Abstract
Because envelope gp120 of various strains of human immunodeficiency virus type 1 (HIV-1) downregulates the expression and function of a variety of chemoattractant receptors through a process of heterologous desensitization, we investigated whether epitopes derived from gp120 could mimic the effect. A synthetic peptide domain, designated F peptide, corresponding to amino acid residues 414-434 in the V4-C4 region of gp120 of the HIV-1 Bru strain, potently reduced monocyte binding and chemotaxis response to macrophage inflammatory protein 1β (MIP-1β) and stromal cell-derived factor 1 (SDF-1), chemokines that use the receptors CCR5 and CXCR4, respectively. Further study showed that F peptide by itself is an inducer of chemotaxis and calcium mobilization in human monocytes and neutrophils. In cross-desensitization experiments, among the numerous chemoattractants tested, only the bacterial chemotactic peptide fMLF, when used at high concentrations, partially attenuated calcium mobilization induced by F peptide in phagocytes, suggesting that this peptide domain might share a 7-transmembrane, G-protein–coupled receptor with fMLF. By using cells transfected with cDNAs encoding receptors that interact with fMLF, we found that F peptide uses an fMLF receptor variant, FPRL1, as a functional receptor. The activation of monocytes by F peptide resulted in downregulation of the cell surface expression of CCR5 and CXCR4 in a protein kinase C-dependent manner. These results demonstrate that activation of FPRL1 on human moncytes by a peptide domain derived from HIV-1 gp120 could lead to desensitization of cell response to other chemoattractants. This may explain, at least in part, the initial activation of innate immune responses in HIV-1–infected patients followed by immune suppression.
HUMAN IMMUNODEFICIENCY virus type 1 (HIV-1) uses CD4 as the primary receptor and the specific chemokine receptors as fusion cofactors.1,2 The acquired immunodeficiency syndrome (AIDS) caused by HIV-1 is characterized by profound immunosuppression with high incidence of certain neoplasms and/or opportunistic infections. Although T-lymphocyte dysfunction is the key feature of AIDS-associated immunosuppression, the function of monocytes and neutrophils in AIDS patients is also impaired.3-7 Our previous studies have shown that monocytes from healthy donors, when preincubated with gp120 of various HIV-1 strains, displayed markedly reduced Ca2+ mobilization and chemotaxis in response to a number of chemoattractants, including the bacterial chemotactic peptide fMLF and chemokines accompanied by receptor downregulation.8 The gp120-induced chemoattractant receptor downregulation apparently resulted from a heterologous desensitization that required activation of protein kinase C (PKC) and was CD4-dependent.8
To define the structural basis for gp120 to attenuate monocyte response to chemoattractants, a series of synthetic peptide domains derived from HIV-1 gp120 were tested for their effect on monocyte activation. One of these synthetic peptide domains, which is designated F peptide and corresponds to amino acid residues 414-434 in the V4-C4 region of gp120 of the HIV-1 Bru strain, potently reduced monocyte binding and chemotaxis in response to CC chemokine MIP-1β and CXC chemokine SDF-1α when used at low micromolar concentrations. Further study showed that F peptide could by itself induce chemotaxis and Ca2+ mobilization in human phagocytic cells. Based on the attenuation of Ca2+ mobilization in phagocytes in response to F peptide by high concentrations of fMLF, we postulated that F peptide might share a phagocyte receptor with fMLF. Two functional receptors, FPR and FPRL1, have been identified for fMLF. Both receptors belong to the 7-transmembrane, G-protein–coupled Rhodopsin superfamily.9-11 Whereas FPR has high affinity for fMLF and is activated by picomolar concentrations of fMLF, FPRL1 is activated by fMLF at micromolar concentration range and was defined as a low-affinity receptor for fMLF.9-11 These activation patterns of fMLF receptors led us to examine whether F peptide was capable of interacting with the low-affinity fMLF receptor, FPRL1. By using human kidney embryonic epithelial 293 cells transfected to express various chemoattractant receptors, we demonstrate that F peptide uses FPRL1 as its functional receptor. Our study suggested that this peptide domain derived from HIV-1 gp120 downregulates the expression and function of chemokine receptors CCR5 and CXCR4 in monocytes by activating FPRL1-mediated signaling cascade.
MATERIALS AND METHODS
Reagents and cells.
F peptide, EGSDTITLPCRIKQFINMWQE, which corresponds to amino acid residues 414-434 in the V4-C4 region of HIV-1 gp120 (strain Bru), was purchased from Intracell (Cambridge, MA) and was also synthesized and purified by the Department of Biochemistry, Colorado State University (Fort Collins, CO) according to the published sequence. The amino acid composition was verified by mass spectrometer. The endotoxin levels in dissolved peptide were undetectable by Limulus amebacyte lysate assays (sensitivity, 0.06 IU/mL; Bio Whittaker, Walkersville, MD). The synthetic chemotactic peptide fMLF was purchased from Sigma (St Louis, MO). Several other synthetic HIV-1 gp120 domains were also purchased from Intracell and tested for their biological effects. These peptide domains include V3 region of gp120 MN (RIHIGPGRAFYTTKN), amino acid 120-135 of gp120 Bru (VKLTPLCVSLKCTDLG), amino acid 302-324 of gp120 SBI-IIIB (TRPNNNTRKSIRIQRGPGRAFVT), amino acid 307-320 of gp120 Bru (NKRKRIHIGPGRAF), and amino acid 460-474 of gp120 Bru (TRDGGNNNNGSEIFR). Radio-iodinated chemokines were purchased from DuPont NEN (Boston, MA). Monoclonal anti-CXCR4 (12G5) and anti-CCR5 (2D7) antibodies were obtained from NIH AIDS Research and Reference Reagent Program (Rockville, MD). Monoclonal anti-CD4 antibody was purchased from Biogenesis (Poole, UK). Fluorescein isothiocyanate (FITC)-conjugated antimouse IgG (whole molecule) was purchased from Sigma.
