Abstract
The common β chain (βc) of the granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-3 (IL-3), and IL-5 receptors is the major signaling subunit of these receptors coupling ligand binding to multiple biological activities. It is thought that these multiple functions arise as a consequence of the recruitment of specific signaling molecules to tyrosine-phosphorylated residues in the cytoplasmic domain of βc. However, the contribution of serine phosphorylation in βc to the recruitment of signaling molecules is not known. We show here the identification of a phosphoserine motif in the cytoplasmic domain of βc that interacts with the adaptor protein 14-3-3ζ. Coimmunoprecipitation and pull-down experiments with a glutathione S-transferase (GST):14-3-3ζ fusion protein showed that 14-3-3 directly associates with βc but not the GM-CSF receptor chain. C-terminal truncation mutants of βcfurther showed that a region between amino acids 544 and 626 in βc was required for its association with 14-3-3ζ. This region contains the sequence 582HSRSLP587, which closely resembles the RSXSXP (where S is phosphorylated) consensus 14-3-3 binding site identified in a number of signaling molecules, including Raf-1. Significantly, substitution of582HSRSLP587 for EFAAAA completely abolished interaction of βc with GST–14-3-3ζ. Furthermore, the interaction of βc with GST–14-3-3 was greatly reduced in the presence of a peptide containing the 14-3-3 binding site, but only when 585Ser was phosphorylated. Direct binding experiments showed that the peptide containing phosphorylated 585Ser bound 14-3-3ζ with an affinity of 150 nmol/L. To study the regulation of 585S phosphorylation in vivo, we raised antibodies that specifically recognized 585Ser-phosphorylated βc. Using these antibodies, we showed that GM-CSF stimulation strongly upregulated 585Ser phosphorylation in M1 myeloid leukemic cells. The proximity of the SHC-binding site (577Tyr) to the 14-3-3–binding site (582HSRSLP587) and their conservation between mouse, rat, and human βc but not in other cytokine receptors suggest that they form a distinct motif that may subserve specialized functions associated with the GM-CSF, IL-3, and IL-5 receptors.
HUMAN granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-3 (IL-3), and IL-5 are pleiotropic cytokines capable of stimulating normal and transformed hematopoietic cells.1-4 With each, the initiating event for signal transduction is the binding of GM-CSF, IL-3, and IL-5 to their surface receptors that are composed of a cytokine-specific α chain and a common β chain (βc).5-7 Engagement of βc by the binding of GM-CSF, IL-3, and IL-5 to surface receptors results in the stimulation of cell survival, proliferation, and differentiation and mature cell effector function in the appropriate lineage, a fact that emphasises the major signaling role played by βc in mediating GM-CSF, IL-3, and IL-5 biological activities.8
One of the first events in GM-CSF, IL-3, and IL-5 activation of their receptors and in the initiation of the signaling cascade is tyrosine phosphorylation of βc.9 This is a common theme among cytokine receptor signaling subunits and can be seen in homodimeric receptors such as the erythropoietin (EPO) receptor, thrombopoietin (TPO) receptor, and granulocyte colony-stimulating factor (G-CSF) receptor as well as in heterodimeric receptors such as in the IL-6 and IL-2 receptors.10-13 In the latter case, as in the GM-CSF, IL-3, and IL-5 receptor system, tyrosine phosphorylation is also restricted to its signaling subunit.14
Tyrosine phosphorylation of cytokine receptor signaling subunits appears to be a critical step in the creation of docking sites for the association of signaling molecules. For example, ligand-induced tyrosine phosphorylation of βc is considered critical for binding of SH2-containing signaling molecules such as STATs, SHP1, SHP2, and SHC. In the case of STAT5, this is recruited to phosphotyrosines in βc and is then phosphorylated by JAK2, resulting in STAT activation, dimerization, and translocation to the nucleus, where it directly regulates gene transcription.15,16 Previously, it has been shown that 6 of the 8 tyrosine residues of βc allow the docking of STAT5 and its subsequent activation, indicating a high degree of redundancy as to which tyrosine residues are critical for STAT5 activation.17 The specific phospho-tyrosine residue responsible for recruitment of SHC to βc is577Tyr. The bound SHC recruits grb2 into the complex and grb2 binds sos, which then activates p21ras nucleotide exchange. This leads to the activation of ras and downstream partners of the mitogen-activated protein (MAP) Kinase pathway, including Raf.17-20 SHP2, a prominent tyrosine phospho-protein, is able to bind 3 tyrosine residues of the activated βc (577Tyr, 612Tyr, and695Tyr), although only 577Tyr and612Tyr allow SHP2 to interact with grb2.17 21
Despite the well-documented importance of tyrosine phosphorylation of cytokine receptors, it is becoming apparent that signaling can proceed in its absence, suggesting that other mechanisms may be at work. This is demonstrated in the EPO and TPO receptors, in which the substitution of all tyrosines failed to abolish their activities.22,23In the case of βc, mutation of all 8 cytoplasmic tyrosine residues does not abolish activation of JAK2 and induction of tyrosine phosphorylation of STATs and many other cellular proteins. The only significant defect in this mutation is the reduction in phosphorylation of SHP2 and SHC.17 In the case of mutant mice that have STAT5a and STAT5b genes deleted, there is no hematopoietic phenotype, suggesting that other pathways may substitute for STAT5 activation.24 We and others have also shown that FDCP-1 cells transduced with a βc mutant in which all intracellular tyrosines were substituted for phenylalanines were able to proliferate in response to GM-CSF.16,17 25
We show here the identification in the common β chain of the GM-CSF, IL-3, and IL-5 receptors of a sequence responsible for its association with the adaptor protein 14-3-3ζ. Furthermore, we show that GM-CSF regulates the phosphorylation of 585Ser within the 14-3-3ζ binding sequence. Given the conservation of this region in βc of different species but not in other cytokine receptors, we suggest that it may be involved in GM-CSF, IL-3, and IL-5 receptor-specific functions.
