Abstract
The mechanisms that regulate hematopoietic progenitor cell (HPC) mobilization from the bone marrow to blood have not yet been defined. HPC mobilization by granulocyte colony-stimulating factor (G-CSF), cyclophosphamide (CY), or interleukin-8 but not flt-3 ligand is markedly impaired in G-CSF receptor–deficient (G-CSFR–deficient) mice. G-CSFR is expressed on mature hematopoietic cells, HPCs, and stromal cells, which suggests that G-CSFR signals in one or more of these cell types was required for mobilization by these agents. To define the cell type(s) responsible for G-CSF–dependent mobilization, a series of chimeric mice were generated using bone marrow transplantation. Mobilization studies in these chimeras demonstrated that expression of the G-CSFR on transplantable hematopoietic cells but not stromal cells is required for CY- or G-CSF–induced mobilization. Moreover, in irradiated mice reconstituted with both wild type and G-CSFR–deficient bone marrow cells, treatment with CY or G-CSF resulted in the equal mobilization of both types of HPCs. This result held true for a broad spectrum of HPCs including colony-forming cells, CD34+lineage− and Sca+ lineage−cells, and long-term culture initiating cells. Collectively, these data provide the first definitive evidence that expression of the G-CSFR on HPCs is not required for their mobilization by G-CSF and suggest a model in which G-CSFR–dependent signals act in trans to mobilize HPCs from the bone marrow.
The use of hematopoietic progenitor cells (HPCs) to reconstitute hematopoiesis following myeloablative therapy has significantly improved the clinical outcome for patients with a variety of diseases. Recently, mobilized peripheral blood HPCs rather than bone marrow–derived HPCs have been used because of their reduced engraftment times, relative ease of collection, and possibly reduced risk of acute graft-versus-host disease (GVHD). Although the great majority of HPCs reside within the bone marrow, a small number of HPCs also continuously circulate in the peripheral blood. This number can be dramatically increased or mobilized by a wide variety of stimuli. The mechanisms that regulate HPC mobilization have not yet been defined.
A notable feature of HPC mobilization is the diversity of stimulating agents, which include hematopoietic growth factors; cytotoxic agents; and certain chemokines, eg, interleukin-8 (IL-8),1,2 macrophage inflammatory protein-2 (MIP-2),3 and BB-10010 (a genetically engineered form of MIP-1α4). A partial list of hematopoietic growth factors capable of mobilizing HPCs includes granulocyte colony-stimulating factor (G-CSF),5-7 granulocyte-macrophage colony-stimulating factor (GM-CSF),8 IL-7,9,10IL-12,11 flt-3 ligand,12,13 stem cell factor,14-16 and thrombopoietin.17 A striking feature of this group is the diversity of their target cell populations. For example, hematopoietic growth factors that predominantly effect myeloid cells (eg, G-CSF), lymphocytes (eg, IL-7), or megakaryocytic cells (eg, thrombopoietin)17 are all potent mobilizing stimuli.
The mobilization of HPCs by hematopoietic growth factors with distinct cellular targets and biological actions suggests a common mechanism of action. Indeed, several common features are observed during mobilization with these agents. First, the kinetics of HPC mobilization are similar, with peak levels of circulating HPCs (increases of 5-fold to 500-fold over baseline) generally achieved after 7-10 days of cytokine treatment. Second, a broad spectrum of HPCs, including primitive pluripotent as well as committed myeloid, megakaryocytic, and erythroid progenitors, are mobilized.18-21 Third, mobilized HPCs have characteristic phenotypic features that are distinct from HPCs that reside in the bone marrow under steady-state conditions. Most notably, relative to bone marrow HPCs, a higher percentage of mobilized blood HPCs are in a quiescent stage of the cell cycle,22-25and the expression of very late activation antigen–4 (VLA-4)26-29 and c-kit19 30 on their cell surface is reduced.
G-CSF is the most commonly used agent to mobilize HPCs in current clinical practice. To explore the mechanisms of G-CSF–induced mobilization, we recently examined the mobilization response of G-CSFR–deficient mice to the 3 major types of mobilizing stimuli: cytotoxic agents (cyclophosphamide [CY]), chemokines (IL-8), and hematopoietic growth factors (G-CSF and flt-3 ligand).31 We showed that HPC mobilization by G-CSF, CY, and IL-8 (but not flt-3) was markedly impaired in G-CSFR–deficient mice, which suggests that G-CSFR signals were required for mobilization by these agents. The G-CSFR is expressed on hematopoietic cells including pluripotent and myeloid-committed progenitors, neutrophils, monocytes, and possibly certain lymphocyte subsets.32 In addition, the G-CSFR is expressed on endothelial cells and can induce their proliferation in vitro.33 To define the cell type(s) responsible for G-CSF–dependent mobilization, a series of chimeric mice was generated using bone marrow transplantation. In this study we show that expression of the G-CSFR on a subset of hematopoietic cells but not on either HPCs themselves or stromal cells is required for G-CSF– or CY-induced mobilization. This suggests that G-CSFR–dependent signals act in trans to mobilize HPCs from the bone marrow.
