The 32D clone 3 (32D Cl3) cell line is a widely used model for the in vitro study of hematopoietic cell proliferation, differentiation, and apoptosis. It was originally described by Valtieri et al as a murine, hematopoietic, immature myeloid, nontumorigenic, diploid cell line that is strictly dependent on exogenously supplied interleukin-3 (IL-3) for maintenance of its growth in culture and that is able to terminally differentiate in vitro into neutrophilic granulocytes by removing IL-3 and by adding granulocyte-colony stimulating factor (G-CSF) to the culture medium.1 In this cell line the neutrophilic granulocytic differentiation can be modulated to an extent that mimics very closely the differentiation pattern that occurs in the normal bone marrow in vivo. These characteristics make such a cell line particularly suitable for in vitro studies on hematopoiesis, compared with other cell lines of leukemic origin, in which the differentiation program is impaired by the molecular events leading to malignancy and thus no longer require the growth factors that control the proliferation and differentiation of normal cells in vivo. The extrapolation in vivo of results obtained in vitro with the 32D Cl3 cell line in experiments performed to study the molecular mechanisms that underlie cell proliferation, signal transduction, growth factor dependence, apoptosis, and differentiation has always been considered feasible because of the nontumorigenicity of this cell line and the presence of a normal diploid karyotype.
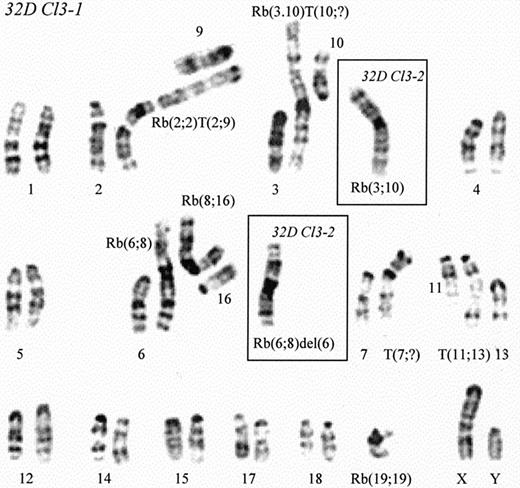
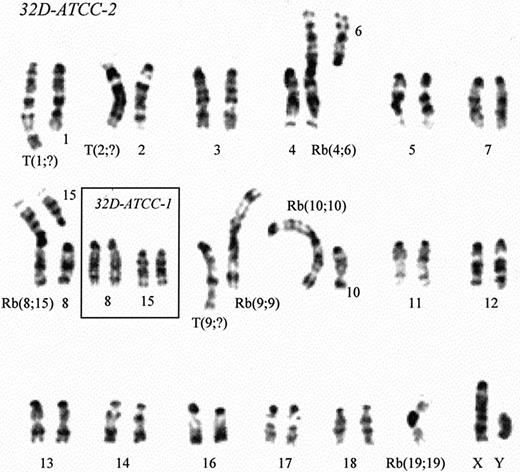
Before transfecting 32D Cl3 cells in our laboratory, we controlled their karyotype because a clone previously was described that, although indistinguishable from the original cell line regarding its absolute requirement of IL-3 and its ability to terminally differentiate along the granulocytic lineage with G-CSF, showed an abnormal karyotype [(39, XY, −2, −4, +der (2) t(2;4) (A1;A1)],2 compared with that originally described by Valtieri,1which corresponds to mouse standard karyotype: 40 acrocentric chromosomes. Our cells (from Dr G. Rovera's laboratory) have been maintained in culture in Iscove modified Dulbecco medium supplemented with 10% heat-inactivated fetal bovine serum and 10% WEHI-3B cell-conditioned medium as a source of murine IL-3. Cytogenetic characterization showed the presence of 2 cell populations with different karyotypes that we named 32D Cl3-1 and 32D Cl3-2, the former representing 70% of the total. In both karyotypes we identified 34 chromosomes: 28 telocentric and 6 chromosomes with 2 arms. The analysis of G-banded metaphaseplates of 32D Cl3-1 allowed us to identify chromosome pairs 1, 4, 5, 12, 14, 15, 17, 18, and XY corresponding to standard mouse chromosomes, while the others showed some marked alterations consisting of Robertsonian fusions: Rb(2;2)T(2;9), Rb(3;10), Rb(6;8), Rb(8;16), Rb(19;19); translocation: t(11;13); partial deletions; and duplications (Figure1). The 32D Cl3-2 karyotype (30% of metaphase plates) differs from the predominant one only for 2 small cytogenetic changes involving Rb(3;10) and Rb(6;8). On the basis of evidence of such chromosomal alterations, our 32D Cl3 cells cannot yet be considered normal hematopoietic diploid murine cells even though they are nontumorigenic in mice, as we verified, and despite their ability to differentiate into mature granulocytes in the presence of G-CSF. For this reason we have renamed our cell line 32D Cl3-A. The remarkable rearrangements involving such a high