MATERIALS AND METHODS
Mutagenesis of human βc and expression plasmid constructs.
Substitution mutations of 2 sequences within the cytoplasmic domain of the human βc cDNA were constructed using oligonucleotide-directed mutagenesis (Altered-sites; Promega, Sydney, New South Wales, Australia), as described previously.26Both mutants were essentially poly-alanine substitutions. Mutagenesis oligonucleotides encoding nonalanine residues were included to facilitate restriction enzyme screening of mutant clones. The first motif was 582HSRSLP587 mutated to582EFAAAA587, and the second was820RSKPSSP826 mutated to820EFAAAAA826. The point mutant S585A was also constructed; however, this mutant created a cryptic proteolytic site in βc and was not able to be used (see Results). The mutations were confirmed by nucleotide sequencing and the mutant βc cDNAs subcloned into the eukaryotic expression vector pcDNA1 (Invitrogen, San Diego, CA). The βcdeletion mutant cDNAs were a kind gift of Dr A. Miyajima (University of Tokyo, Tokyo, Japan). The 14-3-3 zeta clone was isolated from a human monocyte lambda gt11 cDNA library (Clontech Laboratories, Palo Alto, CA) and subcloned into a pGEX-2T vector.
GM-CSF and IL-3.
Recombinant human IL-3 and GM-CSF were produced in Escherichia coli essentially as described before.27,28 Cytokine purity and quantitation was determined by high-performance liquid chromatography (HPLC) analysis and Coomasie staining of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)–separated proteins. The activity of the cytokines based on the ED50 values in a TF-1 proliferation assay29was 0.03 ng/mL for GM-CSF and 0.1 ng/mL for IL-3.
Antibodies.
The monoclonal antibodies (MoAbs) 8E4 and 1C1 directed against the βc were generated as previously described.30The anti–phospho-585Serβc antibody was raised by immunizing New Zealand White rabbits with the phosphorylated CLGPPHSRSLPDILG peptide conjugated to keyhole limpet hemocyanin (Sigma, St Louis, MO). The antipeptide antibody was firstly affinity purified with the immunizing peptide conjugated to sepharose and then absorbed with the nonphosphorylated CLGPPHSRSLPDILG peptide conjugated to sepharose. The specificity of the anti–phospho-585Serβc antibody was verified by dot immunoblots against the CLGPPHSRSLPDILG peptide in either the nonphosphorylated or serine-phosphorylated form and a scrambled peptide LPLSGPDSHIRGPL. The corresponding phosphorylated serine in each peptide is underlined. Peptides were synthesized by Chiron Mimotopes (Melbourne, Australia). The anti–14-3-3ζ antibody was kindly provided by Dr A. Aitken (Edinburgh University, Edinburgh, UK).
Cell culture and DNA transfection.
The HEK293T cell line was maintained in RPMI-1640 supplemented with 10% vol/vol fetal calf serum (FCS). On the day before transfection, 1.4 × 106 cells were plated into 6-cm tissue culture dishes to adhere overnight. Four hours after a medium change, 6 μg of wild-type or mutated βc cDNA was added to cells in the form of a calcium phosphate precipitate,31 and the cells were placed in an incubator for 4 to 6 hours to permit the uptake of the DNA-calcium phosphate precipitate. The cells were then washed, replated, and placed in the incubator for 48 hours before cytokine treatment. M1 cell line expressing GM-CSF receptor α chain and βc wild-type was maintained in RPMI-1640 supplemented with 10% vol/vol FCS. The M1 cell line was kindly provided by Dr N. Nicola (Walter and Eliza Hall Institute of Medical Research, Australia).
Surface marker analysis by flow cytometry.
Expression of receptors on transfected cells was verified by flow cytometry. Briefly, cells were incubated with the anti-βcMoAb (1C1)32 or anti–GM-CSFRα MoAb (4H1)30for 20 minutes on ice, washed, and subsequently incubated with fluorescein isothiocyanate (FITC)-conjugated antimouse IgG antibody (Silenus Laboratories, Hawthorn, Victoria, Australia) for 20 minutes on ice. Cells were then washed and resuspended in FACS FIX and analyzed using a Profile II (Coulter Electronics, Hileah, FL).
Immunoprecipitations.
Cells were lysed in lysis buffer (150 mmol/L NaCl, 10 mmol/L Tris-HCl [pH 7.4], 1% Digitonin with protease inhibitors [10 μg/mL leupeptin, 2 mmol/L phenylmethlysulfonyl fluoride, and 10 μg/mL aprotinin], and 2 mmol/L sodium vanadate) for 30 minutes at 4°C followed by centrifugation of the lysate for 15 minutes at 12,000g at 4°C. After 1 hour of preclearance with Protein-A-sepharose (Pierce, Rockford, IL) at 4°C, the supernatant was incubated for 2 hours with 5 μg/mL antibody. Protein-Ig complexes were captured by incubation for 1 hour with Protein-A-sepharose followed by 6 subsequent washes in lysis buffer. Samples were boiled for 5 minutes in SDS sample load buffer in the presence or absence of 2-mercaptoethanol before separating immunoprecipitated proteins by SDS-PAGE.