Materials and methods
Mice
G-CSFR–deficient mice (129 SvJ mice crossed with outbred C57BL/6J mice) were generated in our laboratory as described previously.34 Wild type mice (F1 generation) were generated by crossing 129 SvJ mice with a congenic strain of C57BL/6J mice (B6.SJL-Ptprc* Pep3b BoyJ, Jackson Laboratory, Bar Harbor, ME) that have the Ly5.1gene. (Both 129 SvJ and C57BL/6J mice have theLy5.2 gene.) We used 6- to 10-week-old mice in all studies. Mice were housed in a specific pathogen-free environment. All experiments were approved by the Washington University Animal Studies Committee.
Bone marrow transplantation
Recipient mice were conditioned with 1200 cGy given in 2 equal divided doses 24 hours apart from a cesium 137 (137Cs) source at a rate of approximately 95 cGy/min. Bone marrow cells were harvested using standard techniques, and the nonadherent (stromal cell–depleted) fraction collected after culturing in tissue culture flasks for 4 hours in α–modified Eagle medium (α-MEM) containing 10% heat-inactivated fetal bovine serum (FBS), 1 mmol/L L-glutamine, and 10 μg/mL ciprofloxacin (Bayer, Kankakee, IL). We injected 2 × 106 nonadherent bone marrow into the tail vein of potentially lethally irradiated sex-matched recipient mice. To generate mixed chimeras, 0.4 × 106 wild type and 2 × 106 G-CSFR–deficient nonadherent bone marrow cells were injected. Prophylactic antibiotics (sulfamethoxazole/trimethoprim; Alpharma, Baltimore, MD) were given during the initial 2 weeks after transplantation. Complete blood counts were determined 4 weeks after transplantation using a Hemavet automated cell counter (CDC Technologies, Oxford, CT).
Mobilization protocols
G-CSF.
Recombinant human G-CSF (Amgen, Thousand Oaks, CA) was administered by daily subcutaneous injection at a dose of 250 μg/kg/d for 5 days. Mice were analyzed 4 hours after the final G-CSF dose.
Cyclophosphamide.
Cyclophosphamide (Sigma, St. Louis, MO) was reconstituted in sterile water and given as a single 200 mg/kg intraperitoneal injection. Mice were analyzed on day 8 following CY administration.
Colony-forming cell assay
Blood, bone marrow, and spleen cells were harvested from mice using standard techniques, and the number of nucleated cells in these tissues were quantified using a Hemavet automated cell counter. We plated 10-20 μL blood, 1 × 105 nucleated spleen cells, or 2.5 × 104 nucleated bone marrow cells in 2.5 mL methylcellulose media supplemented with a cocktail of recombinant cytokines (MethoCult 3434; Stem Cell Technologies, Vancouver, British Columbia, Canada). The cells were then placed in a humidified chamber with 5% carbon dioxide (CO2). Colonies containing at least 50 cells were scored on day 10. In some experiments, Geneticin (G418) (Gibco BRL Life Technologies, Gaithersburg, MD) was added to the cultures to a final concentration of 1 mg/mL (0.708 mg/mL active) drug. G-CSFR–deficient HPCs contain the neomycin phosphotransferase gene and, therefore, are resistant to G418; at this dose, 100% of wild type colony-forming cells (CFCs) and no G-CSFR–deficient CFCs were killed (data not shown).
Flow cytometry
Ly5 gene expression.
Nucleated cells from blood or bone marrow were incubated with fluorescein isothiocyanate–conjugated (FITC-conjugated) rat antimouse Ly5.2 and phycoerythrin-conjugated (PE-conjugated) rat antimouse Ly5.1 at 4°C for 30 minutes in phosphate-buffered saline (PBS) containing 0.1% sodium azide and 0.2% bovine serum albumin. The wild type cells used in this study stained positive for both Ly5.1 and Ly5.2, while G-CSFR–deficient cells stained positive only for Ly5.2 (data not shown).
CD34+ lineage− and Sca+ lineage− cell enumeration.
Nucleated spleen cells were incubated with FITC-conjugated rat antimouse CD34 or antimouse Sca-1; biotin-conjugated rat antimouseLy5.1; and a cocktail of PE-conjugated rat antimouse lineage markers including CD11b (myeloid cells), B220 (B lymphocytes), CD3 (T lymphocytes), and Ter-119 (erythroid cells). After incubation at 4°C for 30 minutes, the cells were washed and incubated with RPE-Cy5–conjugated streptavidin (Dako, Carpinteria, CA). All antibodies were purchased from PharMingen (San Diego, CA). Red blood cells in the various cell preparations were lysed in Tris-buffered (tris[hydroxymethyl] aminomethane–buffered) ammonium chloride (pH 7.2) buffer. All samples were analyzed using a FACSan flow cytometer and CellQuest version 1.2.2 software; Becton Dickinson, Mansfield, MA).
