Abstract
High-dose therapy with autologous peripheral blood stem cell (PBSC) rescue is widely used for the treatment of malignant disease. CD34 selection of PBSC has been applied as a means of reducing contamination of the graft. Although CD34 selection results in a 2 to 3 log reduction in contaminating tumor cells without significantly delaying engraftment, many other types of cells are depleted from the CD34-enriched grafts and immune reconstitution may be impaired. In the present study, 31 cytomegalovirus (CMV)-seropositive patients who received myeloablative therapy followed by the infusion of CD34-selected autologous PBSC were assessed for the development of CMV disease in the first 100 days posttransplant. Seven patients (22.6%) developed CMV disease and 4 patients (12.9%) died from complications of their infection. In a contemporaneous group of 237 CMV-seropositive patients receiving unselected, autologous PBSC, only 10 patients (4.2%) developed CMV disease, with 5 deaths (2.1%). In a multivariate logistic regression analysis, the use of CD34-selected autologous PBSC after high-dose therapy was associated with a marked increase in the incidence of CMV disease and CMV-associated deaths.
HIGH-DOSE THERAPY followed by autologous transplantation of peripheral blood stem cells (PBSC) improves initial response rates and overall survival for several categories of cancer patients.1-3 However, the major cause of treatment failure remains relapse. Because PBSC products frequently contain detectable contaminating tumor cells,4,5 investigators have attempted to reduce the incidence of relapse by selecting the CD34+cells, thereby depleting tumor cells. A number of phase I, II, and III studies6-13 have been conducted with CD34-selected PBSC infused after myeloablative therapy. Because these studies demonstrate effective hematopoietic recovery and a reduction in the number of contaminating tumor cells in the PBSC product, an increasing number of patients are being offered treatment with autologous CD34-selected PBSC.
The issue of infectious complications and immune reconstitution after the infusion of CD34-selected PBSC has been less completely studied. There are suggestions that immune reconstitution may be delayed with CD34 selection with an associated increased risk for infections. This increased risk may be due to the removal of T cells, natural killer (NK) cells, and monocytes. Among CD34-selected allogeneic transplant recipients, a higher incidence of infectious complications, including cytomegalovirus (CMV) disease,14-16 has been reported. Additionally, case reports have described CMV disease, cryptosporidiosis, and other serious opportunistic infections among patients receiving autologous CD34-selected PBSC.17-20However, no systematic review of common opportunistic infections such as CMV has been reported for autologous CD34-selected PBSC.
CMV disease is a well-described infection in patients with T-cell deficiencies, including allogeneic stem cell transplant recipients and human immunodeficiency virus (HIV)-infected individuals.21-24 However, CMV disease is relatively uncommon after conventional autografting with unmodified bone marrow or PBSC and is reported to occur in only 2% to 9% of such patients.25-28 Little has been published about the impact of CD34 selection on the incidence of CMV infections after autologous transplantation.
In this report, we describe our experience with CMV disease among CMV-seropositive autologous PBSC transplant patients who received CD34-selected stem cell products. Thirty-one patients transplanted for hematological or nonhematological diseases received CD34-selected autologous PBSC after myeloablative therapy and were assessed for the development of CMV disease during the first 100 days posttransplant. These patients were compared with a nonrandomized control group of 237 CMV-seropositive patients who were contemporaneously transplanted with unselected PBSC.
MATERIALS AND METHODS
Study design.
Between April 1995 and November 1998, 268 CMV-seropositive patients underwent a myeloablative conditioning regimen followed by infusion of autologous PBSC. According to the specific protocol active at the time of patient enrollment or at the discretion of the attending physician, 31 patients received CD34-selected PBSC. The remaining 237 patients received unselected PBSC. Of the CD34-selected patients, 25 were treated at the Fred Hutchinson Cancer Research Center (FHCRC; Seattle, WA) or an affiliated academic center (University of Washington and Veteran's Affairs Medical Center, Seattle, WA) and 6 patients were treated at Oregon Health Sciences (Portland, OR) or Swedish Medical Center (Seattle, WA) under the auspices of the Puget Sound Oncology Consortium (Seattle, WA). Of the patients receiving unselected PBSC product, 187 patients were treated at the FHCRC, Veteran's Affairs Medical Center, or University of Washington, and 50 patients were treated at either the Oregon Health Sciences or Swedish Medical Center. After we had obtained informed consent, all patients were treated on an FHCRC or Puget Sound Oncology Consortium protocol approved by the institutional review board of the hospital where the therapy was administered. Patients were prospectively evaluated for the first 100 days posttransplant for the development of CMV infection or disease. The data used for patients in the present report were information available as of March 30, 1999.