Precipitations.
Cells were lysed in lysis buffer for 30 minutes at 4°C followed by centrifugation of the lysate for 15 minutes at 12,000g at 4°C. After 1 hour of preclearance with glutathione S-transferase (GST)-sepharose at 4°C, the supernatant was incubated for 2 hours with GST–14-3-3-sepharose followed by 3 subsequent washes in lysis buffer. Samples were boiled for 5 minutes in SDS sample load buffer in the presence of 2-mercaptoethanol before separating precipitated proteins by SDS-PAGE.
Competition of precipitations and immunoprecipitations by peptides.
Cell lysates were precipitated or immunoprecipitated in the presence of various concentrations of the following peptides: βcpeptide sequence CLGPPHSRSLPDILG in either the nonphosphorylated or serine-phosphorylated form and a scrambled peptide CLPLSGPDSHIRGPL. Raf1 peptides corresponding to the sequence CLSQRQRSTSTPNVHM were also used and were either nonphosphorylated or serine-phosphorylated. The corresponding phosphorylated serine in each peptide is underlined. Peptides were synthesized by Chiron Mimotopes. The presence of βc in either the immunoprecipitation or precipitation experiment was determined by Western blotting with anti-βc antibody (MoAb 1C1).
SDS-PAGE, immunoblot, and enhanced chemiluminescence (ECL).
Immunoprecipitated proteins separated by SDS-PAGE were transferred to nitrocellulose membrane by electroblotting. Dot blots of peptides were performed by spotting either 10 or 100 ng of each peptide onto nitrocellulose membranes. Routinely, nitrocellulose membranes were blocked in a solution of phosphate-buffered saline (PBS)/0.05% (vol/vol) Tween 20 (PBT) containing 1% (wt/vol) blocking reagent 1096 176 (Boehringer Mannheim, Mannheim, Germany) and probed with anti-βc (1C1), anti–14-3-3ζ,33 or anti–phospho-585Serβc, followed by either anti-mouse or anti-rabbit peroxidase-conjugated antibodies. Immunoreactive proteins were detected by chemiluminescence using the ECL kit (Amersham, Little Chalfont, UK) following the manufacturer’s instructions.
Binding of the 125I-labeled 14-3-3ζ to synthetic peptides.
The recombinant 14-3-3ζ protein was 125I-labeled using IODOBEADS (Pierce). The synthetic peptides were solubilized in distilled water and then diluted to 50 μg/mL in 0.1 mol/L NaHCO3, pH 9.2. The peptides were coated onto microtiter wells (Immunolon II Removawells; Dynatech Laboratories, Chantilly, VA) by incubation at 22°C for 6 hours and then at 4°C overnight. The peptide-coated microtiter wells were blocked at 22°C with 5% bovine serum albumin for 2 hours and then with125I-labeled 14-3-3ζ protein for 2 hours. After 3 washes, microtiter well-bound radioactivity were estimated in a γ-counter.
RESULTS
Association of βc with 14-3-3ζ.
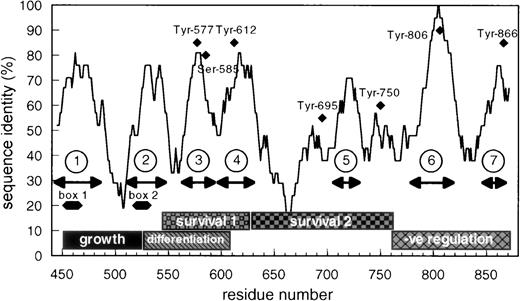
In examining the cytoplasmic region of βc, we identified a small region encompassing amino acids 566 to 589 that exhibits unusually high sequence identity (72%) between mouse and human βc when compared with the overall identity of the cytoplasmic domain of βc (55% identity; Fig 1). Previous studies have implicated this region as being important for cell survival and differentiation.34 Closer examination of this region showed, in addition to Tyr577 (binding site for SHC), a possible 14-3-3 binding motif, 582HSRSLP587that is conserved in βc from different species. This sequence closely resembles the prototypic 14-3-3 binding consensus (RSXSXP) identified in Raf-1 (where S is phosphorylated) and in other proteins involved in signal transduction (Fig 2).
Schematic representation of the cytoplasmic domain of βc showing the overall sequence identity between human and mouse βc and the seventh region of highest identity. The positions of box-1 and box-2 are shown as well as the functional role ascribed to different parts of human βc.34 The position of the conserved tyrosine residues and of 585Ser is also shown.
Schematic representation of the cytoplasmic domain of βc showing the overall sequence identity between human and mouse βc and the seventh region of highest identity. The positions of box-1 and box-2 are shown as well as the functional role ascribed to different parts of human βc.34 The position of the conserved tyrosine residues and of 585Ser is also shown.
Comparison of sequences in signaling molecules known to bind 14-3-3ζ and the derived consensus 14-3-3 binding motif. The sequences in human, mouse, and rat β chains similar to the consensus are also shown.
Comparison of sequences in signaling molecules known to bind 14-3-3ζ and the derived consensus 14-3-3 binding motif. The sequences in human, mouse, and rat β chains similar to the consensus are also shown.
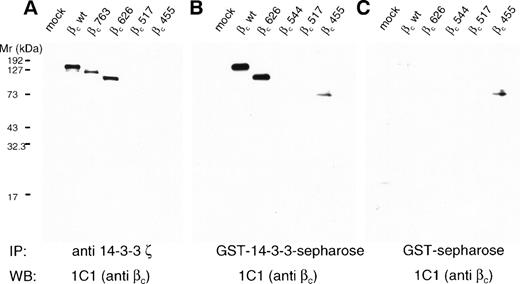
To test the possibility that human βc was able to associate with 14-3-3 and to determine the region of interaction, we transfected HEK 293T cells with wild-type βc and a series of C-terminal truncation mutants and examined the ability of βc to associate with 14-3-3 by coimmunoprecipitation. Whereas wild-type βc and C-terminal truncation mutants up to amino acid 626 coimmunoprecipitated with 14-3-3, further truncations resulted in no detectable association of βc and 14-3-3 (Fig 3A). Similar results were obtained in GST–14-3-3 pull-down experiments. When compared with GST controls, GST–14-3-3 interacted with wild-type βc and C-terminal truncation mutants up to amino acid 626. However, additional truncations resulted in a loss of detectable association of βc and GST–14-3-3 (Fig 3). These experiments indicate that (1) βc interacts with 14-3-3 and (2) that the region of interaction lies between amino acids 544 and 626.
Human βc associates with 14-3-3ζ, and this association is mediated by the 544-626 region of βc. HEK 293T cells were either mock-transfected (mock) or transfected with wild-type βc (wt) or βc containing C-terminal deletions. Lysates were prepared from transfected cells and either immunoprecipitated with anti–14-3-3ζ antibody (A) or precipitated with either 14-3-3–GST sepharose (B) or GST-sepharose (C). All proteins were separated on 7.5% SDS-PAGE under reducing conditions before Western blotting with anti-βc antibody (MoAb 1C1).
Human βc associates with 14-3-3ζ, and this association is mediated by the 544-626 region of βc. HEK 293T cells were either mock-transfected (mock) or transfected with wild-type βc (wt) or βc containing C-terminal deletions. Lysates were prepared from transfected cells and either immunoprecipitated with anti–14-3-3ζ antibody (A) or precipitated with either 14-3-3–GST sepharose (B) or GST-sepharose (C). All proteins were separated on 7.5% SDS-PAGE under reducing conditions before Western blotting with anti-βc antibody (MoAb 1C1).
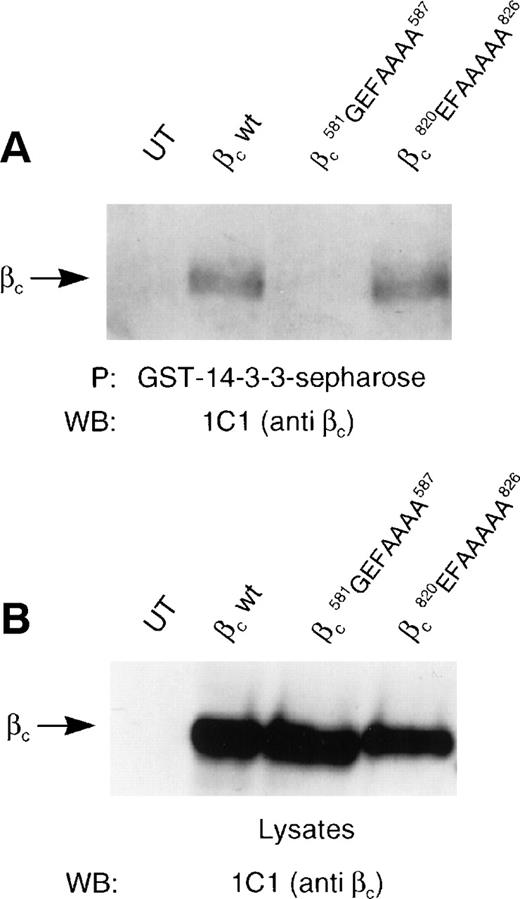
We then examined whether 14-3-3 interacted with βc via the 582HSRSLP587 motif that lies within the 544-626 region identified in Fig 3. A substitution mutant (βc-582HSRSLP587→EFAAAA) and a point mutant (βc-585S→A) in the putative 14-3-3 binding site were constructed as well as a control mutant (βc820RSKPSSP826→EFAAAAA). These mutants were expressed in HEK 293T cells and examined for their ability to interact with GST–14-3-3 in pull-down experiments. Whereas wild-type βc and the control mutant βc interacted with GST–14-3-3, no detectable interaction was observed for the βc582HSRSLP587→EFAAAA mutant (Fig 4). These results indicate that 14-3-3 associates with βc via the582HSRSLP585 sequence. Experiments examining the association of βc-585S→A point mutant with GST–14-3-3 were not possible, because it is likely that this mutation introduced a cryptic proteolysis cleavage site. Flow cytometry and Western blot analysis indicated that this mutant was proteolysed and failed to be expressed on the cell surface (data not shown).
14-3-3ζ specifically binds the HSRSLP motif of the βc cytoplasmic domain. (A) HEK 293 cells were used either untransfected (UT) or transfected with wild-type βc (wt), with βc containing the sequence581PHSRSLP587 mutated to581GEFAAAA587, or with βccontaining the sequence 820RSKPSSP826 mutated to 820EFAAAAA826. Lysates were made and immunoprecipitations were performed using GST–14-3-3-sepharose. The presence of βc was determined by Western blotting with an anti-βc antibody (MoAb 1C1). (B) The level of expression βc in the lysates was determined by Western blotting with an anti-βc antibody (MoAb 1C1).
14-3-3ζ specifically binds the HSRSLP motif of the βc cytoplasmic domain. (A) HEK 293 cells were used either untransfected (UT) or transfected with wild-type βc (wt), with βc containing the sequence581PHSRSLP587 mutated to581GEFAAAA587, or with βccontaining the sequence 820RSKPSSP826 mutated to 820EFAAAAA826. Lysates were made and immunoprecipitations were performed using GST–14-3-3-sepharose. The presence of βc was determined by Western blotting with an anti-βc antibody (MoAb 1C1). (B) The level of expression βc in the lysates was determined by Western blotting with an anti-βc antibody (MoAb 1C1).
Role of 585Ser in βc interaction with 14-3-3.
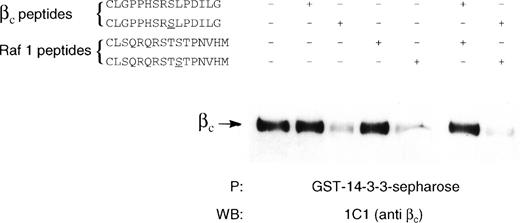
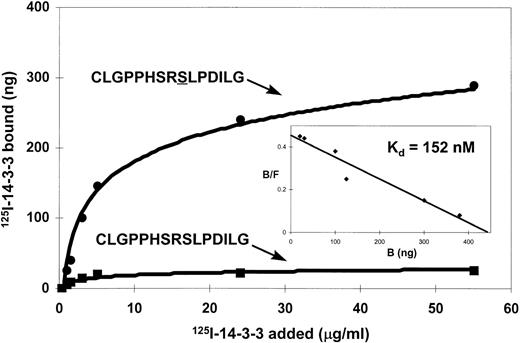
14-3-3 is known to be a phospho-serine binding protein that interacts with the RSXSXP motif, where S is phosphorylated. It would then be expected in the case of βc that585Ser phosphorylation within the582HSRSLP587 motif would be required for 14-3-3 association. The inability to express full-length βc-585S→A precluded using this mutant in either coimmunoprecipitation or pull-down experiments to examine the requirement of βc585Ser phosphorylation for 14-3-3 interaction. As an alternative, we synthesized a βc peptide containing a nonphosphorylated585Ser (C578LGPPHSRSLPDILG591) and a βc peptide containing a phosphorylated585Ser and examined their ability to inhibit βc interaction with GST–14-3-3 in a pull-down experiment. Whereas the peptide containing phosphorylated585Ser inhibited βc association with GST–14-3-3, no inhibition of association was observed for the peptide containing the nonphosphorylated 585Ser (Fig 5). As a comparison, nonphosphorylated and phosphorylated peptides corresponding to the 14-3-3 binding site in Raf-1 were also tested. We found that the serine-phosphorylated Raf-1 peptide was also able to inhibit βc association with 14-3-3, whereas the nonphosphorylated peptide did not (Fig 5). Furthermore, the ability of the βc-phosphorylated peptide to inhibit the association of βc with 14-3-3 was dose-dependent and specific as another phosphorylated peptide with sequence corresponding to a different region of βc failed to inhibit this association (Fig 6). Direct binding experiments and Scatchard analysis demonstrated that the phosphorylated peptide (C578LGPPHSRSLPDILG591) bound to 14-3-3 with an affinity of approximately 150 nmol/L (Fig 7). This affinity is comparable to the affinities reported by other investigators (55 to 190 nmol/L)35 36 for 14-3-3 binding. Together, these experiments show that the 582HSRSLP587 sequence in βc directly binds to 14-3-3 and that this binding is dependent on 585Ser being phosphorylated.
Inhibition of βc association with 14-3-3ζ by phosphorylated but not by unphosphorylated βc and raf-1 peptides. Lysates of HEK293T cells transfected with βc were immunoprecipitated with GST–14-3-3-sepharose in the absence or in the presence of chemically synthesized peptides (100 μmol/L) containing a βc sequence (CLGPPHSRSLPDILG) or a Raf-1 sequence (CLSQRQRSTSTPNVHM). In the phosphorylated peptides, the relevant phosphorylated serine is underlined. The experiment was performed on 7.5% SDS-PAGE under reducing conditions. The presence of βc in the precipitation experiment was determined by Western blotting with anti-βc antibody (MoAb 1C1).
Inhibition of βc association with 14-3-3ζ by phosphorylated but not by unphosphorylated βc and raf-1 peptides. Lysates of HEK293T cells transfected with βc were immunoprecipitated with GST–14-3-3-sepharose in the absence or in the presence of chemically synthesized peptides (100 μmol/L) containing a βc sequence (CLGPPHSRSLPDILG) or a Raf-1 sequence (CLSQRQRSTSTPNVHM). In the phosphorylated peptides, the relevant phosphorylated serine is underlined. The experiment was performed on 7.5% SDS-PAGE under reducing conditions. The presence of βc in the precipitation experiment was determined by Western blotting with anti-βc antibody (MoAb 1C1).
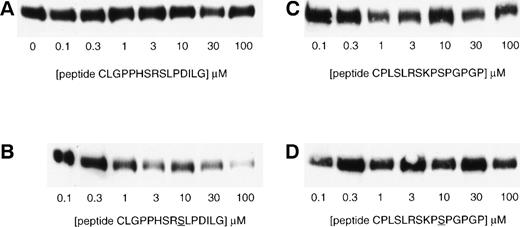
Specific inhibition of βc association with 14-3-3ζ by a phosphorylated peptide encompassing the 579-592 region of βc. Lysates of HEK293T cells transfected with βc were precipitated with GST–14-3-3-sepharose in the absence or in the presence of various concentrations of chemically synthesized peptides. Two βc peptide sequences were used, CLGPPHSRSLPDILG in either the nonphosphorylated (A) or serine585-phosphorylated (B) form and CPLSLRSKPSPGPGP in either the nonphosphorylated (C) or serine-phosphorylated (D) form. The appropriate phosphorylated serine in each peptide is underlined. The experiment was performed on 7.5% SDS-PAGE under reducing conditions. The presence of βc in the immunoprecipitates was determined by Western blotting with anti-βc antibody (MoAb 1C1).
Specific inhibition of βc association with 14-3-3ζ by a phosphorylated peptide encompassing the 579-592 region of βc. Lysates of HEK293T cells transfected with βc were precipitated with GST–14-3-3-sepharose in the absence or in the presence of various concentrations of chemically synthesized peptides. Two βc peptide sequences were used, CLGPPHSRSLPDILG in either the nonphosphorylated (A) or serine585-phosphorylated (B) form and CPLSLRSKPSPGPGP in either the nonphosphorylated (C) or serine-phosphorylated (D) form. The appropriate phosphorylated serine in each peptide is underlined. The experiment was performed on 7.5% SDS-PAGE under reducing conditions. The presence of βc in the immunoprecipitates was determined by Western blotting with anti-βc antibody (MoAb 1C1).
Binding of 125I-labeled 14-3-3ζ to synthetic peptides corresponding to the 14-3-3 binding region of βc. Microtiter wells were coated with synthetic peptides CLGPPHSRSLPDILG either nonphosphorylated or phosphorylated on the second serine (underlined). Various concentrations of125I-labeled recombinant 14-3-3ζ protein were added to microtiter wells and incubated at 22°C for 2 hours. (Insert) Scatchard analyses of 14-3-3 interaction with the serine-phosphorylated peptide.
Binding of 125I-labeled 14-3-3ζ to synthetic peptides corresponding to the 14-3-3 binding region of βc. Microtiter wells were coated with synthetic peptides CLGPPHSRSLPDILG either nonphosphorylated or phosphorylated on the second serine (underlined). Various concentrations of125I-labeled recombinant 14-3-3ζ protein were added to microtiter wells and incubated at 22°C for 2 hours. (Insert) Scatchard analyses of 14-3-3 interaction with the serine-phosphorylated peptide.
In vivo regulation of 585Ser phosphorylation.
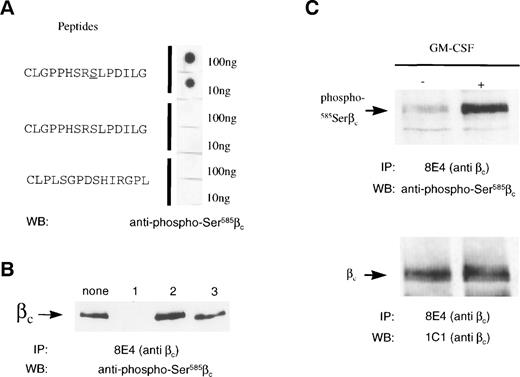
Having identified the requirement for βc585Ser to be phosphorylated to allow 14-3-3 binding, we then examined whether 585Ser was phosphorylated in vivo and whether its phosphorylation was regulated by GM-CSF. These possibilities were initially addressed using32P-orthophosphate–labeled HEK 293T cells transfected with the GM-CSF receptor (α and βc subunits). These cells were stimulated with GM-CSF, and total βc was immunoprecipitated and examined for 32P-labeling. No detectable change in βc serine/threonine phosphorylation in response to GM-CSF stimulation was observed (data not shown). This is most likely due to the presence of 60 serine and threonine residues in the intracellular region of βc, some of which may be constitutively phosphorylated. To directly address the phosphorylation status of βc585Ser in vivo, we raised a rabbit antiserum against a peptide containing the 14-3-3 binding site identified in βc: C578LGPPHSRSLPDILG591. This antibody preparation, termed anti–phospho-585Ser, specifically recognized the CLGPPHSRSLPDILG peptide containing a phosphorylated 585Ser but not a peptide containing a nonphosphorylated 585Ser or a peptide containing a scrambled 14-3-3 binding site (Fig 8). The specificity of the anti–phospho-585Ser antibody was further confirmed by Western blotting of immunoprecipitated βc from GM-CSF receptor-transfected HEK 293T cells. In these experiments, the phosphorylated CLPPHSRSLPDILG peptide was able to inhibit anti–phospho-585Ser recognition of βc, whereas the nonphosphorylated and scrambled peptides did not (Fig 8B). In addition, pretreatment of βcimmunoprecipitates with calf intestinal phosphatase before Western blot analysis completely abolished the anti–phospho-585Ser signal, and immunoprecipitation of either the wild-type βc or the582HSRSLP587→EFAAAA mutant from HEK293T transfected cells followed by Western blot analysis with the anti–phospho-585Ser antibody demonstrated that this antibody specifically recognized the wild-type but not the mutant receptor (data not shown).
585Ser in βc is phosphorylated in vivo by GM-CSF. (A) The anti–phospho-585Serβc antibody specifically recognizes the phosphorylated CLGPPHSRSLDILG peptide. Dot blots were prepared on nitrocellulose filters of either the nonphosphorylated or the serine-phosphorylated CLGPPHSRSLPDILG peptide and the scrambled peptide CLPLSGPDSHIRGPL before probing with anti–phospho-585Serβc antibody. (B) The serine-phosphorylated CLGPPHSRSLPDILG peptide specifically inhibits the binding of anti–phospho-585Serβc antibody to βc. Lysates of HEK293T cells transfected with wild-type βc were immunoprecipitated with anti-βcantibody (MoAb 8E4). Immunoprecipitates were run on 7.5% SDS-PAGE under reducing conditions. Anti–phospho-585Serβc antibody was then preincubated with either medium (none), 100-fold molar excess of the serine-phosphorylated (1) or nonphosphorylated (2) CLGPPHSRSLPDILG peptide, or the scrambled peptide CLPLSGPDSHIRGPL (3). The filters were then Western blotted and probed with the pretreated anti–phospho-585Serβc antibody. (C) Upregulation of 585Ser phosphorylation by GM-CSF. M1 cells expressing GM-CSFR and βc were either untreated (−) or stimulated with GM-CSF (2 ng/mL) for 30 seconds (+). Lysates of the M1 cells were immunoprecipitated with anti-βcantibody (MoAb 8E4) and the immunoprecipitates were run on 10% SDS-PAGE under reducing conditions. The filters were then Western blotted with either anti–phospho-585Serβcantibody or the anti-βc antibody (MoAb 1C1).
585Ser in βc is phosphorylated in vivo by GM-CSF. (A) The anti–phospho-585Serβc antibody specifically recognizes the phosphorylated CLGPPHSRSLDILG peptide. Dot blots were prepared on nitrocellulose filters of either the nonphosphorylated or the serine-phosphorylated CLGPPHSRSLPDILG peptide and the scrambled peptide CLPLSGPDSHIRGPL before probing with anti–phospho-585Serβc antibody. (B) The serine-phosphorylated CLGPPHSRSLPDILG peptide specifically inhibits the binding of anti–phospho-585Serβc antibody to βc. Lysates of HEK293T cells transfected with wild-type βc were immunoprecipitated with anti-βcantibody (MoAb 8E4). Immunoprecipitates were run on 7.5% SDS-PAGE under reducing conditions. Anti–phospho-585Serβc antibody was then preincubated with either medium (none), 100-fold molar excess of the serine-phosphorylated (1) or nonphosphorylated (2) CLGPPHSRSLPDILG peptide, or the scrambled peptide CLPLSGPDSHIRGPL (3). The filters were then Western blotted and probed with the pretreated anti–phospho-585Serβc antibody. (C) Upregulation of 585Ser phosphorylation by GM-CSF. M1 cells expressing GM-CSFR and βc were either untreated (−) or stimulated with GM-CSF (2 ng/mL) for 30 seconds (+). Lysates of the M1 cells were immunoprecipitated with anti-βcantibody (MoAb 8E4) and the immunoprecipitates were run on 10% SDS-PAGE under reducing conditions. The filters were then Western blotted with either anti–phospho-585Serβcantibody or the anti-βc antibody (MoAb 1C1).
Using these anti–phospho-585Ser specific antibodies, we then examined the regulation of βc585Ser phosphorylation after GM-CSF stimulation of MI myeloid leukemic cells. M1 myeloid leukemic cells were stimulated with 2 ng/mL GM-CSF, βc was immunoprecipitated, and immunoprecipitates were probed with the anti–phospho-585Ser antibody. As shown in Fig 8, GM-CSF stimulation strongly upregulated 585Ser phosphorylation of βc.
DISCUSSION
We show here the identification of a 14-3-3–binding motif in the common β chain of the GM-CSF/IL-3/IL-5 receptors. It is shown that this motif mediates the association of βc with 14-3-3 in vitro and in vivo and requires the phosphorylation of585Ser, but it diverges from the canonical 14-3-3 binding sequence, having an His at position 1 instead of the usual Arg. Furthermore, we obtained evidence that phosphorylation of585Ser is regulated by GM-CSF, demonstrating for the first time that not only tyrosine, but also serine phosphorylation of βc is subjected to ligand-mediated regulation. Given the proximity of the 14-3-3 binding sequence (582HSRSLP587) to the SHC-binding sequence (577Tyr), we suggest that these form a novel motif perhaps involved in certain specialized functions associated with the GM-CSF, IL-3, and IL-5 receptors.
We have demonstrated βc:14-3-3 interaction using several approaches. Firstly, we were able to coimmunoprecipitate βc with 14-3-3, indicating that these proteins interact in vivo. Secondly, we have shown in pull-down experiments that GST–14-3-3 interacts with βc. Using a series of C-terminally truncated mutants, we have localized this interaction to a region between amino acids 544 and 626. Closer examination of this region showed a possible 14-3-3–binding motif,582HSRSLP587. This sequence closely resembles the 14-3-3–binding consensus identified in Raf-1 and several other signaling molecules (582RSXSXP587, where S is phosphorylated). Thus, phosphorylation of Ser585 within 582HSRSLP587 was an expected prerequisite for 14-3-3 binding to βc. A substitution mutant of this motif (582EFAAAA587) abolished the interaction of βc with 14-3-3. A S585A mutant was not able to be tested for its ability to associate with 14-3-3 due to its poor expression (see Results); however, phosphopeptide competition experiments suggest that the association is dependent on Ser585phosphorylation.
Although the phosphorylation of the second serine in the HSRSLP sequence and the presence of a proline at the end are consistently found in 14-3-3 binding sequences, from the 14-3-3 crystal structure it is predicted that the proline in the docking protein produces a critical 90° bend in the chain, allowing the remainder of the protein to exit the binding cleft.36 The presence of an His at the beginning of the sequence is unusual, because the most frequent residue found at this position is Arg (Fig 2), although other amino acids can be found and, in fact, the sequence seen in IRS-1 contains an His. It is becoming clear that there is no fixed 14-3-3 binding sequence but instead a defined hierarchy in which certain amino acids are preferred over others.36 In terms of the sequence found in βc, the affinity of GST–14-3-3 for a peptide encompassing the phosphorylated 14-3-3 binding motif was 152 nmol/L, which is in line with the affinity of other previously reported 14-3-3 binding motifs.35 36
The notion that the 14-3-3 binding motif in βc may be functionally important is supported by the ability of GM-CSF to regulate the phosphorylation of 585Ser in the M1 myeloid cell line (Fig 8). This was shown through the development of antibodies specific for the CLGPPHSRSLPDILG sequence in which the second serine was phosphorylated. In previous experiments, we have found that the overall serine/threonine phosphorylation of the cytoplasmic region of βc (which contains 60 serines and threonines) was similar before and after stimulation by GM-CSF (data not shown), indicating a substantial basal level of phosphorylation that obscured any specific serine and threonine phosphorylation event regulated by ligand. These results make 585Ser more akin to tyrosine residues in βc, because the phosphorylation of these is ligand-dependent. Analogous to tyrosine-phosphorylated sites in βc that serve as docking sites for STAT-517, SHC,16,17,19 and PTP137 with clear functional consequences, the ligand-dependent phosphorylation of585Ser may also initiate distinct signaling events. In other experiments using a CTL-EN cell line expressing the human IL-3 receptor, we found that stimulation with IL-3 increased the association of βc to 14-3-3 by approximately 5-fold (data not shown). The importance of serine/threonine phosphorylation in mediating the biological activity of cytokines and growth factors is underscored by the recent findings of several groups that several receptor (GHR, EPOR, TPOR, and G-CSF) mutants in which all cytoplasmic tyrosines have been substituted for phenylalanine are able to mediate most, if not all, of the biological activity of the wild-type receptors. We25and others16 17 have also shown that a mutant βc in which all 8 cytoplasmic tyrosines are substituted for phenylalanine is able to support both proliferation and survival of FDCP1 and BaF3 cells in response to GM-CSF. Thus, it is tempting to speculate that this may be achieved, at least in part, by ligand-dependent 585Ser phosphorylation, which activates either the same or alternative and redundant pathways.
Although our results show that 14-3-3 binds 585Ser of βc, little is known about the possible role of 14-3-3 in receptor signaling. The 14-3-3 family of proteins consists of 7 different isoforms and is expressed ubiquitously from yeast to humans.38,39 The ability of 14-3-3 to bind phosphoserine motifs in a wide range of signaling molecules40-50 suggests that 14-3-3 proteins participate in a number of cell signaling pathways that may include mitogenesis, transformation, and survival.
Although 14-3-3 has been shown to bind a number of signaling molecules, it has been more difficult to determine how 14-3-3 can regulate signaling events. However, the recent x-ray crystallographic structure of 14-3-3 has provided several hints.51,52 The 14-3-3 proteins dimerize through an N-terminal domain to form 2 large acidic grooves. It is proposed that this groove can bind 2 separate 14-3-3–binding motifs, raising the possibility that 14-3-3 could function as an adaptor, bringing together 2 different cellular proteins.41 53 Thus, one possible function of 14-3-3 could be to act as an adaptor linking βc to other 14-3-3–binding molecules involved in GM-CSF signaling. Another possibility is that 14-3-3 could be involved in the regulation of βc cytoplasmic domain contact with cellular membranes in a manner similar to that described for BAD and Raf-1.
Although the exact functions and signaling pathways involved remain to be elucidated, it is noteworthy that the582HSRSLP587 14-3-3 binding sequence in βc lies proximal to the 577Tyr binding site for SHC.16,17 Because these sequences form a distinct motif conserved in βc of different species and not found in other cytokine receptors, we propose that they subserve specific functions associated to the GM-CSF, IL-3, and IL-5 receptors. In support of this concept, we note that this new motif lies within a region of βc involved in myeloid cell survival and differentiation34; however, functional studies will be necessary to test this hypothesis.
ACKNOWLEDGMENT
The authors thank Anna Nitschke for excellent secretarial assistance and Dr A. Aitken for a gift of antibodies to 14-3-3 and useful discussions.
Supported by grants from the NH&MRC of Australia.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to A.F. Lopez, MD, PhD, The Hanson Centre for Cancer Research, IMVS, Frome Road, Adelaide, SA 5000, Australia; e-mail: angel.lopez@imvs.sa.gov.au.