LTC-IC assay
Individual LTC-ICs in the spleen were identified by limiting dilution, as described previously,35 with the following modifications. A feeder layer of irradiated AFT024 stromal cells (gift from Dr Ihor Lemischka, Princeton University, Princeton, NJ)36 was established in 96-well plates. Light density spleen cells were isolated by centrifugation density gradient (Histopaque 1077, Sigma) per manufacturer's recommendations. We plated 8 different dilutions (range, 0.15-5.0 × 104 cells per well) of these cells onto the feeder layer. Cultures were maintained at 33°C for 5 weeks, with weekly half-media exchanges of Myelocult (Stem Cell Technologies). Cells were harvested from each well using trypsin, divided into 2 equal parts, and analyzed using the CFC assay with and without G418.
Statistical analysis
Statistical significance was assessed by a 2-sided Student ttest.
Results
Generation of G-CSFR–deficient radiation chimeras
Wild type or G-CSFR–deficient bone marrow cells were used to reconstitute hematopoiesis in potentially lethally irradiated (1200 cGy) G-CSFR-deficient or wild type recipient mice, respectively. Nonadherent bone marrow cells were used to minimize stromal cell contamination. The wild type mice used in these studies were generated by crossing 129 SvJ mice with a congenic strain of C57BL/6J mice that has the Ly5.1 gene. (129 SvJ and C57BL/6J mice have theLy5.2 gene.) F1 generation wild type mice were used exclusively to minimize the risk of GVHD because the G-CSFR–deficient mice are an outbred C57BL/6J × 129 SvJ Fn generation. Importantly, no evidence of GVHD was observed in these experiments; all mice appeared healthy and demonstrated a weight gain of at least 10% during the 4-5 week period after transplantation. Hematopoietic reconstitution was assessed 4-5 weeks following transplantation using complete blood counts, and allelic differences in the Ly5 gene were analyzed by flow cytometry to determine the percentage of G-CSFR–deficient blood leukocytes (data not shown). Mobilization studies were limited to mice with more than 75% donor circulating leukocytes.
Expression of G-CSFR on transplantable hematopoietic cells but not stromal cells is required for CY- or G-CSF–induced mobilization
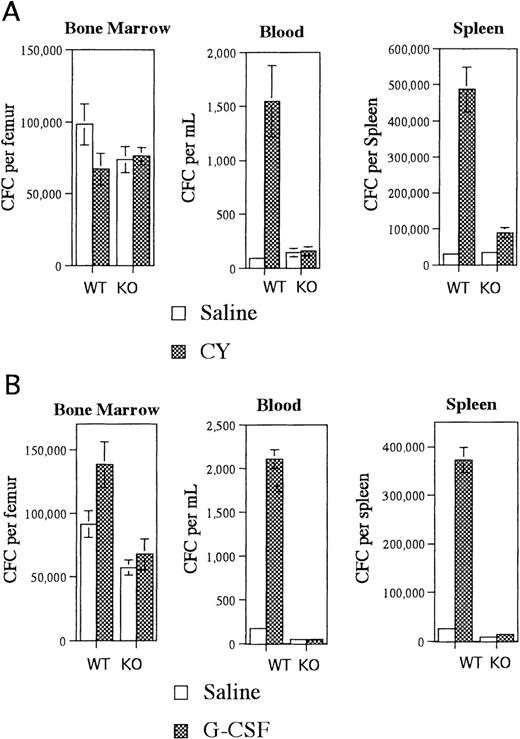
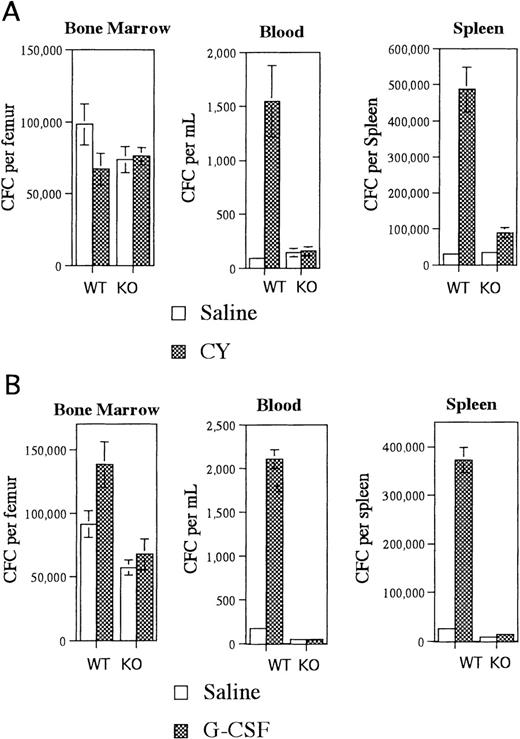
Irradiated G-CSFR–deficient mice reconstituted with wild type bone marrow cells had near normal CY-induced HPC mobilization (Figure1A). An 18-fold increase in blood CFCs and a 16-fold increase in spleen CFCs were observed in CY-treated versus saline-treated mice. In contrast, CY treatment of irradiated wild type mice reconstituted with G-CSFR–deficient bone marrow cells did not result in a significant increase in blood or splenic CFCs (Figure 1A). This mobilization defect was not secondary to impaired HPC regeneration after CY treatment because comparable numbers of CFC were detected in the bone marrow of both types of radiation chimeras (Figure 1A). Similar results were observed after treatment of these radiation chimeras with G-CSF. In mice reconstituted with wild type bone marrow cells, G-CSF treatment induced a 12-fold and 15-fold increase in blood and spleen CFCs, respectively (Figure 1B). In contrast, there was no significant increase in blood or splenic CFCs in mice reconstituted with G-CSFR–deficient bone marrow cells (Figure 1B). Collectively, these data demonstrate that expression of the G-CSFR on transplantable hematopoietic cells but not stromal cells is required for CY- or G-CSF–induced mobilization.