number of chromosomes in our cells and the structural chromosomal abnormalities observed by Laneuville in his clone2 led us to consider the 32D Cl3 cell line chromosomically very unstable, thus suggesting that the karyotype could presumably be modified in a different way from the original one in each laboratory. In this view we performed a cytogenetic analysis of a 32D Cl3 clone from the American Type Culture Collection (ATCC) (ATCC number CRL-11346) and of a 32D Cl3 clone with the same source as our clone (Dr Rovera) but cultured in another laboratory. ATCC 32D Cl3 metaphase analyses showed the presence of 2 equally represented cell populations (here, 32D-ATCC-1 and 32D-ATCC-2). In 32D-ATCC-1 we identified 38 chromosomes: 33 telocentric and 5 chromosomes with 2 arms. G-banding showed chromosome pairs 3, 5, 7, 8, 11, 12, 13, 14, 15, 16, 17, 18, and XY as standard mouse chromosomes, while the others displayed Rb(4;6), Rb(9;9)+9, Rb(10;10)+10, Rb(19;19), and some additions of unidentified genetic materials (Figure 2). The 32D-ATCC-2 (2n = 37 chromosomes, of which 6 have 2 arms) possess the same chromosomal characteristics with the addition of an Rb(8;15).
G-banded karyotype of our 32D Cl3 cell line (32D Cl3-A).
Shown are the features of 32D Cl3-1 (representing 70% of the metaphase plates) and 32D Cl3-2 (representing the remaining 30%).
G-banded karyotype of our 32D Cl3 cell line (32D Cl3-A).
Shown are the features of 32D Cl3-1 (representing 70% of the metaphase plates) and 32D Cl3-2 (representing the remaining 30%).
G-banded karyotype of 32D Cl3 cells from American Type Culture Collection (ATCC).
32D-ATCC-1 and 32D-ATCC-2 indicate the 2 equally represented cell populations in the cell line.
G-banded karyotype of 32D Cl3 cells from American Type Culture Collection (ATCC).
32D-ATCC-1 and 32D-ATCC-2 indicate the 2 equally represented cell populations in the cell line.
The 32D Cl3 cell line with the same source as our cells displayed the presence of at least 5 different cell populations characterized by a variety of Rb translocations, most frequently involving chromosome 6 and various additions of unidentified chromosomic materials.
In conclusion, although all of the 32D Cl3 cells we examined were strictly IL-3 dependent for growth in culture and were able to differentiate into mature granulocytes in the presence of G-CSF, they showed a highly variable karyotype characterized by several complex chromosomal rearrangements. Our findings lead us to consider the 32D Cl3 cell line chromosomically very unstable and thus induce the speculation that each laboratory may possess its own clone of 32D Cl3 genetically different from the original one and from other clones of other laboratories, even if all clones maintain at least the 2 phenotypic characteristics that represent the peculiarity of this cell line. Nevertheless, other phenotypic features not yet considered could be different in different clones, and some metabolic pathways could be altered as a result of chromosomal alterations. We wish to remind all those utilizing this cell line for scientific research of the possible consequence of chromosomal instability.
In general, in matters of cell culture, the possibility of selection in vitro of cellular clones with different genotypic characteristics should always be evaluated (even if the original karyotype is conserved).
Acknowledgment
This study was partially supported by grants from Ministero della Universitá e della Ricerca Scientifica e Tecnologica (MURST) 40% and 60%.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal