Achieving early, complete, and sustained reperfusion after acute myocardial infarction does not occur in approximately 50% of patients, even with the most potent established thrombolytic therapy. Bleeding is observed with increased concentrations of thrombolytics as well as with adjunctive antithrombotic and antiplatelet agents. A novel approach to enhance thrombolytic therapy is to inhibit the activated form of thrombin-activatable fibrinolysis inhibitor (TAFI), which attenuates fibrinolysis in clots formed from human plasma. Identification of TAFI in rabbit plasma facilitated the development of a rabbit arterial thrombolysis model to compare the thrombolytic efficacy of tissue-plasminogen activator (tPA) alone or with an inhibitor, isolated from the potato tuber (PTI), of activated TAFI (TAFIa). Efficacy was assessed by determining the time to patency, the time the vessel remained patent, the maximal blood flow achieved during therapy, the percentage of the original thrombus, which lysed, the percentage change in clot weight, the net clot accreted, and the release of radioactive fibrin degradation products into the circulation. The results indicate that coadministration of PTI and tPA significantly improved tPA-induced thrombolysis without adversely affecting blood pressure, activated partial thromboplastin time, thrombin clotting time, fibrinogen, or -2-antiplasmin concentrations. The data indicate that inhibitors of TAFIa may comprise novel and very effective adjuncts to tPA and improve thrombolytic therapy to achieve both clot lysis and vessel patency.
ACUTE MYOCARDIAL infarction is triggered by rupture of an atherosclerotic plaque and superimposed thrombus.1 Although thrombolytic therapy increases survival of patients with acute myocardial infarction, rapid and sustained reperfusion of the infarct-related artery is achieved in approximately 50% of patients.2-4 Development of better thrombolytic agents or more effective adjunctive antithrombotic therapy5has improved overall efficacy of coronary thrombolysis marginally.6,7 There are minimally 2 possible explanations for these disappointing results. First, rethrombosis may be induced by the procoagulant state that accompanies the thrombolytic process. Second, coronary thrombi may be inherently resistant to thrombolysis.8-10 Although thrombin generated during fibrinolysis may contribute to rethrombosis by inducing clot accretion, it may also contribute to the production of inherently more resistant thrombi by activating an inhibitor of fibrinolysis, referred to as thrombin-activatable fibrinolysis inhibitor (TAFI). This would necessarily decrease the efficacy of fibrinolytic agents in vivo. Potentially, inactivation of thrombin or activated TAFI (TAFIa) during thrombolysis would improve outcome.
Antithrombotics most widely used potentiate inhibition of thrombin by antithrombin III. The limitations of antithrombin III-dependent thrombin inhibitors, such as heparin and its derivatives, have been attributed to their inability to completely inhibit activation of prothrombin and inhibit thrombin bound to fibrin.11Thrombin bound to fibrin may represent a pool that may be active, protected from inhibition, and released to stimulate and amplify its own generation by activating platelets,12 factors V and VIII,13 and factor XI.10 To circumvent limitations associated with indirect inhibitors, efforts have been directed towards developing new direct thrombin inhibitors, including hirudin and its derivatives.14,15 The high concentrations of these agents required for efficacy is associated with increase in spontaneous bleeding complications.16-18 A novel alternative to inhibition of thrombin or its generation as adjunctive thrombolytic therapy may be to reduce the inherent resistance of a thrombus to tissue-plasminogen activator (tPA)-induced thrombolysis by inhibiting TAFIa.10 19 In contrast to adjuvants comprising anticoagulants, this approach would preserve both thrombin’s activity and the ability to generate thrombin distal to the target thrombus potentially overcoming safety issues.
The zymogen TAFI, also known as plasma procarboxypeptidase B, is found in human plasma and circulates as a precursor of an exopeptidase with carboxypeptidase B-like specificity.20,21 TAFI is also referred to as procarboxypeptidase U21a; however, this remains unproven. It is activated by thrombin alone, although thrombomodulin enhances the rate of thrombin catalyzed activation of TAFI by 1,250-fold.22-26 TAFIa is inhibited by a carboxypeptidase inhibitor isolated from the potato tuber (PTI).22,26,27 This 39 amino acid peptide is a specific inhibitor of both the carboxypeptidase A and B family of proteases.28,29 It has been postulated that PTI potentiates fibrinolysis by inhibiting TAFIa-dependent removal of C-terminal lysine residues exposed on fibrin partially degraded by plasmin.26,30,31 These C-terminal lysine residues are considered to be potent cofactors in tPA-mediated activation of plasminogen.32,33 Presumably, C-terminal lysines increase the efficacy of tPA to activate plasminogen and induce thrombolysis by facilitating both colocalization of the components and stabilization of the ternary complex comprising tPA-fibrin-plasminogen.34 In view of this mechanistic advantage, we designed experiments to determine whether administration of PTI would improve tPA-induced thrombolysis in vivo and, therefore, could be used as an adjunct with tPA. We first established the presence of TAFI in rabbit plasma and subsequently developed a rabbit arterial thrombolysis model, a modified rat model,35 that permits assessment of both thrombolysis and net thrombus accreted during tPA-induced thrombolysis. Using this model, we compared the relative effects of tPA alone or tPA in combination with PTI on lysis of the original thrombus, thrombus growth (net clot accreted), and vessel patency.
MATERIALS AND METHODS
Reagents
PTI was purchased from Calbiochem (La Jolla, CA) and dissolved in 0.2-μm filtered 20 mmol/L N-[2-Hydroxyethyl]piper-azine-N′-[2-ethanesulfonic acid] (HEPES), 150 mmol/L NaCl, pH 7.4 (HBS), to a concentration of 2.0 mg/mL. Barbiturate Euthanyl was purchased from MTC Pharmaceuticals (Cambridge, ON). Rabbit fibrinogen was purchased from Sigma (Oakville, Ontario, Canada) and labeled using Na125I from NEN Life Science Products (Guelph, Ontario, Canada) and iodobeads from Pierce (Rockford, IL). tPA (Activase, lot no. C9038A) was purchased from Genentech (Burlington, Ontario, Canada). New Zealand White Specific Pathogen Free male rabbits (25, including 3 rabbits for pharmacokinetics and 3 rabbits for evaluation of consistency between clots generated in test tubes compared with those generated in vivo) with an average weight of 3.4 ± 0.1 kg were purchased from Charles River Laboratories (St. Constant, Quebec, Canada). The protocol for these studies was approved by the Animal Research Ethics Committee of McMaster University (Hamilton, Ontario, Canada).
Surgery
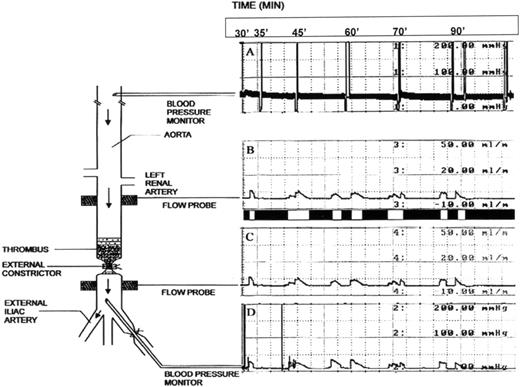
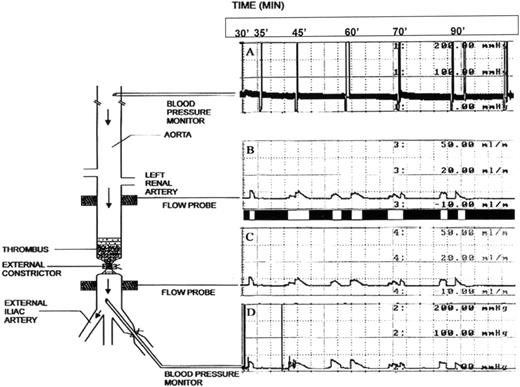
Anesthetics included a mixture of 50 mg/kg ketamine and 2 mg/kg xylazine administered intramuscularly (induction) and a mixture of 1% to 3% isoflurane, 1.5 L/min oxygen, and 0.5 L/min nitrous oxide (maintenance). The right common carotid artery was cannulated with a polyethylene tubing (PE # 90) for arterial blood pressure monitoring and blood sampling. A midline laparotomy exposed the entire abdominal aorta. All aortic side branches below the renal arteries were ligated. Two tourniquets were placed around the aorta 1.8 cm apart 2 cm distally to the right renal artery. Both external iliac arteries were isolated and a tourniquet was placed around each of them. Under segmental occlusion, a thrombectomy catheter (Fogarty catheter # 4; Baxter Healthcare Corp, Irvine, CA) was inserted via the left iliac artery into the aorta to induce 12 cm of damage by 30 passes of the balloon inflated with 0.4 mL of air (Fig 1). The aortic wall between the tourniquets was externally damaged by 16 parallel clampings using a straight vascular clamp. Damage of this magnitude was empirically determined to prevent spontaneous complete thrombolysis, expose tissue factor to induce coagulation,36and produce a platelet-rich thrombus36; this damage, superimposed on a high grade stenosis (see below), assures cyclic reocclusion (Fig 1, flow recordings). All of these are properties of arterial thrombosis that are observed clinically.1Continuous measurement of blood flow and pressure was accomplished using 2 flow probes placed above and below the damaged aortic segment and cannulas in the carotid and iliac artery, respectively. Thereafter, the cranial aortic tourniquet was snared and all blood below it was expressed into the right external iliac artery, which was then occluded using an iliac tourniquet.
Schematic representation of the rabbit thrombolysis model. A thrombus, formed from rabbit whole blood, is depicted superimposed on a stenosis in a denuded and damaged segment of the aorta. The external constrictor, by itself, restricts blood flow by at least 66%, reducing the flow from 15 mL/min to 3 to 5 mL/min, thereby producing a critical stenosis. Blood flow is not observed after production of the thrombus. The spatial relationship of both the blood pressure and flow probes with respect to the thrombus is indicated. To the right of the schematic representation of the rabbit are tracings from both blood pressure and flow probes, which indicate a representative recording of cyclic flow observed during treatment with tPA and PTI.
Schematic representation of the rabbit thrombolysis model. A thrombus, formed from rabbit whole blood, is depicted superimposed on a stenosis in a denuded and damaged segment of the aorta. The external constrictor, by itself, restricts blood flow by at least 66%, reducing the flow from 15 mL/min to 3 to 5 mL/min, thereby producing a critical stenosis. Blood flow is not observed after production of the thrombus. The spatial relationship of both the blood pressure and flow probes with respect to the thrombus is indicated. To the right of the schematic representation of the rabbit are tracings from both blood pressure and flow probes, which indicate a representative recording of cyclic flow observed during treatment with tPA and PTI.
Thrombus and Stenosis Generation
A 0.6-mL blood sample was withdrawn from the carotid artery cannula and mixed with 125I-labeled rabbit fibrinogen (∼1,000,000 counts per minute [CPM]) and dispensed into three 0.1-mL aliquots. One aliquot was injected via a catheter (PE tubing # 50) into the damaged and occluded aortic segment and the remaining 2 aliquots were allowed to clot spontaneously. The aortic radioactive clot was allowed to age for 30 minutes in situ. The remaining 2 aliquots served as a control for both clot weight and radioactivity. To demonstrate that the whole blood clot formed in vitro was representative of the thrombus formed in situ, thrombi were excised immediately after 30 minutes of maturation without being subjected to the systemic vasculature in 3 control rabbits. The difference between both the weight and the radioactivity of clots induced in tubes or in the aorta (n = 3) was 7.2% ± 13% and 2% ± 2%, respectively. An aortic stenosis, greater than 96% of cross-sectional area, was created by two 4-0 silk ligatures placed around a 23-gauge needle at the distal end of the thrombus. One intravenous (IV) line (Angicath 22 gauge; Becton Dickinson, Sandy, UT) was inserted into the marginal vein of each ear for separate IV administration of each agent. Aortic thrombi were occlusive after clot maturation, because no blood flow was detected in the aorta above or below the clot when all tourniquets were released.
Treatment Groups
In group I—SAL/SAL (n = 6), equivalent volumes (1.0 mL) of saline were administered in place of tPA or PTI where appropriate. In group II—tPA/SAL (n = 4), an IV bolus of 0.25 mg/kg tPA and an infusion of 0.25 mg/kg tPA per 60 minutes were administered. In group III—SAL/PTI (n = 5) , and IV bolus of 0.5 mg/rabbit PTI was administered. In group IV—tPA/PTI (n = 4), IV boluses of 0.5 mg/rabbit PTI and 0.25 mg/kg tPA followed by IV infusions of 0.25 mg/kg per 60 minutes were administered. Harvard apparatus (pump 44, model 4200-005; Harvard Apparatus Inc, South Natick, MA) was used for the infusion of treatments.
Blood Collection
Blood (1.8 mL) was collected into 0.2 mL of 3.8% sodium citrate immediately before clot induction (0 minute), postinjection of PTI (30 minutes), postadministration of a bolus and initiation of an infusion of tPA (35 minutes), and at 45, 60, 75, and 90 minutes. A schematic diagram of the procedure is shown in Fig 2. Blood samples were centrifuged at 1,700g for 15 minutes in an eppendorf centrifuge (Netheler & Hinz GmbH, Hamburg, Germany), and the plasma was stored on ice until the end of each experiment. They were subsequently stored at −70°C for later analysis of activated partial thromboplastin time (APTT), thrombin clotting time (TCT), α-2 antiplasmin, and fibrinogen concentrations and determination of ex vivo lysis time using a turbidometric fibrinolysis assay.
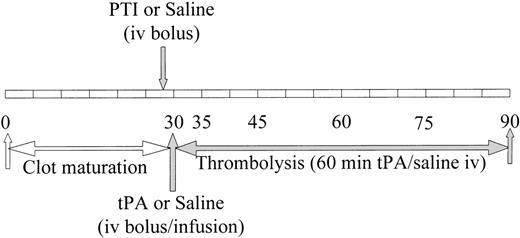
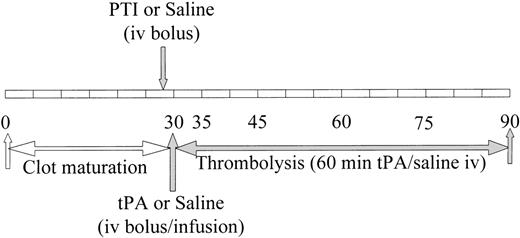
Schematic representation of the time course of thrombus formation and subsequent treatment. The time course indicates initiation of thrombus formation, thrombus maturation during the first 30 minutes, and the following 60 minutes of treatment. The duration of experiment extends for a total of 90 minutes, during which 7 separate blood samples are obtained at the indicated times. Thirty minutes were allowed for tissue factor exposed on the denuded aorta to produce a thrombus from rabbit whole blood containing radiolabeled rabbit fibrinogen. PTI or saline was administered immediately before obtaining the second blood sample at 30 minutes. After the blood sample was obtained at the 30-minute time point, but before the next blood sample at 35 minutes, a bolus of tPA or saline was administered and an infusion of each was initiated.
Schematic representation of the time course of thrombus formation and subsequent treatment. The time course indicates initiation of thrombus formation, thrombus maturation during the first 30 minutes, and the following 60 minutes of treatment. The duration of experiment extends for a total of 90 minutes, during which 7 separate blood samples are obtained at the indicated times. Thirty minutes were allowed for tissue factor exposed on the denuded aorta to produce a thrombus from rabbit whole blood containing radiolabeled rabbit fibrinogen. PTI or saline was administered immediately before obtaining the second blood sample at 30 minutes. After the blood sample was obtained at the 30-minute time point, but before the next blood sample at 35 minutes, a bolus of tPA or saline was administered and an infusion of each was initiated.
Endpoints
Thrombolysis in vivo.
(1) The percentage change in clot weight (PCCW) is the net result of clot lysis and net accretion calculated from the clot weight (in milligrams) generated in tubes before treatment and clot weight (in milligrams) explanted from the aorta at 90 minutes after clot formation as follows: PCCW = ([explanted clot weight/tube clot weight] × 100) − 100. A positive number indicates a net increase in clot weight during the experiment.
(2) Clot lysis (CL; in percentage) represents the lysis of the original thrombus and was calculated from clot radioactivity (CPM) as follows: CL = 100 − ([explanted clot radioactivity/tube clot radioactivity] × 100). Clot radioactivity was measured in CPM on Clinigamma model 1272 (LKB-Wallac, Turku, Finland).
(3) Net clot accreted (NCA; in percentage) is a measure of net thrombus production after exposure of the original thrombus to the circulation and was expressed as follows: NCA = PCCW (%) + CL (%). Because CL is a positive number, ranging from 0% to 100%, representing a loss in clot mass CL (%) is added to PCCW (%) to compensate for the loss in mass due to lysis of the original clot. This value represents the net mass accreted and not the total mass accreted, because we cannot account for concomitant loss of mass due to lysis.
(4) 125I-labeled fibrin degradation products (125I-FDPs) released from the clot into circulation during the experiment were monitored. Values are expressed as the ratio between radioactivity in 1 mL of citrated whole blood times the total blood volume of the rabbit and radioactivity present in the control thrombus calculated as follows: 125I-FDPs in percentage = ([total blood volume × radioactivity in 1 mL of whole blood (CPM) at 90 minutes]/[tube clot radioactivity (CPM)] × 100), where the total blood volume was determined for each rabbit, individually, assuming 61 mL of whole blood per killogram of rabbit (average, 206 ± 8 mL/rabbit).
Flow.
(1) Time to aortic patency (TP; in minutes) is the time required to achieve aortic blood flow of ≥0.2 mL/min. Aortic blood flow was measured using the Transonic Blood Flow Monitoring System, which consisted of 2 transonic flow probes (model no. 2.5SB232) connected to the CH-10 extension cables and an AT 206 Medical Flowmeter, dual channel (Transonic Systems Inc, Ithaca, NY). Results from 1 rabbit in group IV were excluded due to flow probe malfunction.
(2) Cumulative aortic patency in percent (CAP) during 60 minutes is calculated as follows: CAP in % = ([Σ of patent intervals during 60 minutes in the aorta (min)/60 minutes] × 100), where 60 minutes of patency is 100% and Σ of patent intervals (in minutes) in the aorta with blood flow of ≥0.2 mL/min during 60 minutes is X %. Again, results from 1 rabbit in group IV were excluded due to flow probe malfunction; however, all other parameters were included for data analysis.
(3) Mean blood pressure is measured in millimeters of Hg. Blood pressure was monitored on the Blood Pressure Monitoring System, which consisted of a transducer (Cobe Custom CDXIII Kit; Cobe, Lakewood, CO) and an extension cable (Cobe CDX 3) connected to a pressure monitor (model no. 78 205D; Hewlett-Packard, Waltham, MA). Blood pressures and aortic blood flows were continuously recorded and also analyzed using the data acquisition program AT CODAS (Dataq Instruments Inc, Akron, OH).
Coagulation and Fibrinolytic Parameters
APTT was determined according to the method of Proctor and Rapaport,37 TCT was determined according to Seegers and Smith,38 fibrinogen concentrations were determined using the method of Clauss,39 and α-2 antiplasmin concentrations were determined using the method of Teger-Nilsson et al.40 These parameters were determined for each plasma sample at each time interval by the Hemostasis Reference Laboratory (HCHRC, Hamilton, Ontario, Canada).
Fibrinolysis Assay (In Vitro)
Quantification of fibrinolysis was performed according to methodology previously described for clots formed from human plasma.41Briefly, citrated rabbit plasma was diluted one third with HBS. Diluted plasma (95 μL) was added to the wells of a microtiter plate containing 2 μL of 0.3 μmol/L thrombin dissolved in 0.5 mol/L Ca2+, HBS, and 0.01% Tween 80 in the presence or absence of 3 μL of 0.5 μg/mL tPA dissolved in HBS/0.01% Tween 80. Turbidity was monitored at 405 nm and 37°C for 5 hours at 2-minute intervals using a SpetraMAX Plus (Molecular Devices, Sunnyvale, CA). Duplicate lysis time determinations were calculated for each time point by identifying the time required to achieve the half-maximal absorbance observed during the dissolution of the turbid fibrin clot. Additionally, the absolute effect of PTI on lysis time in vitro was assessed for the 0 time point only, for each rabbit, by determining the lysis time in the absence and presence of a saturating concentration of PTI (10 μg/mL). Comparison of lysis times of clots formed from plasma withdrawn from the rabbit at later time points during treatment was the basis for the determination of the presence of PTI infused into the rabbit. In instances after the infusion of tPA lysis, time was too short, due to the excess of infused tPA, to unequivocally assess the presence/absence of PTI functionally in the rabbit.
Gel Electrophoresis and Western Blotting
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed as described by Neville42 and Western blotting was performed as indicated by Towbin et al.43Briefly, reduced samples were subjected to electrophoresis on a 5% to 15% gradient gel and subsequently transferred to nitrocellulose and blocked with 5% nonfat dry milk in HBS/0.05% Tween 20. Antigen was probed for 2 hours with 1.0 μg/mL horseradish peroxidase-conjugated goat anti-TAFI polyclonal antibody (Affinity Biologicals Inc, Hamilton, Ontario, Canada) dissolved in HBS/Tween 20 and detected using chemiluminescence ECL reagents (Amersham Life Sciences, Oakville, Ontario, Canada) and X-Omat film (Kodak Scientific Imaging, Amersham).
Statistical Analysis
The data are expressed as the mean ± SEM. Significance (P< .03) was assessed using the Student’s t-test for data within the treatment groups and a 2-tailed ANOVA test for data between treatment groups.
RESULTS
Presence of TAFI in Rabbit Plasma
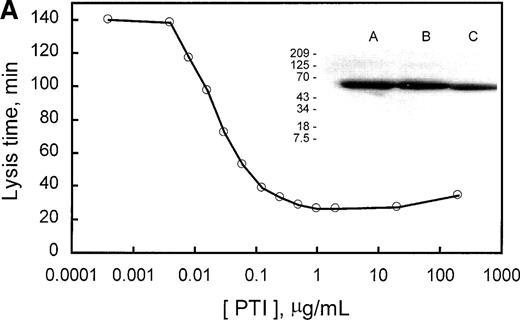
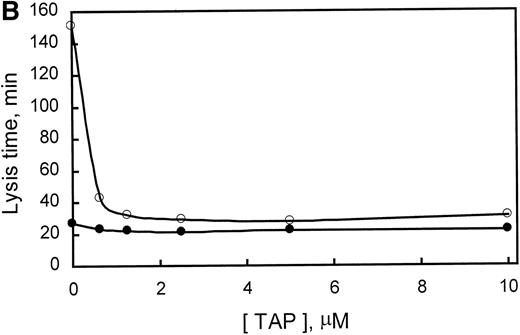
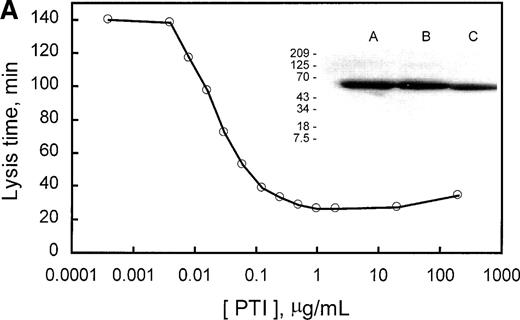
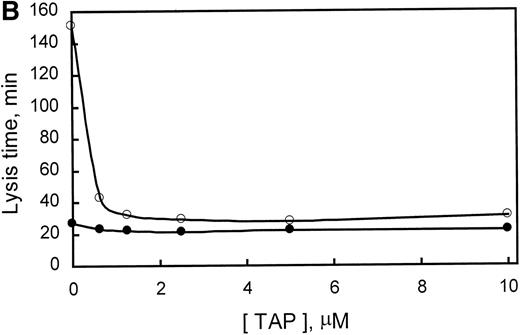
PTI specifically potentiates tPA-induced fibrinolysis in clots of human plasma. Similarly, PTI reduced the lysis time of clots formed from rabbit plasma by a factor of 5.6 at saturating concentrations. In the absence of PTI, lysis time was 140 minutes and was saturably reduced to approximately 25 minutes with 0.25 μg/mL PTI (Fig 3A). Because PTI was homogeneous, as assessed by SDS-PAGE, and lysis time increased, marginally, upon subsequent addition of PTI to 200 μg/mL, it is concluded that PTI neither contains an inhibitor of fibrinolysis as a contaminant nor inhibits fibrinolysis at the concentrations used. The data also provide indirect evidence for the presence of TAFI in rabbit plasma. The presence of TAFI in rabbit plasma is further evidenced by a Western blot of rabbit plasma (Fig 3A, inset), which indicates that a polyclonal antibody, raised against human TAFI, cross-reacts with a protein of similar molecular mass in rabbit plasma. Additionally, inhibition of thrombin formation during fibrinolysis, using factor Xa inhibitor from the tick (Tick anticoagulant peptide [TAP]), also shortened lysis time from 150 to 30 minutes at saturating concentrations (>2 μmol/L; Fig 3B). Inclusion of a saturating concentration of PTI did not appreciably shorten lysis time further. These results are consistent with the requirement for the generation of high concentrations of thrombin during fibrinolysis to activate rabbit TAFI. Furthermore, because PTI is unable to shorten lysis time any further than TAP alone, the effect of PTI on lysis time is solely mediated through the inhibition of TAFI. Therefore, rabbit plasma contains an inhibitor of fibrinolysis that requires activation by thrombin, is inhibited by PTI, and whose zymogen comigrates with human TAFI and is recognized by antibodies directed at human TAFI.
Demonstration of the presence of TAFI in rabbit plasma. (A) The concentration-dependent potentiation of lysis time by the carboxypeptidase inhibitor from the potato (PTI). Lysis times were determined using a turbidometric assay as indicated in Materials and Methods. The inset represents a Western blot of purified human TAFI, human plasma, and rabbit plasma in lanes A, B, and C, respectively. (B) The concentration-dependent potentiation of lysis time by the factor Xa inhibitor, TAP. Clots were formed as indicated in Materials and Methods, but with 294 pmol/L tPA. The effect of various concentrations of TAP (0 to 10 μmol/L) on lysis time was determined for clots produced in the absence (○) or presence (•) of 5 μg/mL PTI. Indicated values are the average from 2 experiments, in which the range did not exceed 5% of the mean. These data indicate that rabbit plasma contains TAFI, which, when activated by thrombin, is able to prolong lysis time and is inhibited by the carboxypeptidase inhibitor PTI.
Demonstration of the presence of TAFI in rabbit plasma. (A) The concentration-dependent potentiation of lysis time by the carboxypeptidase inhibitor from the potato (PTI). Lysis times were determined using a turbidometric assay as indicated in Materials and Methods. The inset represents a Western blot of purified human TAFI, human plasma, and rabbit plasma in lanes A, B, and C, respectively. (B) The concentration-dependent potentiation of lysis time by the factor Xa inhibitor, TAP. Clots were formed as indicated in Materials and Methods, but with 294 pmol/L tPA. The effect of various concentrations of TAP (0 to 10 μmol/L) on lysis time was determined for clots produced in the absence (○) or presence (•) of 5 μg/mL PTI. Indicated values are the average from 2 experiments, in which the range did not exceed 5% of the mean. These data indicate that rabbit plasma contains TAFI, which, when activated by thrombin, is able to prolong lysis time and is inhibited by the carboxypeptidase inhibitor PTI.
Pharmacokinetics of PTI
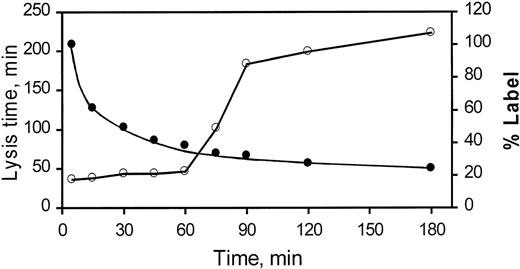
Because PTI inhibits TAFIa-dependent prolongation of lysis of clots formed from rabbit plasma in vitro, we wished to determine whether PTI potentiates fibrinolysis in vivo. As a first step, the pharmacokinetics of PTI were determined both by monitoring I125-labeled PTI (I125-PTI) and by assessing function through the use of an ex vivo clot lysis assay. The half-life of both the labeled I125-PTI and the functional presence of infused PTI is approximately 25 minutes (Fig 4). High counts, associated with I125-PTI, were measured in urine after infusion, indicating that clearance may be mediated by the kidney. Because 0.5 mg of PTI was infused into the rabbits, the calculated initial plasma concentration of PTI is 4.9 μg/mL. After 3 half-lives (75 minutes), the expected concentration of 0.6 μg/mL is near, but exceeds, the concentration of PTI needed to maximally inhibit TAFI-dependent prolongation of lysis time (Fig 3A). Therefore, PTI would be present and able to completely inhibit TAFI-dependent prolongation of lysis time for the duration of 60 minutes of treatment in an experiment in vivo. This is supported by the functional data, shown in Fig 4, in which lysis time was observed to become prolonged only after 60 minutes subsequent to the IV injection of PTI.
Pharmacokinetics of PTI. One milliliter of PTI (0.5 mg/mL containing 1 × 106 cpm/mL 125I-PTI) was injected IV into each of 3 conscious rabbits. At the indicated times, blood was obtained and plasma was produced by centrifugation. The presence of PTI in each plasma sample was assessed by determination of lysis time (○) and the results are plotted as a function of time. The amount of 125I-PTI (•) in each plasma sample was also assessed and is similarly plotted. Indicated values are the mean of 3 experiments, in duplicate, in which the SEM did not exceed 8% for lysis times and 3% for radioactivity. The data indicate that the PTI concentration maximally potentiates fibrinolysis ex vivo for up to 60 minutes after its administration to the rabbit and exhibits a half-life of approximately 25 minutes.
Pharmacokinetics of PTI. One milliliter of PTI (0.5 mg/mL containing 1 × 106 cpm/mL 125I-PTI) was injected IV into each of 3 conscious rabbits. At the indicated times, blood was obtained and plasma was produced by centrifugation. The presence of PTI in each plasma sample was assessed by determination of lysis time (○) and the results are plotted as a function of time. The amount of 125I-PTI (•) in each plasma sample was also assessed and is similarly plotted. Indicated values are the mean of 3 experiments, in duplicate, in which the SEM did not exceed 8% for lysis times and 3% for radioactivity. The data indicate that the PTI concentration maximally potentiates fibrinolysis ex vivo for up to 60 minutes after its administration to the rabbit and exhibits a half-life of approximately 25 minutes.
Ex Vivo Fibrinolysis
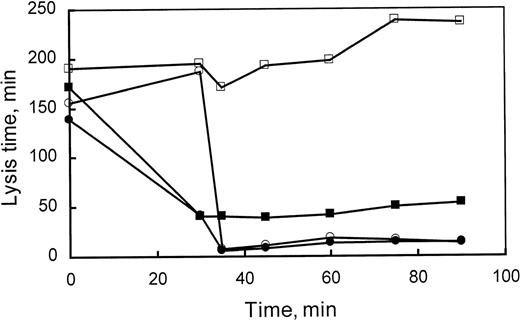
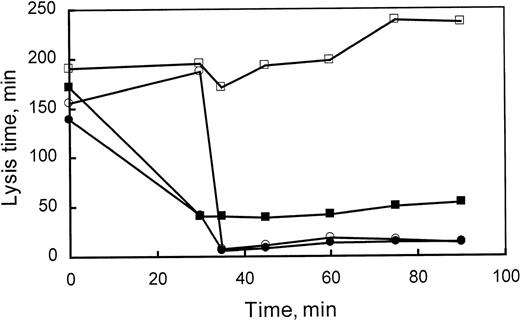
Compliance with the treatment protocol from experiments performed in vivo was assessed using an ex vivo clot lysis assay. As indicated in Materials and Methods, a turbidometric fibrinolysis assay was used to demonstrate the presence of both PTI and tPA. Plasma clots were formed in duplicate, both in the presence and absence of exogenously added tPA to the wells of the microtiter plate, and lysis times were determined (Fig 5). In the presence of exogenously added tPA, clots from the plasma of rabbits receiving PTI alone reduced the average lysis time from 160 to 30 minutes. Furthermore, PTI activity was observed for the duration of the experiment, because the ex vivo rates of fibrinolysis were still enhanced significantly (160 to ≤50 minutes) at the final time point. Infusion of tPA reduced ex vivo lysis time to approximately 10 minutes and was apparently unaffected by coadministration of PTI.
Compliance with treatment. Ex vivo clot lysis time was used to assess the presence of both PTI and tPA administered to rabbits. Lysis times were determined in duplicate for 6 rabbits treated with Sal/Sal (□), 5 rabbits treated with Sal/PTI (▪), 4 rabbits treated with tPA/Sal (○), and 4 rabbits treated with tPA/PTI (•). Lysis time is reduced to approximately 40 minutes after the administration of PTI to the rabbit at 30 minutes. Subsequent administration of tPA, at 35 minutes, induces a reduction in lysis time to approximately 10 minutes. Each point reflects either 8, 10, or 12 individual measurements, in which the standard error of the mean is less than 10% of the indicated lysis time.
Compliance with treatment. Ex vivo clot lysis time was used to assess the presence of both PTI and tPA administered to rabbits. Lysis times were determined in duplicate for 6 rabbits treated with Sal/Sal (□), 5 rabbits treated with Sal/PTI (▪), 4 rabbits treated with tPA/Sal (○), and 4 rabbits treated with tPA/PTI (•). Lysis time is reduced to approximately 40 minutes after the administration of PTI to the rabbit at 30 minutes. Subsequent administration of tPA, at 35 minutes, induces a reduction in lysis time to approximately 10 minutes. Each point reflects either 8, 10, or 12 individual measurements, in which the standard error of the mean is less than 10% of the indicated lysis time.
In the absence of exogenous tPA, ex vivo lysis times exceeded the duration of the experiment (≥300 minutes), except when rabbits were treated with tPA either with or without PTI cotreatment. This indicates that release of significant concentrations of endogenous plasminogen activator was not stimulated by the injection of PTI, because treatment with PTI alone did not facilitate ex vivo clot lysis in the absence of exogenous tPA. If tPA was released in rabbits treated with PTI, the plasma concentration of tPA did not exceed 0.660 nmol/L, because the lysis time of clots formed from nontreated control rabbit plasma, diluted one third, was measurable (≤200 minutes) with 0.220 nmol/L tPA. When tPA was administered to the rabbit, ex vivo lysis times were approximately 10 minutes, despite the absence of exogenous tPA. The ex vivo lysis times were similar when both tPA and PTI were administered.
Efficacy of Treatment
Clot lysis, perhaps the most important endpoint, was calculated as the percentage difference between I125-Fibrin in the control and residual I125-Fibrin remaining in the thrombus after treatment. This measurement is indicative of lysis of the initial clot, where counts are proportional to initial clot mass. PTI significantly potentiated tPA-induced clot lysis, achieving 89% degradation of the initial clot (Table 1). In contrast, only 54% of the clot had lysed with tPA alone. Although tPA induced 15% greater lysis than the endogenous thrombolysis observed with Sal/Sal treatment, this value does not approach significance. Endogenous plasminogen activators appear to induce spontaneous clot lysis and account for approximately 39% clot degradation, which was only slightly elevated in the presence of PTI (44%). Release of I125-FDPs into the blood exhibited a similar trend when compared with clot lysis produced with each treatment. However, only treatment with both tPA and PTI achieved a significant elevation of the blood concentration of I125-FDPs released from the initial clot. Although quantification of released I125-FDPs is not a particularly sensitive method to demonstrate clot lysis, it appears to confirm clot lysis determinations. A second parameter, percentage change in clot weight, is composed of at least 3 components and indicates the net result of an increase in weight due to clot accretion and a decrease in clot weight due to lysis of both the accreted clot and the original radiolabeled clot. Only treatment comprising both tPA and PTI was able to reduce the endpoint clot mass compared with the original clot mass. In contrast, a 21% increase in endpoint clot mass was observed with tPA treatment alone, even though 54% of the original clot was lysed. However, tPA alone reduced the percentage change in clot weight by at least a factor of 2 when compared with either Sal/Sal (increase of 49.7%) or Sal/PTI (increase of 54.7%). Therefore, compared with tPA alone, coadministration of PTI significantly enhanced the efficacy of tPA-induced thrombolysis.
Although the calculated NCA values do not achieve significance, they appeared to be reduced when both tPA and PTI were administered. This is most likely a result of enhanced lysis of newly accreted thrombus rather than by directly affecting thrombus formation. Regardless of mechanism, the amount of I125-Fibrin clot lysis that occurred after administration of both tPA and PTI exceeded the reduction observed in clot weight. This is consistent with the conclusion that clot accretion must have occurred and accounts for 41.9% of the final clot mass. This compares with 74.5% of the final clot mass accreted when treatment was tPA alone. Interestingly, Sal/PTI did not decrease the NCA (98.3%) when compared with Sal/Sal (88.4%). Therefore, at the concentrations of tPA and PTI tested, a greater than additive effect on NCA suggests, but does not prove, a synergistic effect of coadministration.
Previously described parameters are indicative of fibrinolysis; however, they may not completely reflect resolution of the thrombus resulting in vessel patency, because they only reflect degradation of the protein matrix within the thrombus. Even a small thrombus at the site of a critical stenosis may prevent blood flow. Therefore, an effective thrombolytic must establish reasonable flow (TIMI-3 grade) through the previously occluded vessel. In addition to determining thrombolysis, therefore, we also monitored vessel patency using flow probes both proximal and distal to the occlusive thrombus. As a result, 3 further parameters were determined and include time to aortic patency, total accumulated time of aortic patency, and maximal blood flow. The time required to achieve patency was significantly shortened when rabbits were treated with both tPA and PTI. Patency was observed by 6.8 minutes with both tPA and PTI, compared with 22.9 minutes with tPA alone. Corresponding to the reduction in time to patency observed with tPA and PTI, this same treatment maintained an open vessel for 30 minutes (56% of the remaining treatment time after patency), compared with 11 minutes (29% of remaining treatment time) observed with tPA alone. Also, maximal blood flow observed with coadministration of tPA and PTI (1.9 mL/min) was 2-fold greater than that observed with tPA alone (0.9 mL/min). Because of the stenosis, the maximum attainable flow would not be expected to exceed 3 to 5 mL/min. In contrast to both Sal/Sal or Sal/PTI treatment, in which aortic patency was not achieved, treatment with tPA, with or without PTI, was able to achieve patency, although cyclic flow (patency and reocclusion) was observed. Cumulatively, these data indicate that tPA and PTI administered together are more effective than tPA alone at degrading the fibrin matrix of the platelet-rich thrombus and establishing blood flow through the aorta.
Effect of PTI on APTT, TCT, Both Fibrinogen and Antiplasmin Concentrations, and Blood Pressure
PTI alone does not affect APTT (22.5 ± 0.7 seconds), TCT (8.3 ± 0.1 seconds), fibrinogen concentration (2.45 ± 0.04 g/L), or antiplasmin concentrations (0.97 ± 0.02 U/mL) when compared with Sal/Sal treatment (23.8 ± 0.5 seconds, 8.1 ± 0.1 seconds, 2.45 ± 0.05 g/L, and 1.06 ± 0.02 U/mL, respectively), where mean values are expressed ± SEM for all 42 samples, which represents all time points for all 6 rabbits. In contrast, tPA induces an initial 50% increase in APTT at 35 minutes over that observed with Sal/Sal, which returns to baseline by 45 minutes, and an initial 500% increase in TCT, over that observed with Sal/Sal, which decreases by 45 minutes to a sustained 300% increase for the remainder of the experiment. Administration of both PTI and tPA did not exacerbate the pronounced prolongation of either APTT or TCT induced by tPA. Both tPA and tPA with PTI significantly decreased the concentrations of both fibrinogen and antiplasmin to a similar extent (∼80% of initial concentration), which indicates that PTI does not potentiate tPA-induced consumption of these components. Furthermore, mean blood pressure (∼46 mmHg), measured in the carotid artery, was not affected during the full 90 minutes of the experiment for any treatment group. These data, although limited, indicate that an inhibitor of TAFIa, such as PTI, does not affect either the production of thrombin or the actions of thrombin to form a clot or to induce any significant potentiation of tPA-induced fibrinogenolysis.
DISCUSSION
In our study, we compared the efficacy of tPA-induced lysis in both the absence and presence of an inhibitor of TAFIa in a rabbit arterial thrombolysis model. We attempt to assess the clinical relevance of a TAFIa inhibitor on thrombolysis and indirectly establish the (patho)physiological role of TAFIa in this process. To date, TAFI has been isolated only from human plasma.20,22 However, TAFI has been inferred to be present in the plasma of other mammalian species.44-46 Before initiation of in vivo experimentation using a rabbit model, we confirmed the presence of TAFI in rabbit plasma using an antibody raised against human TAFI and Western blotting (Fig 3). The presence of TAFI was also demonstrated functionally in 3 ways. First, TAP (an inhibitor of factor Xa that inhibits prothrombin activation47 48) enhances lysis time, demonstrating that thrombin production during fibrinolysis prolongs lysis time (Fig 3), providing evidence for the existence of an inhibitor of fibrinolysis that was activated by thrombin. Second, PTI shortened lysis time in ex vivo fibrinolysis assay, but only when thrombin is produced during fibrinolysis (Fig 3). Thus, rabbit TAFI is similar in these respects to human TAFI.
From these in vitro results, we reasoned that PTI could be used as a TAFI inhibitor in potentiation of thrombolysis in vivo. Pharmacokinetics of IV bolus injection of PTI in conscious rabbits did not induce any visible side effects, such as behavioral change, cardiorespiratory change, or anaphylaxis. Because PTI was functionally present for at least 60 minutes (Fig 4), we limited the duration of thrombolytic therapy to 60 minutes. PTI significantly improved tPA-induced arterial thrombolysis in rabbits (Table 1). This is reflected by a significant increase in original clot lysis (1.7-fold), a decrease in the percentage of upward change in clot weight (3.2-fold), a decrease in the time to aortic patency (3.4-fold), an increased duration of patency (2.7-fold), and an increase in postlysis blood flow (2.1-fold). However, PTI alone did not appear to significantly affect endogenous thrombolysis in this model. This may be due to a lack of sensitivity in this model, because others have shown potentiation of endogenous thrombolysis in a jugular vein thrombosis model in rabbits10 potentially indicating differences between venous versus arterial thrombi. With regard to safety, no measurable effect of PTI on blood pressure or on systemic concentrations of both fibrinogen and α-2-antiplasmin, and the coagulation parameters, APTT and TCT, in the absence of tPA was observed. Furthermore, PTI did not exacerbate effects of tPA on these parameters. Although blood pressure, fibrinogen concentrations, and blood coagulation were not affected by PTI, further work is required to unequivocally establish PTI safety.
Our results indicate that an inhibitor of TAFIa, delivered systemically, is capable of potentiating tPA-induced lysis of a platelet-rich arterial thrombus. These results are in accordance with the observations of others10,19; however, significant differences exist between the experimental procedures previously published and those reported here. Minnema et al10demonstrated that incorporation of PTI in a radioactive clot, created in the isolated segment of the jugular vein, potentiated endogenous thrombolysis, as assessed by loss of 125I-human fibrin from the clot. We observed a similar profibrinolytic effect in an arterial thrombolysis model using a systemic injection of PTI alone, but it was not significant. However, tPA-induced thrombolysis was significantly potentiated with PTI in our model. This is in counter-distinction to the results of Colucci et al,49 which did not demonstrate the ability of TAFI to participate in the regulation of fibrinolysis using “pharmacologically relevant” concentrations of tPA in vitro. Refino et al19 also demonstrate an effect of PTI on tPA-induced thrombolysis arterial-venous shunt model in rabbits. Our results are in agreement with those of Refino et al,19despite differences between their model, in which nonocclusive thrombi were induced in an A-V shunt, and our model, in which occlusive thrombus produced in a damaged artery. However, Refino et al19 did not evaluate the effect of PTI alone in their model. Unlike both Minnema et al10 and Refino et al,19 we do not report on the effect of incorporation of PTI into the thrombus. Furthermore, neither of these previous investigators identified the presence of TAFI in rabbit plasma nor assessed and confirmed the function of PTI in plasma samples obtained during treatment.
Despite the positive results obtained with a single-dose regime, optimization of both tPA and PTI concentrations may lead to further improvements. Furthermore, as a representative drug for adjunctive thrombolytic therapy, PTI may be used to investigate the potential for early prophylactic administration in patients suspected of thromboembolic complications. In these instances, newly accreted clot occurring during the time interval between diagnosis and treatment would comprise PTI and, therefore, may be more susceptible to tPA-induced thrombolysis. Additionally, PTI may also enhance spontaneous thrombolysis, as suggested by others.10 19 Of course, in view of our data, this would be feasible only if a higher concentration of PTI was able to significantly enhance endogenous arterial thrombolysis. Because PTI is a peptide comprising 39 amino acids, antigenicity is a considerable concern. At this point, therefore, PTI may only be regarded as a representative inhibitor of TAFIa that could be used to assess the viability and impact of inhibiting carboxypeptidases, including TAFIa, during thrombolytic therapy. Inhibition of carboxypeptidases such as TAFIa may potentiate thrombolytic therapy without the risk associated with other adjuvants that inhibit thrombin or its production. Work currently in progress includes both the optimization of dose regime as well as the study of safety issues. We anticipate that adjunctive therapy comprising inhibition of TAFIa could achieve superior thrombolytic efficacy compared with that achieved by current tPA therapy used in patients with acute myocardial infarction.
ACKNOWLEDGMENT
The authors thank Dr G. Klement for statistical analyses, Dr S. Krisnaswamy for kindly providing rTAP, Dr M.E. Nesheim for providing human thrombin, and Vicky Miller for technical assistance.
Supported by Grants No. NA-3568 and T-3644 from the Heart and Stroke Foundation of Ontario. L.B. is a Research Scholar of the Heart and Stroke Foundation of Canada.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Laszlo Bajzar, PhD, McMaster University and the Hamilton Civic Hospitals Research Centre, 711 Concession St, Hamilton, Ontario, Canada L8V 1C3; e-mail:lbajzar@thrombosis.hhscr.org.