Mobilization of radiation chimeras.
Irradiated G-CSFR–deficient mice reconstituted with wild type (WT) bone marrow cells or irradiated wild type mice reconstituted with G-CSFR–deficient bone marrow cells (KO) were treated with (A) CY versus saline or (B) G-CSF versus saline, and the number of CFCs in the bone marrow, blood, and spleen were quantified. A total of 6-8 mice were analyzed for each data point. Data represent the mean plus or minus standard error of the mean (SEM).
Mobilization of radiation chimeras.
Irradiated G-CSFR–deficient mice reconstituted with wild type (WT) bone marrow cells or irradiated wild type mice reconstituted with G-CSFR–deficient bone marrow cells (KO) were treated with (A) CY versus saline or (B) G-CSF versus saline, and the number of CFCs in the bone marrow, blood, and spleen were quantified. A total of 6-8 mice were analyzed for each data point. Data represent the mean plus or minus standard error of the mean (SEM).
Functional G-CSFR on hematopoietic progenitor cells is not required for their mobilization by CY or G-CSF
Within the transplantable hematopoietic cell compartment, G-CSFR is expressed on HPCs, neutrophils, and monocytes and possibly natural killer (NK) cells and B lymphocytes.37-39 G-CSFR signals in any or all of these cell types could potentially be required for HPC mobilization. To determine whether a functional G-CSFR on HPCs is required, a series of “mixed” chimeras were generated in which both wild type and G-CSFR–deficient hematopoietic cells contributed equally to hematopoiesis. If expression of G-CSFR on HPCs is required, then mobilization of these mixed chimeras would be predicted to mobilize only wild type (G-CSFR–positive) HPCs.
Irradiated wild type mice were reconstituted with both wild type and G-CSFR–deficient bone marrow cells. Analyses of Ly5 gene expression were used to identify mice that contained similar numbers of circulating wild type and G-CSFR–deficient lymphocytes. In addition, the percentage of wild type versus G-CSFR–deficient HPCs was measured by determining the percentage of CFCs that produced colonies in vitro in the presence of G418 (1 mg/mL); G-CSFR–deficient HPCs contain the neomycin phosphotransferase gene and, therefore, are resistant to G418. Interestingly, approximately 5-fold greater numbers of G-CSFR–deficient bone marrow cells than wild type bone marrow cells were required to achieve equal hematopoietic reconstitution in mixed chimeras. This suggests that either fewer primitive HPCs are present in the bone marrow of G-CSFR–deficient mice or that G-CSFR–deficient HPCs are at a competitive disadvantage to reconstitute hematopoiesis.
CY-induced mobilization of HPCs in mixed chimeras.
Mixed chimeras in which both wild type and G-CSFR–deficient hematopoietic cells contributed to hematopoiesis were treated with saline or CY (n = 6 for each), and the number of CFCs in the bone marrow, blood, and spleen were quantified. The growth of colonies in the absence or presence of 1 mg/mL G418 was used to discriminate between wild type (G418-sensitive [G418S]) and G-CSFR–deficient (G418-resistant [G418R]) CFCs. Data represent the mean plus or minus SD.
CY-induced mobilization of HPCs in mixed chimeras.
Mixed chimeras in which both wild type and G-CSFR–deficient hematopoietic cells contributed to hematopoiesis were treated with saline or CY (n = 6 for each), and the number of CFCs in the bone marrow, blood, and spleen were quantified. The growth of colonies in the absence or presence of 1 mg/mL G418 was used to discriminate between wild type (G418-sensitive [G418S]) and G-CSFR–deficient (G418-resistant [G418R]) CFCs. Data represent the mean plus or minus SD.
The mixed chimeras were treated with CY or G-CSF, and their mobilization response was characterized. Surprisingly, after CY treatment a similar number of wild type and G-CSFR–deficient CFCs were mobilized into the blood and spleen (Figure2). A 14.5-fold versus a 13.1-fold increase in wild type versus G-CSFR–deficient CFCs was detected in the spleen after CY treatment. Likewise, CY treatment of mixed chimeras induced a 5.3-fold increase in wild type CFCs in the blood versus a 5.5-fold increase in G-CSFR–deficient CFCs. With G-CSF–treated mixed chimeric mice, 2 consistent observations were made (Table1). First, G-CSF treatment induced a selective increase in wild type CFCs in the bone marrow of mixed chimeric mice. Second, treatment with G-CSF induced a significant increase in both wild type and G-CSFR–deficient CFCs in the blood and spleen. In fact, after accounting for the decreased number of G-CSFR–deficient CFCs in the bone marrow, it appears that wild type and G-CSFR–deficient CFCs are mobilized equally well by G-CSF. These data indicate that although expression of the G-CSFR on HPCs is required for G-CSF–induced proliferation of CFCs, it is not required for CY- or G-CSF–induced mobilization.
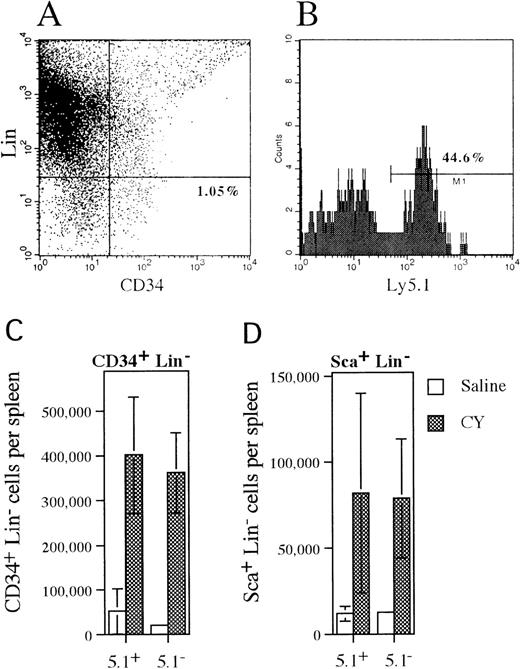
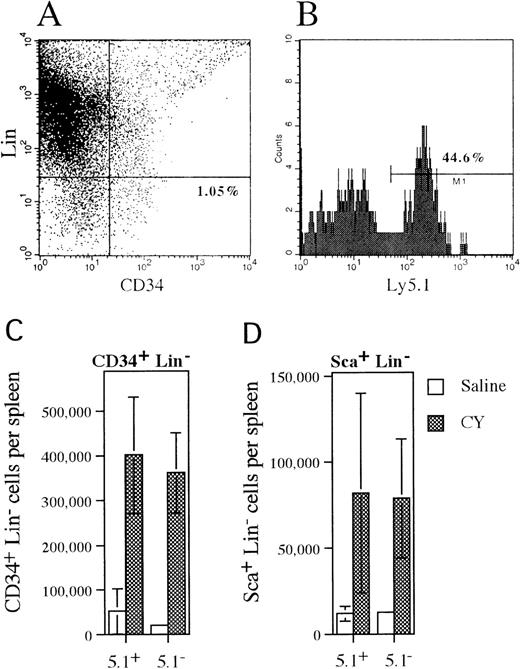
Mobilization of CD34+lineage− and Sca+ lineage−cells in mixed chimeras.
(A) Representative histogram of spleen cells isolated from CY-treated mice that were stained with antibodies against a cocktail of lineage markers and CD34. The percentage of CD34+lineage− cells is shown. (B) Ly 5.1expression on CD34+ lineage− cells that were identified in the previous histogram. The percentage of CD34+ lineage− cells that expressLy5.1 is shown. (C, D) The absolute number of wild type (Ly5.1+) and G-CSFR–deficient (Ly5.1−) CD34+lineage− or Sca+ lineage−cells present in the spleen of saline- or CY-treated mice is shown. Data represent the mean plus or minus SD.
Mobilization of CD34+lineage− and Sca+ lineage−cells in mixed chimeras.
(A) Representative histogram of spleen cells isolated from CY-treated mice that were stained with antibodies against a cocktail of lineage markers and CD34. The percentage of CD34+lineage− cells is shown. (B) Ly 5.1expression on CD34+ lineage− cells that were identified in the previous histogram. The percentage of CD34+ lineage− cells that expressLy5.1 is shown. (C, D) The absolute number of wild type (Ly5.1+) and G-CSFR–deficient (Ly5.1−) CD34+lineage− or Sca+ lineage−cells present in the spleen of saline- or CY-treated mice is shown. Data represent the mean plus or minus SD.
To determine whether more primitive HPCs followed a similar pattern of mobilization, the number and phenotype of CD34+lineage− and Sca+ lineage−cells and LTC-ICs present in the spleen of mixed chimeras following CY treatment were analyzed (Figure3). The CD34+lineage− cell population contains both primitive and lineage-committed HPCs,40 while the Sca+lineage− cell population is thought to contain a more primitive subset of HPCs including hematopoietic stem cells.41 Expression of Ly5.1 was used to discriminate between wild type (Ly5.1+) and G-CSFR–deficient (Ly5.1−) cells (Figure 3A, B). Treatment with CY induced a similar increase in both wild type (7.7-fold) and G-CSFR–deficient (12.7-fold) CD34+ lineage− cells in the spleen of mixed chimeras (Figure 3C). Likewise, a similar increase in wild type (6.7-fold) and G-CSFR–deficient (6.6-fold) Sca+lineage− cells in the spleen was detected after treatment with CY (Figure 3D). Individual LTC-IC clones in the spleen of CY-treated mixed chimeras were identified by limiting dilution. The G418-sensitivity of CFCs derived from these LTC-IC clones was used to discriminate between wild type (G418-sensitive) and G-CSFR–deficient (G418-resistant) LTC-ICs. From a total of 24 LTC-IC clones analyzed, 11 were found to be derived from G-CSFR–deficient cells (data not shown). Collectively, these data demonstrate that a broad spectrum of primitive and committed wild-type and G-CSFR–deficient HPCs are mobilized by CY in these mixed chimeras.
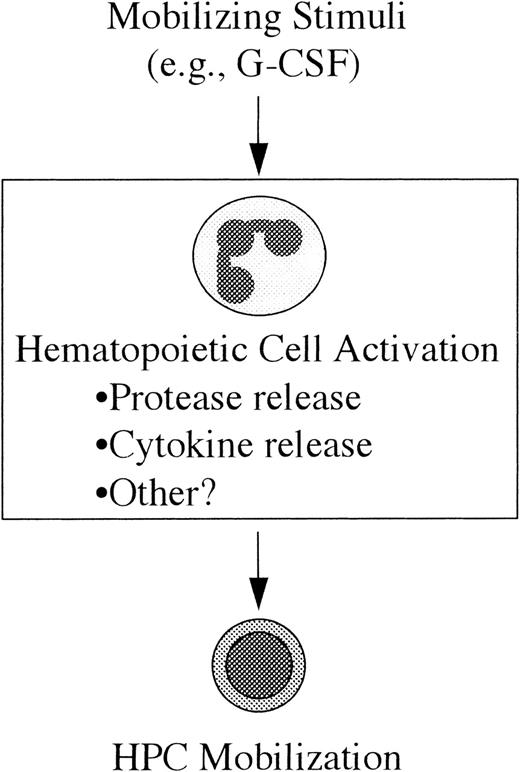
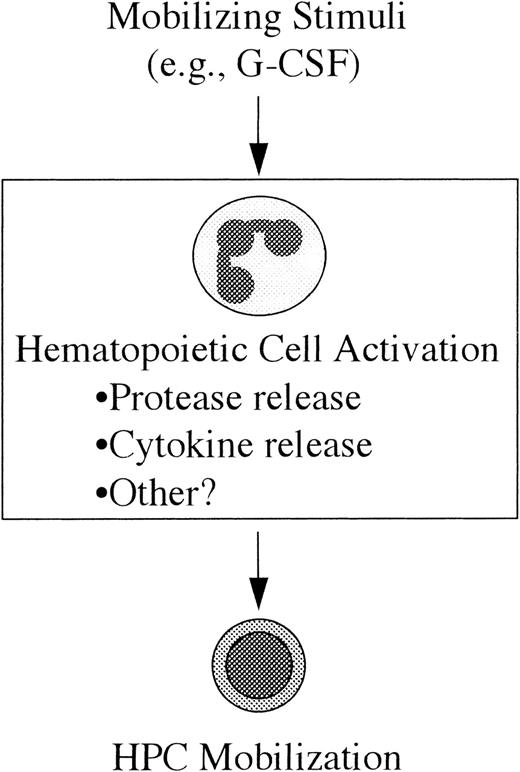
Model of HPC mobilization.
In this model the mobilizing stimulus (eg, G-CSF) acts directly on a mature subset of hematopoietic cells resulting in their activation; in the case of G-CSF, the likely (but unproven) target cell population is neutrophils. The mechanism by which these activated cells lead to HPC mobilization is not yet clear, but it may include the release of cytokines or extracellular matrix-degrading proteases. (See text for discussion.)
Model of HPC mobilization.
In this model the mobilizing stimulus (eg, G-CSF) acts directly on a mature subset of hematopoietic cells resulting in their activation; in the case of G-CSF, the likely (but unproven) target cell population is neutrophils. The mechanism by which these activated cells lead to HPC mobilization is not yet clear, but it may include the release of cytokines or extracellular matrix-degrading proteases. (See text for discussion.)
Discussion
The mobilization of HPCs could potentially occur by 3 general mechanisms. The mobilizing stimulus (eg, hematopoietic growth factor) could result in phenotypic changes in the HPCs themselves, which lead to enhanced migration into the intravascular space. Alternatively, the mobilizing stimulus could lead to changes in the bone marrow microenvironment that facilitate HPC release. Finally, it is theoretically possible that the increase in the level of blood HPCs could be secondary to a prolongation of their half-life in the circulation; this possibility seems unlikely because mobilized peripheral blood HPCs collected by apheresis are rapidly cleared from the circulation after transfusion into recipients.
The first model predicts that the phenotype of mobilized peripheral blood HPCs versus HPCs that reside in the bone marrow under steady-state conditions should be different. In fact, consistent differences have been detected between these 2 groups. First, expression of the very late activation antigen–4 (VLA-4) integrin is consistently lower in peripheral blood HPCs,26-29 a potentially important finding given reports that anti–VLA-4 antibodies can mobilize HPCs in mice or nonhuman primates.42,43Second, relative to bone marrow HPCs, a higher percentage of peripheral blood HPCs appear to be in a quiescent stage of the cell cycle.22-25 For example, in one study, 7% of peripheral blood versus 47% of bone marrow progenitors was observed to be in the S phase.23 Third, mobilized HPCs have decreased expression of c-kit,19,30,44 another potentially important finding given reports that HPC mobilization by G-CSF45 or anti-vascular cell adhesion molecule (VCAM) antibodies46 is impaired in W/Wv(c-kit–deficient) mice. Whether any of these phenotypic differences is responsible for the release of HPCs from the bone marrow is not yet clear.
Recent studies of IL-8 administration in mice suggest that changes in the bone marrow microenvironment may contribute to HPC mobilization. IL-8 is a CXC chemokine that is produced by a wide variety of cell types including neutrophils, monocytes, fibroblasts, and endothelial cells. It is a potent activator of neutrophils and leads to their migration, degranulation, and up-regulation of Mac-1.47-50IL-8 administration in mice and nonhuman primates induces a rapid increase in the level of circulating HPCs that are detectable 5 minutes after parenteral administration, peak at 15-30 minutes, and return to baseline within 2 hours.1,2 A role for neutrophil activation in IL-8–induced mobilization has been suggested based on the following observations. First, the receptor for IL-8 (CXCR-2) in mice does not appear to be expressed on HPCs.51 Second, the induction of neutropenia in mice blocks subsequent IL-8–induced HPC mobilization.52 Finally, pretreatment of rhesus monkeys with neutralizing antibodies directed against MMP-9 (gelatinase B), a protease released by neutrophils after IL-8 stimulation, also blocks IL-8–induced HPC mobilization.53 The relevance of these findings to hematopoietic growth factor– or cytotoxic agent–induced HPC mobilization is not clear. The kinetics of IL-8–induced HPC mobilization (minutes) are distinct from that observed with hematopoietic growth factors or cytotoxic agents (days). Moreover, a recent report showed that IL-8–induced mobilization of neutrophils and band cells occurs from the bone marrow sinusoids rather than from the extravascular hematopoietic compartment, which suggests that IL-8 may mobilize HPCs from the same intravascular compartment.54
In the present study we have examined the contribution of certain bone marrow cell populations to CY- or G-CSF–induced HPC mobilization. We previously showed that HPC mobilization by G-CSF, CY, or IL-8 (but not flt-3 ligand) is markedly impaired in G-CSFR–deficient mice.31 Because G-CSFR is expressed on neutrophils, monocytes, HPCs, and stromal cells (endothelial cells) and possibly B lymphocytes and NK cells,37-39 this observation suggested that G-CSFR signals in one or more of these cell types was required for mobilization by these agents. To identify this cell population, the mobilization response in a series of radiation chimeras generated by bone marrow transplantation was characterized. These studies demonstrated that expression of G-CSFR on bone marrow stromal cells is not required for HPC mobilization by CY or G-CSF. Irradiated G-CSFR–deficient mice reconstituted with wild type bone marrow cells (in which stromal cells had been depleted) demonstrated near normal mobilization with these agents. Conversely, in irradiated wild type mice reconstituted with G-CSFR–deficient bone marrow cells, mobilization by G-CSF or CY was markedly impaired. We next examined HPC mobilization in radiation chimeras reconstituted with both wild type and G-CSFR–deficient bone marrow cells. Remarkably, CY or G-CSF treatment of these mixed chimeras resulted in the equal mobilization of both wild type and G-CSFR–deficient HPCs. This result held true for a broad spectrum of HPCs including CFCs, CD34+lineage− and Sca+ lineage−cells, and LTC-ICs. Collectively, these data demonstrate that expression of G-CSFR on a subset of hematopoietic cells but not on either the HPCs themselves or stromal cells is required for HPC mobilization.
A model of HPC mobilization based on these observations is presented in Figure 4. In this model, the first step toward HPC mobilization is the activation of a subset of mature hematopoietic cells by the mobilizing stimulus (eg, G-CSF). The second step is the generation of secondary signals by these activated cells that, in turn, leads to HPC mobilization. The cell type(s) responsible for the generation of these signals has not been definitively identified. In fact, it is likely that distinct primary cellular targets exist for the different mobilizing stimuli. In the case of G-CSF, there is evidence to suggest that neutrophils are generating these secondary signals. First, as described above, neutrophil activation has been implicated in IL-8–induced mobilization. Second, the major cellular targets of G-CSF are cells of the neutrophil lineage. G-CSF treatment results in an increase in the number and activation state of neutrophils in the bone marrow and blood.55
The nature of the secondary signal(s) that are responsible for the actual mobilization of HPCs from the bone marrow is not known. One intriguing possibility is that activation of hematopoietic cells may lead to their release of proteases with subsequent degradation of the bone marrow extracellular matrix and disruption of the normal barriers to HPC egress into the vascular compartment. Consistent with this hypothesis, MMP-9 release by neutrophils has been implicated in IL-8–induced HPC mobilization (see above).53 Another potential secondary signal is the release of cytokines by activated hematopoietic cells. For example, G-CSF treatment results in an increase in the serum concentration of IL-8.56Interestingly, the kinetics of the increase in serum IL-8 levels and the level of circulating HPCs are similar.56 Moreover, serum IL-8 levels correlate with the magnitude of the HPC mobilization response.
In summary, these data provide the first definitive evidence that expression of G-CSFR on HPCs is not required for their mobilization by G-CSF and suggest a model in which G-CSFR–dependent signals act in trans to mobilize HPCs from the bone marrow. This model is consistent with the observation that the phenotype of mobilized HPCs is similar for mobilizing stimuli with distinct biological actions and target cell populations.
Acknowledgments
We thank Dr Ihor Lemishka for his generous gift of the AFT024 stromal cell line and Drs Timothy Graubert, Timothy J. Ley, and Monica Bessler for their critical review of this manuscript.
Supported by grant R01 HL60772-01A1 (D.C.L) from the National Institutes of Health, National Heart, Lung, and Blood Institute, Bethesda, MD.
Reprints:Daniel C. Link, Washington University School of Medicine, Division of Bone Marrow Transplantation and Stem Cell Biology, Campus Box 8007, 660 South Euclid Ave, St. Louis, MO 63110-1093; e-mail: dlink@im.wustl.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Fig. 2. CY-induced mobilization of HPCs in mixed chimeras. / Mixed chimeras in which both wild type and G-CSFR–deficient hematopoietic cells contributed to hematopoiesis were treated with saline or CY (n = 6 for each), and the number of CFCs in the bone marrow, blood, and spleen were quantified. The growth of colonies in the absence or presence of 1 mg/mL G418 was used to discriminate between wild type (G418-sensitive [G418S]) and G-CSFR–deficient (G418-resistant [G418R]) CFCs. Data represent the mean plus or minus SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/10/10.1182_blood.v95.10.3025/5/m_bloo01032002x.jpeg?Expires=1764472725&Signature=VuVd51UV3CQzeTAEwAd1WlhK7gU~kyXrRznok1NUNUyRpstIkvBcasGvI8GX6jGiaUUebH2m5D1~VBNQR1w-Gl8RTwTQ0vs~GtrjtU51M26ufPvbD8QPDvdsr8dCHNbTPC4uuqPFxd6T0oCSbT~LdNvIm8b36dZpKeOa6DPu9CgxF4WymewQTVor7sleRB6nGh0vsBcNxSoN3DnnOEwDwMOEl85EP3ykwPahQW7KhyRv-MgLiSKPSjh8k0sCFw4S25S9GNDrAkBRwa0HI7zfMp88XdI-dRS7d0Cx4R-5vFkQNyvXYTk7Jfdbw~2OBTUvHd1ChvlS9fdl7jphn2JNpg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. CY-induced mobilization of HPCs in mixed chimeras. / Mixed chimeras in which both wild type and G-CSFR–deficient hematopoietic cells contributed to hematopoiesis were treated with saline or CY (n = 6 for each), and the number of CFCs in the bone marrow, blood, and spleen were quantified. The growth of colonies in the absence or presence of 1 mg/mL G418 was used to discriminate between wild type (G418-sensitive [G418S]) and G-CSFR–deficient (G418-resistant [G418R]) CFCs. Data represent the mean plus or minus SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/10/10.1182_blood.v95.10.3025/5/m_bloo01032002x.jpeg?Expires=1764472726&Signature=ismH6GvDrQ5pCeUOxXCbX-e0IC7n7dziNz3qLbLPNHUagQBbKKaTuupILwT47uyVCeq~LqW5sn3~COjUnbEXGFlxxcN55~CDM-JpDzT4hki1WebLpkWE8oNN4hmRcXGVIRSr85Hh63VBGF070eB2qy~T3Z35bW0~r-Kr-4RALKW4hRM6YDapG2qr664U3outHSiKgnlCr2y2fMjcLONY1rtMvwdQZa~JgEUDwvnC61ntrSG3Jou3DntSDRlU3RR2hbSAHcv4ttf3ok2lEfQdxPMf0BACJsCasSdg4EvWCKrtpC~2AMqOtMU8PkHJTcxdc1GkQCOIWwV2LQavPfcjKQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)