Human peripheral blood monocytes were isolated from buffy coats (NIH Clinical Center, Transfusion Medicine Department, Bethesda, MD) enriched for mononuclear cells by using iso-osmotic Percoll gradient as previously described.12 Neutrophils were isolated from buffy coat blood with dextran sedimentation as described.12The purity of the cell preparations was examined by morphology and was greater than 90% for monocytes and greater than 98% for neutrophils. Rat basophilic leukemia cells (RBL-2H3) transfected with epitope-tagged FPR (designated ETFR) were a kind gift of Drs H. Ali and R. Snyderman (Duke University, Durham, NC). FPRL1-transfected HEK/293 cells (designated FPRL1/293) were established as previously described.13 All the transfected cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS; Hyclone, Logan, UT), 1 mmol/L glutamine (GIBCO-BRL, Grand Island, NY) and 800 μg/mL geneticin (G418; GIBCO-BRL).
Chemotaxis assays.
Chemotaxis assays were performed using 48-well chemotaxis chambers (Neuro Probe, Cabin John, MD) as described previously.8Twenty-six to 28 μL of chemoattractants at different concentrations was placed in the wells of the lower compartment of the chamber, and 50 μL of cells (monocytes or neutrophils at 2 × 106/mL and transfected cells at 1 × 106/mL) were placed in the wells of the upper compartment. The upper and lower compartments were separated by a polycarbonate filter (Osmonics, Livermore, CA; 5-μm pore size for monocytes and neutrophils and 10-μm pore size for transfected cells). For migration of the transfected cells, the filters were precoated with 50 μg/mL collagen type I as described.14 After incubation at 37°C for different times (90 minutes for monocytes, 60 minutes for neutrophils, or 5 hours for transfected cells), the filters were removed and stained and the cells migrated across the filters were counted under light microscope after coding the samples. The results were expressed as chemotaxis index (CI), which represents the fold increase in the number of migrated cells in 3 high-powered fields in response to chemoattractants over the spontaneous cell migration in response to control medium.
Binding assays.
Binding assays were performed by preincubating duplicate samples of human monocytes (2 × 106/sample) with various concentrations of F peptide for 60 minutes at 37°C in a volume of 200 μL/sample of binding medium (RPMI1640, 1% bovine serum albumin [BSA], 5 mmol/L HEPES, and 0.05% NaN3).125I-labeled chemokines (0.12 nmol/L) were then added to each sample. After incubation for 40 minutes at room temperature, the cells were washed once with cold phosphate-buffered saline (PBS) and centrifuged through a 10% sucrose/PBS cushion. The tips of each tube containing cell pellets were cut and the cell-associated radioactivity was measured in a gamma counter. Unlabeled native chemokines at 1,000-fold excess were used as positive controls to yield nonspecific binding. Specific binding was obtained by subtraction of nonspecific binding (in the presence of unlabeled chemokine) from total binding (in the absence of unlabeled chemokine). The percentage of inhibition of specific binding by F peptide treatment was calculated as follows: % inhibition = 1 − (specific binding on F peptide-treated cells)/(specific binding on medium-treated cells) × 100%.
Calcium mobilization.
Ca2+ mobilization was measured by incubating 107/mL cells in loading medium (DMEM, 10% FBS, and 2 mmol/L glutamine) with 5 μmol/L Fura-2 AM (Molecular Probes, Eugene, OR) for 30 minutes at room temperature. The dye-loaded cells were washed and resuspended in saline buffer (138 mmol/L NaCl, 6 mmol/L KCl, 1 mmol/L CaCl2, 10 mmol/L HEPES [pH 7.4], 5 mmol/L glucose, and 0.1% BSA) or Hanks’ balanced salt solution (HBSS) at a density of 1 × 106/mL. The cells were then transferred into quartz cuvettes (1 to 2 × 106 cells in 2 mL) that were placed in a luminescence spectrometer (LS-50B; Perkin-Elmer, Beaconsfield, UK). Stimulants at different concentrations were added in a volume of 20 μL to each cuvette at the indicated time points. The ratio of fluorescence at 340 and 380 nm was calculated using a FL WinLab program (Perkin-Elmer).
Fluorescence-activated cell sorting (FACS) analysis.
Peripheral blood monocytes (0.5 × 106) treated with medium or F peptide were washed and blocked with 50 μg/mL human IgG for 15 minutes at 4°C. The cells were then incubated with 5 μg/mL anti-CXCR4 (12G5) or anti-CCR5 (2D7) monoclonal antibody (Courtesy of NIH AIDS Research and Reagent Program) in ice-cold PBS for 30 minutes. For PKC inhibitor treatment, the cells suspended in medium were incubated with 1 ng/mL staurosporine (Kamiya Biomedical Co, Thousand Oaks, CA) for 10 minutes at 37°C or with 40 ng/mL calphostin C (Sigma) for 2 hours at 37°C with an 8-W fluorescent light source located 15 cm away from the samples, followed by incubation with F peptide and anti-CXCR4 or anti-CCR5 antibody. The cells were then washed three times with PBS and stained with 1:200 diluted FITC-conjugated antimouse IgG for 30 minutes at 4°C. After washing, the cells were fixed in 1% paraformaldehyde and analyzed by a Coulter flow cytometer (courtesy of Flow Cytometry Laboratory, SAIC Frederick, NCI-FCRDC, Frederick, MD).
Statistical analysis.
All experiments were performed at least 3 times and the results presented are from representative experiments. The significance of the difference between test and control groups was analyzed using the Student’s t-test.
RESULTS
F peptide inhibits monocyte binding and chemotactic response to chemokines.
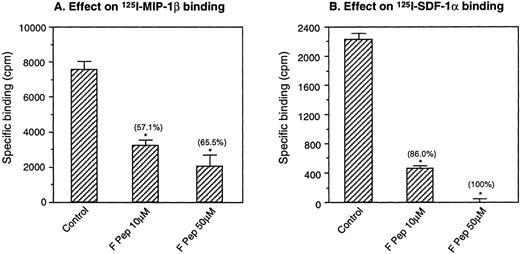
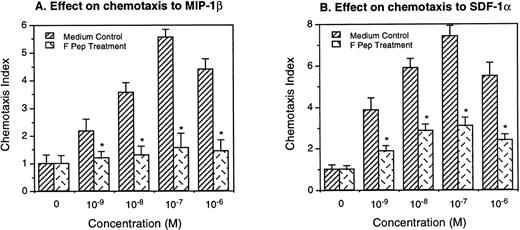
In the first series of experiments, we tested several synthetic gp120 domains available to us, as listed in Materials and Methods, for their effect on human monocyte activation by chemokines. Only 1 of these peptide domains, designated F peptide, which is derived from the V4-C4 region (amino acid 414-434) of gp120 of the HIV-1 Bru strain, could affect the ability of monocytes to bind MIP-1β or SDF-1α, chemokines that use CCR5 and CXCR4 as the functional receptors. When treated with F peptide at 37°C for 60 minutes, monocytes showed markedly decreased capacity to bind radiolabeled MIP-1β and SDF-1α (Fig 1). Monocytes preincubated with F peptide also showed reduced chemotaxis response to both MIP-1β and SDF-1α (Fig 2), suggesting that the reduction of chemokine binding on monocytes was associated with impaired cell function in response to chemokines. In parallel experiments, monocytes incubated with F peptide also showed reduced binding and chemotaxis induced by other chemokines such as MIP-1α and RANTES (data not shown), suggesting that the effect of F peptide on monocyte activation by chemokines is broad.
Downregulation of iodinated chemokine binding to monocytes by F peptide. Monocytes were preincubated with F peptide at the indicated concentrations for 60 minutes at 37°C. The cells were then incubated with 125I-MIP-1β (A) or125I-SDF-1 (B) at room temperature for 40 minutes and pelleted through a sucrose cushion. The cell-associated radioactivity was determined. Unlabeled MIP-1β or SDF-1 at 1,000-fold excess was used to define the level of maximal direct competition by native ligand. The percentage of inhibition of specific chemokine binding is shown in parentheses. *P < .05 compared with binding to cells incubated with medium only.
Downregulation of iodinated chemokine binding to monocytes by F peptide. Monocytes were preincubated with F peptide at the indicated concentrations for 60 minutes at 37°C. The cells were then incubated with 125I-MIP-1β (A) or125I-SDF-1 (B) at room temperature for 40 minutes and pelleted through a sucrose cushion. The cell-associated radioactivity was determined. Unlabeled MIP-1β or SDF-1 at 1,000-fold excess was used to define the level of maximal direct competition by native ligand. The percentage of inhibition of specific chemokine binding is shown in parentheses. *P < .05 compared with binding to cells incubated with medium only.
Inhibition of monocyte chemotaxis to chemokines by F peptide. Monocytes were preincubated with or without F peptide (5 × 10−6 mol/L) for 60 minutes at 37°C and washed 2 times with PBS. Different concentrations of MIP-1β or SDF-1 were placed in the lower wells of the chemotaxis chamber. Monocytes were placed in the upper wells. After incubation for 90 minutes at 37°C, the cells that migrated across the polycarbonate filter were counted. The chemotactic activity was expressed as CI. *A significantly reduced migratory response of F peptide-pretreated monocytes to chemokines compared with medium-treated cells (P < .05).
Inhibition of monocyte chemotaxis to chemokines by F peptide. Monocytes were preincubated with or without F peptide (5 × 10−6 mol/L) for 60 minutes at 37°C and washed 2 times with PBS. Different concentrations of MIP-1β or SDF-1 were placed in the lower wells of the chemotaxis chamber. Monocytes were placed in the upper wells. After incubation for 90 minutes at 37°C, the cells that migrated across the polycarbonate filter were counted. The chemotactic activity was expressed as CI. *A significantly reduced migratory response of F peptide-pretreated monocytes to chemokines compared with medium-treated cells (P < .05).
