed 15 minutes later by a second injection of 20 mL/kg buffer (n = 11); (2) the rFVIII group received 40 mL/kg buffer followed by 200 U/kg human rFVIII in a volume of 20 mL/kg (n = 15); (3) the RAP preadministration group received 40 mg/kg RAP in a volume of 40 mL/kg followed by 200 U/kg rFVIII in a volume of 20 mL/kg (n = 18). In vivo recovery was determined at 15 minutes as described,1 and FVIII levels were measured with an ELISA that is specific for human FVIII (Immunozym FVIII:Ag, Baxter, Vienna, Austria). Statistical comparisons were based on repeated measure analysis of variance. Because of the difficulty in drawing blood at frequent intervals from vWf knockout mice, sufficient material for analysis was not available from each animal at each data point and the number of data points was limited, which allowed only fitting of a 1-compartment model for calculation of the half-life of rFVIII.6
As expected for detection of a human protein in mice, FVIII levels were below the limit of detection in all animals before infusion of rFVIII and in the control group at all time points. Mean recovery (±SD) was only 5.8% (±3.7%) in the rFVIII group (6 males, 7 females), but 12.6% (±5.9%) in the group with preadministration of RAP (8 males, 8 females), P = .0023. There was neither an effect of sex (P > .1) nor an interaction between sex and group.
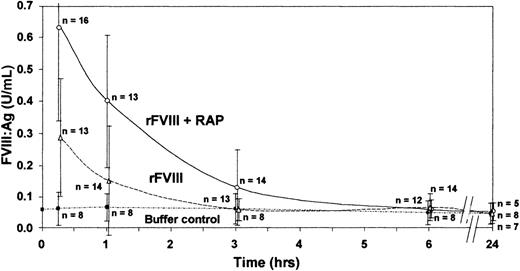
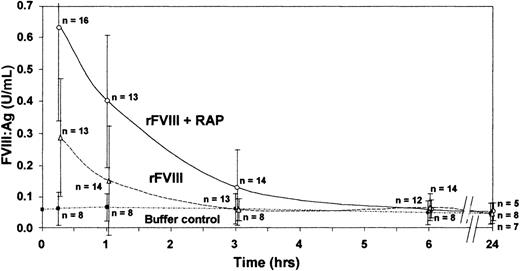
FVIII was maintained at higher levels and was detectable in plasma for a longer period of time with preadministration of RAP (6 hours) than without preadministration of RAP (3 hours) (Figure).
Inhibition of LRP by RAP improves survival of infused human recombinant FVIII in vWf knockout mice.
Animals were treated with 200 U/kg human rFVIII with and without preadministration of RAP (40 mg/kg). FVIII antigen (FVIII:Ag) was measured by an ELISA specific for human FVIII. Group means are shown ± SD.
Inhibition of LRP by RAP improves survival of infused human recombinant FVIII in vWf knockout mice.
Animals were treated with 200 U/kg human rFVIII with and without preadministration of RAP (40 mg/kg). FVIII antigen (FVIII:Ag) was measured by an ELISA specific for human FVIII. Group means are shown ± SD.
Blocking LRP by preadministration of RAP prolonged the half-life of the infused rFVIII from only 42 minutes with rFVIII alone to 67 minutes with preadministration of RAP.
Saenko et al7 reported that RAP slows the clearance of infused plasma-derived FVIII/vWf in normal mice. Our extended study now demonstrates that inhibition of LRP by a single bolus administration of RAP has a significant inhibitory effect on the clearance of infused FVIII in the absence of vWf, thus further supporting the involvement of LRP in the clearance mechanisms of FVIII.