F peptide induces chemotaxis and calcium mobilization in human phagocytes.
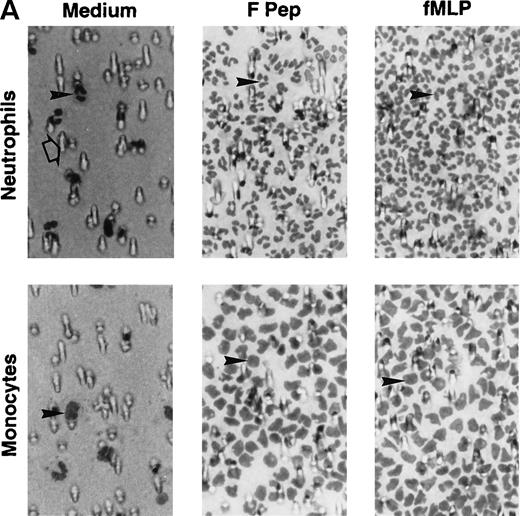
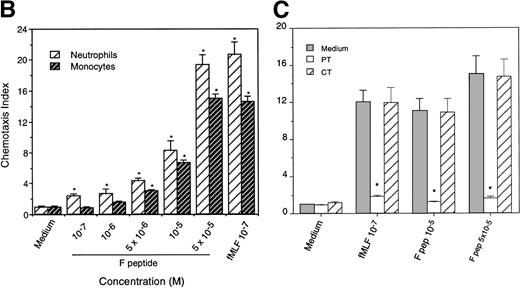
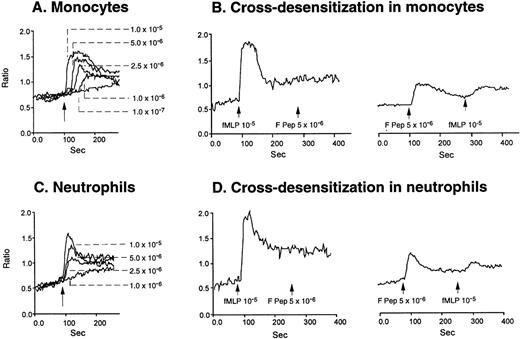
Because a period of preincubation (60 minutes at 37°C) with monocytes was required for F peptide to downregulate the expression and function of chemokine binding sites, we asked whether F peptide by itself might be an activator of phagocytes, thereby desensitizing cell response to chemokines. As shown in Fig 3A and B, human neutrophils and monocytes migrated in a dose-dependent manner in response to F peptide. F peptide induced phagocyte migration at high nanomolar to low micromolar concentration range, and at 5 × 10−5 mol/L, the cell response reached maximum and was comparable to the level induced by an optimal concentration of fMLF (10−7 mol/L). Checkerboard analyses showed that the effect of F peptide on phagocyte migration was chemotactic rather than chemokinetic, because the cells migrated only in response to higher concentrations of the F peptide present in the lower wells of the chemotaxis chamber (Table 1). No enhanced cell migration was observed in the presence of negative F peptide concentration gradients (higher concentrations in the upper wells) or when equal concentrations of the peptide were present in both upper and lower wells (Table 1). The chemotactic response of both monocytes and neutrophils to F peptide was not inhibited by treatment of the cells with PKC inhibitors staurosporine or calphostin C (data not shown), but was markedly inhibited by pretreatment of the cells with pertussis toxin (Fig 3C), suggesting that a 7-transmembrane, G-protein–coupled receptor might be involved in the signaling triggered by F peptide. This was supported by the observation that F peptide induced Ca2+ mobilization in both monocytes and neutrophils at a low micromolar concentration range (Fig 4A and C), comparable to its chemotactic activity. We next tested cross-desensitization of calcium mobilization in monocytes between F peptide and a number of chemokines of both CXC and CC subfamilies to characterize the putative receptor(s) that mediates F peptide signaling. None of the chemokines tested, including monocyte chemotactic protein 1 (MCP-1), RANTES, MIP-1α, interleukin-8 (IL-8), and SDF-1α, could desensitize subsequent cell response to F peptide. F peptide also did not desensitize the subsequent cell response to these chemokines (data not shown), suggesting that F peptide does not share a receptor with any of these chemokines. However, the bacterial chemotactic peptide fMLF, when used at high concentrations (10−5 mol/L and higher), attenuated F peptide-induced calcium mobilization in both monocytes and neutrophils. Conversely, F peptide also partially attenuated the subsequent cell response to fMLF (Fig 4B and D). These results suggest that F peptide might use a phagocyte receptor for which fMLF has low affinity.
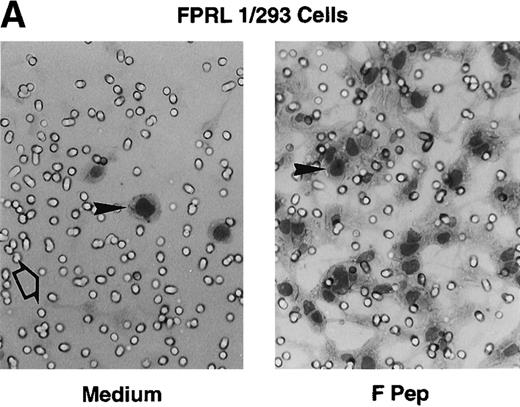
Chemotactic activity of F peptide for monocytes and neutrophils. Different concentrations of F peptide were placed in the lower wells of the chemotaxis chamber. Monocytes or neutrophils were placed in the upper wells. After incubation at 37°C, the cells that migrated across the polycarbonate filter were counted and photographed. (A) Visualization (original magnification × 400) of neutrophil (upper panels) and monocyte (lower panels) migration in response to control medium (Medium), F peptide (F pep; 5 × 10−5 mol/L), and fMLF (fMLP; fMLP 10−7 mol/L). Solid arrows in the figure denote cells migrated across the filters. An open arrow in the upper-left panel indicates one of the micropores in the filter. (B) Fold increase (chemotaxis index) of phagocyte migration in response to F peptide over control medium. *P < .01 compared with spontaneous migration. (C) Inhibition of monocyte migration in response to F peptide by pertussis toxin. Cells preincubated with 100 ng/mL pertussis toxin (PT) at 37°C for 30 minutes were washed and examined for migration induced by different concentrations of F peptide. Cholera toxin (CT) at 100 ng/mL had no effect on monocyte migration induced by F peptide. *P < .01 compared with migration of cells incubated with medium alone.
Chemotactic activity of F peptide for monocytes and neutrophils. Different concentrations of F peptide were placed in the lower wells of the chemotaxis chamber. Monocytes or neutrophils were placed in the upper wells. After incubation at 37°C, the cells that migrated across the polycarbonate filter were counted and photographed. (A) Visualization (original magnification × 400) of neutrophil (upper panels) and monocyte (lower panels) migration in response to control medium (Medium), F peptide (F pep; 5 × 10−5 mol/L), and fMLF (fMLP; fMLP 10−7 mol/L). Solid arrows in the figure denote cells migrated across the filters. An open arrow in the upper-left panel indicates one of the micropores in the filter. (B) Fold increase (chemotaxis index) of phagocyte migration in response to F peptide over control medium. *P < .01 compared with spontaneous migration. (C) Inhibition of monocyte migration in response to F peptide by pertussis toxin. Cells preincubated with 100 ng/mL pertussis toxin (PT) at 37°C for 30 minutes were washed and examined for migration induced by different concentrations of F peptide. Cholera toxin (CT) at 100 ng/mL had no effect on monocyte migration induced by F peptide. *P < .01 compared with migration of cells incubated with medium alone.
Calcium mobilization induced by F peptide in phagocytes and attenuation by fMLF. Monocytes (A) or neutrophils (C) were loaded with Fura-2 and stimulated with different concentrations of F peptide. The ratio of fluorescence at 340 and 380 nm was recorded and calculated with the FLWinLab program. (B) and (D) show sequential stimulation of monocytes (B) or neutrophils (D) with fMLF and F peptide or vice versa.
Calcium mobilization induced by F peptide in phagocytes and attenuation by fMLF. Monocytes (A) or neutrophils (C) were loaded with Fura-2 and stimulated with different concentrations of F peptide. The ratio of fluorescence at 340 and 380 nm was recorded and calculated with the FLWinLab program. (B) and (D) show sequential stimulation of monocytes (B) or neutrophils (D) with fMLF and F peptide or vice versa.
F peptide uses FPRL1 as a functional receptor.
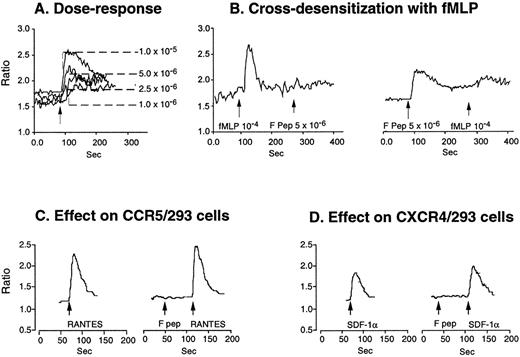
Two receptors that interact with fMLF have been identified.9-11 The prototype 7-transmembrane, G-protein–coupled receptor FPR binds and is activated by low nanomolar concentrations of fMLF and was thus defined as a high-affinity fMLF receptor. An FPR variant, FPRL1, mediates calcium mobilization by high concentrations (micromolar range) of fMLF, thus exhibiting a low-affinity interaction with fMLF.9-11 We then tested the signaling of F peptide on cells transfected with cDNAs coding either for FPR or FPRL1. FPRL1/293 cells, but not cells transfected with FPR (not shown), and chemokine receptors CCR5 or CXCR4 were responsive to F peptide in both chemotaxis (Fig 5A through C) and Ca2+ mobilization experiments (Fig 6). Parental 293 cells also did not respond to F peptide in both chemotaxis and Ca2+mobilization assays (not shown). In addition, whereas the Ca2+ mobilization induced by F peptide in FPRL1/293 cells was attenuated by high concentrations of fMLF, F peptide conversely attenuated fMLF-induced Ca2+ mobilization in these cells (Fig 6B). The concentrations of F peptide required to activate FPRL1/293 cells were in the low micromolar range, comparable to those for phagocyte activation. In contrast, F peptide had no effect on Ca2+ flux induced in CCR5/293 or CXCR4/293 cells by their respective chemokine ligands RANTES (Fig 6C) or SDF-1α (Fig 6D). These results indicate that F peptide indeed uses FPRL1 as a functional receptor.
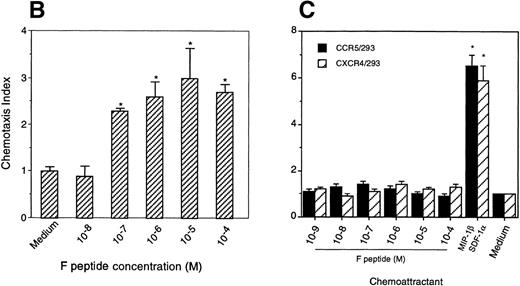
Chemotactic activity of F peptide for FPRL1/293 cells. Different concentrations of F peptide were placed in the lower wells of the chemotaxis chamber. FPRL1/293 cells were placed in the upper wells. After incubation for 5 hours at 37°C, the cells that migrated across the polycarbonate filter were counted and photographed. (A) Visualization (original magnification × 200) of FPRL1/293 cell migration in response to control medium (Medium; left panel) or F peptide (F pep; 5 × 10−5 M; right panel). Solid arrows in the figure denote cells migrated across the filters. An open arrow in the left panel indicates one of the micropores in the filter. (B) Fold increase (chemotaxis index) of FRPL1/293 cell migration in response to F peptide over control medium. (C) Lack of chemotactic activity of F peptide for 293 cells transfected to express CCR5 or CXCR4. MIP-1β and SDF-1 at 10 ng/mL were used as positive controls. *P < .01 compared with spontaneous migration.
Chemotactic activity of F peptide for FPRL1/293 cells. Different concentrations of F peptide were placed in the lower wells of the chemotaxis chamber. FPRL1/293 cells were placed in the upper wells. After incubation for 5 hours at 37°C, the cells that migrated across the polycarbonate filter were counted and photographed. (A) Visualization (original magnification × 200) of FPRL1/293 cell migration in response to control medium (Medium; left panel) or F peptide (F pep; 5 × 10−5 M; right panel). Solid arrows in the figure denote cells migrated across the filters. An open arrow in the left panel indicates one of the micropores in the filter. (B) Fold increase (chemotaxis index) of FRPL1/293 cell migration in response to F peptide over control medium. (C) Lack of chemotactic activity of F peptide for 293 cells transfected to express CCR5 or CXCR4. MIP-1β and SDF-1 at 10 ng/mL were used as positive controls. *P < .01 compared with spontaneous migration.
Calcium mobilization induced by F peptide in FPRL1/293 cells and attenuation by fMLF. FPRL1/293 cells were loaded with Fura-2 and stimulated with different concentrations of F peptide (A), and the ratio of fluorescence at 340 and 380 nm was recorded and calculated. (B) shows sequential stimulation of FPRL1/293 cells with F peptide and fMLF or vice versa. F peptide (5 × 10−6mol/L) did not induce calcium flux in 293 cells transfected with CCR5 (C) or CXCR4 (D) and neither did F peptide (5 × 10−6mol/L) attenuate the calcium flux induced by RANTES (200 ng/mL) or SDF-1 (200 ng/mL) in these cells.
Calcium mobilization induced by F peptide in FPRL1/293 cells and attenuation by fMLF. FPRL1/293 cells were loaded with Fura-2 and stimulated with different concentrations of F peptide (A), and the ratio of fluorescence at 340 and 380 nm was recorded and calculated. (B) shows sequential stimulation of FPRL1/293 cells with F peptide and fMLF or vice versa. F peptide (5 × 10−6mol/L) did not induce calcium flux in 293 cells transfected with CCR5 (C) or CXCR4 (D) and neither did F peptide (5 × 10−6mol/L) attenuate the calcium flux induced by RANTES (200 ng/mL) or SDF-1 (200 ng/mL) in these cells.
F peptide downregulates expression of CCR5 and CXCR4 on monocytes.
We next used FACS analysis to investigate whether activation of monocytes by F peptide through FPRL1 could result in downregulation from cell surface of chemokine receptors. As shown in Tables 2 and 3, monocytes preincubated with F peptide showed markedly reduced CCR5 and CXCR4 expression on the cell surface. The reduction of CCR5 and CXCR4 staining on monocyte cell surface involved activation of PKC, because monocytes pretreated with PKC inhibitors staurosporine and calphostin C maintained CCR5 and CXCR4 expression despite incubation with F peptide. As expected, MIP-1β and SDF-1α competitively inhibited the staining of CCR5 and CXCR4 on monocytes by their respective antibodies. These observations suggest that binding of F peptide to FPRL1 on monocytes activates intracellular PKC, which, in turn, downregulates the expression and function of the chemokine receptors. Thus, the action of F peptide on CCR5 and CXCR4 resembles the mechanism of receptor heterologous desensitization in which PKC is believed to play an important role.15 In contrast, the reduction of CCR5 or CXCR4 staining on monocytes by their native ligands did not require the activation of PKC, because the expression of CCR5 or CXCR4 on monocytes preincubated with staurosporine or calphostin C was equally downregulated by MIP-1β or SDF-1α (Tables 1 and 2).
DISCUSSION
In this study, we report that a peptide domain derived from the V4-C4 region of HIV-1 gp120 was capable of downregulating chemokine receptors CCR5 and CXCR4 on human monocytes. We further observed that this peptide domain of gp120 by itself is a potent chemoattractant for both human monocytes and neutrophils by activating a 7-transmembrane G-protein–coupled receptor FPRL1, which is a low-affinity receptor for the bacterial chemotactic peptide fMLF.9-11 The interaction of FPRL1 in monocytes with F peptide initiates a pertussis toxin-sensitive G-protein signaling cascade involving the activation of PKC, which may be responsible for the inhibitory effect of F peptide on monocyte expression and function of chemokine receptors CCR5 and CXCR4. Despite being essential fusion coreceptors for HIV-1 and mediating migration, the full spectrum of the role of CCR5 and CXCR4 in immune responses remains to be elucidated. However, although CCR5-depleted mice developed normally, they showed significantly reduced clearance ofListeria infection, suggesting a defect in monocyte/macrophage function.16 On the other hand, CXCR4−/− mice had severely reduced B lymphopoiesis, reduced myelopoiesis in fetal liver, and absence of myelopoiesis in bone marrow.17 18These results indicate that both CCR5 and CXCR4 are important in maintaining the homeostasis of the immune system. Therefore, modulation of the expression and function of these receptors may have signifcant impact on the host defense against infection and inflammation.
Leukocyte infiltration at the sites of inflammation in vivo is considered to be based on migration of cells toward a gradient of chemoattractant(s), either derived from microorganisms or the local tissue. The discovery of synthetic N-formyl oligopeptide chemoattractants for phagocytes represented a major advance in the study of leukocyte locomotion.19 Several natural N-formyl peptide chemoattractants, including the prototype N-formyl peptide, fMLF, have since been purified from bacterial supernatants, providing evidence in support of them being biologically relevant ligands for formyl peptide receptors on phagocytic cells. The prototype receptor for formyl peptides designated FPR was shown to have a broad spectrum of agonists, including N-formylated, N-acetylated, N-urea–substituted, or carbonate-modified peptides.20,21 Structural analysis of FPR suggests that the binding pocket of this receptor is able to accommodate an amino terminal group larger than a formyl group,20 21 and this may explain the capacity of this receptor to interact with a great variety of ligands.
The FPR variant, FPRL1, was identified and molecularly cloned from human phagocytic cells by low stringency hybridization of the cDNA library with the FPR sequence and was initially defined as an orphan receptor.13,22-24 The cloning of the same receptor termed FPRH2 from a genomic library was also described.25 FPRL1 possesses 69% identity at the amino acid level to FPR,9-11and both receptors are expressed by monocytes and neutrophils and are clustered on human chromosome 19q13.25,26 Although fMLF is a high-affinity agonist for FPR, it interacts with and induces Ca2+ flux in FPRL1 only at high concentrations.13,25,26 In our study, fMLF did not induce significant migration of FPRL1/293 cells at a concentration as high as 50 μmol/L (data not shown), suggesting that fMLF is not a fully functional agonist for FPRL1. In contrast, F peptide induces migration of FPRL1/293 cells at low micromolar concentrations. Thus, compared with fMLF, F peptide is a more potent and efficacious agonist for FPRL1. Although FPRL1 is mainly expressed in monocytes and neutrophils, cells other than phagocytes, such as hepatocytes, have also been shown to express FPRL1.9 Recently, the expression of this receptor has been reported to be highly inducible in epithelial cells by specific cytokines such as IL-13 and interferon-γ.27Therefore, FPRL1 may play an important role in inflammatory and immunological responses in human cells. In support of this notion, we recently identified FPRL1 to be a functional receptor for a normal serum protein, serum amyloid A (SAA),28 which increases its concentration by up to several hundred-fold during acute-phase responses and is a potent phagocyte chemoattractant and activator.29,30 SAA, similar to F petide, specifically induced Ca2+ flux and chemotaxis of FPRL1/293 cells28 and desensitized the cell response to F peptide and vice versa (data not shown), indicating that these 2 stimulants indeed share FPRL1 as a functional receptor. It should be noted that F peptide does not bear any significant sequence homology to either fMLF or SAA. Therefore, FPRL1, like its prototype FPR, also is capable of hosting a broad spectrum of agonists.
Although FPRL1 can be activated by protein or peptide agonists, a lipid metabolite LXA4 was also reported to be a potent agonist for FPRL1.31 LXA4 is an eicosanoid generated during multiple cellular events such as inflammation, thrombosis, and atherosclerosis and was initially reported as an anti-inflammatory agent.32LXA4 binds to FPRL1 (or otherwise termed LXA4R) transfected CHO cells with high affinity and activates G proteins in these cells.31 In neutrophils, LXA4 inhibits cell migration in response to fMLP or leukotriene B4 through epithelial cell monolayers.33,34 However, in monocytic cells, LXA4 induces potent calcium mobilization and chemotaxis.35 36 Therefore, the signaling cascade induced by LXA4 in monocytes versus neutrophils seems divergent and a further structure-functional study is necessary to delineate similarities and differences in the interaction of FPRL1 with lipid agonist(s) versus protein/peptide agonists.
The signal transduction pathways mediated by FPRL1 have not been extensively studied. The high level of homology to FPR, sensitivity to pertussis toxin, and mediation of cell migration and activation suggest that FPRL1 and FPR may share some signal transduction events after interaction with agonists. The binding of FPR by agonists results in a G-protein–mediated signaling cascade leading to calcium mobilization, cell adhesion, chemotaxis, release of oxygen intermediates, enhanced phagocytosis, and bacterial killing, as well as MAP kinase activation and gene transcription.9-11 Activation by fMLF can also lead to heterologous desensitization of the subsequent cell response to other G-protein–coupled receptor ligands, including chemokines.37 38 In our study, F peptide, although being an agonist for FPRL1, downregulates the surface expression and function of chemokine receptors on monocytes. This effect of F peptide requires preincubation with monocytes at 37°C for 1 hour and is sensitive to PKC inhibitors staurosporine and calphostin C, suggesting a similar mechanism of receptor heterologous desensitization. Because human phagocytic leukocytes express a number of 7-transmembrane, G-protein–coupled receptors that can be activated by a great array of chemoattractants, heterologous desensitization of some of the receptors during pathological states may serve to prevent the overactivation of the cells by multiple stimulants.
Although F peptide is derived from the sequence of HIV-1 envelope gp120, the relevance of our current findings to HIV-1 pathogenesis, if any, remains to be established. However, our observations may suggest some speculative possibilities. It has been reported that monocytes and neutrophils isolated from HIV-1–infected patients responded poorly in vitro to a variety of chemoattractants, including fMLF.3,7We have found that recombinant soluble gp120 of HIV-1 is able to downregulate the expression and function of the receptors for a variety of chemokines on monocytes, including CCR5 and CXCR4, 2 major HIV-1 fusion cofactors.8 The expression of receptors for chemokine IL-8 on neutrophils of HIV-1–infected patients was also downregulated.6 In addition, soluble gp120 was able to downregulate the surface expression of the receptors for fMLF and complement component C5a in normal human monocytes.39
The effect of gp120 on monocytes requires the presence of CD4 molecules and is dependent on a PKC-mediated heterologous chemoattractant receptor desensitization.8 It is not clear whether soluble gp120 itself at higher concentrations (in the micromolar range) is capable of interacting with formyl peptide receptors or, alternatively, conjugation with CD4 may cause exposure of its domains to interact with these receptors. Also, because in this study high nanomolar to low micromolar concentrations of F peptide are needed to activate phagocytes, further study is needed to examine whether HIV-1 envelope proteins undergo proteolytic cleavage in vivo to yield peptide fragments that interact with FPR and/or FPRL1. Nevertheless, it has been estimated that a concentration of HIV-1 envelope protein per virus particle could be equal to 1,300 μmol/L.40 Therefore, at local infection sites, the concentration of HIV-1 envelope and possibly their peptide segments could be present at a high level. In another study, we found that a synthetic peptide domain of HIV-1 envelope gp41, T20/DP178, which exhibits potent anti–HIV-1 activity both in vitro and in vivo, is a selective agonist of the prototype fMLF receptor FPR.41 This, together with the present observation with F peptide, suggests that HIV-1 envelope contains multiple domains that may potentially interact with cellular receptors, thus affecting the immune responses. Although the accessibility of such HIV-1 envelope domain(s) in vivo to host immune cells remains to be determined, it has been reported that antibodies recognizing various epitopes of gp120 appear at early stages of HIV-1 infection.42 Therefore, whereas the receptors for formylated peptides such as FPR and FPRL1 are not used by HIV-1 for fusion, they may participate in the regulation of host innate immune responses seen in AIDS patients characterized by a stimulation of immune system in the early stage of the disease followed by progressive immunosuppression. Further study is currently going on to clarify the signaling events involved in F peptide-induced CCR5 or CXCR4 desensitization by using cell lines cotransfected to express these receptors. In addition, the impact of FPRL1 activation on the capacity of CCR5 and CXCR4 to act as HIV-1 fusion coreceptors is also of great interest. These studies may provide a novel approach to the development of anti-inflammation and anti–HIV-1 agents.
ACKNOWLEDGMENT
The authors thank J.J. Oppenheim for his critical review of this manuscript, and O.M.Z. Howard, R. Salcedo, W. Shen, D. Yang, H. Dong, S. Strobl, L. Finch, C. Maloney, and R. Turner for technical assistance. The clerical assistance by C. Fogle is gratefully acknowledged.
Supported in whole or in part with Federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. NO1-CO-56000.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. The publisher or recipient acknowledges right of the US Government to retain a nonexclusive, royalty-free license in and to any copyright covering this article.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Ji Ming Wang, MD, Laboratory of Molecular Immunoregulation, Division of Basic Sciences, National Cancer Institute-Frederick Cancer Research and Development Center, Bldg 560, Room 31-40, Frederick, MD 21702-1201; e-mail:wangji@mail.ncifcrf.gov.