Patient characteristics.
Of the 31 patients who received CD34-selected PBSC, 23 (74.2%) were transplanted for a hematological malignancy, 3 (9.7%) for an autoimmune disease, and 5 (16.1%) for a solid tumor. Of the 237 patients who received unselected PBSC, 90 (38%) were transplanted for a hematological malignancy and 147 (62%) for a solid tumor. As Table 1 shows, all patients treated for autoimmune disorders, chronic lymphocytic leukemia (CLL), and acute lymphoblastic leukemia (ALL) received CD34-selected PBSC. A similar percentage of both selected and unselected PBSC recipients received 1 to 2 mg/kg methylprednisolone steroid therapy posttransplant for regimen-related toxicities.
Mobilization, collection, and cryopreservation of PBSC.
For CD34 selection, PBSC were mobilized with either recombinant granulocyte colony-stimulating factor (G-CSF; Amgen, Thousand Oaks, CA) alone (n = 6) or intermediate-dose chemotherapy followed by either G-CSF (n = 23) or recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF; Immunex, Seattle, WA) (n = 2). Second mobilizations were required in 7 patients because of either tumor contamination (n = 4) or insufficient number of stem cells collected (n = 3).
For the patients receiving unselected grafts, PBSC were mobilized with either G-CSF alone (n = 33) or intermediate-dose chemotherapy followed by G-CSF (n = 198) or GM-CSF (n = 6). Nineteen patients required more than 1 mobilization either because an insufficient number of cells were collected (n = 8) or because there was tumor contamination of the product (n = 11). All PBSC collections were performed using the COBE Spectra (COBE BCT, Lakewood, CO) and all products were cryopreserved with dimethyl sulfoxide (DMSO) as previously described.29
CD34 selection.
The Baxter 300 Isolex System (Baxter, Inc, Irvine, CA) was used to select the CD34+ cells in 19 cases,30 and the Cellpro Ceprate System (Cellpro, Seattle, WA) was used in 12 cases.13 Both systems were used according to the manufacturer's specifications. Two of 12 patients whose cells were separated with the Cellpro system also underwent an initial B-cell purging of their PBSC product. For the B-cell purging technique, the collected cell products were incubated with a combination of biotinylated antihuman CD19 and CD20 antibodies and then passed through a column of avidin-conjugated gel to bind the CD19/20-positive cells. The unbound cells were then sequentially incubated with avidin and biotin solutions to prevent any CD19/20 antibody-labeled cells that remained from rebinding in the CD34 selection process.
Transplant conditioning.
Patients in both groups were transplanted with a variety of high-dose myeloablative regimens. As compared with recipients of unselected PBSC grafts, a higher proportion of patients in the CD34-selected group received total body irradiation (TBI)-based conditioning regimens. Fourteen of the 31 (45.2%) CD34-selected patients received a TBI-based conditioning regimen, compared with only 34 of the 237 (14.3%) unselected PBSC patients (Table 1).
Supportive care.
After myeloablative therapy, all patients received prophylactic intravenous antibiotics when the absolute neutrophil count (ANC) decreased to less than 0.5 × 109/L and were treated with additional antibiotics when neutropenic fever occurred. Patients who were serologically positive for herpes simplex virus received prophylactic low-dose acyclovir. Because of limitations in drug availability, prophylactic intravenous Ig was administered to only 6 of the 10 CLL and multiple myeloma (MM) patients who received CD34-selected PBSC and 15 of 26 MM patients who received unselected PBSC, despite the administration of prophylactic Ig being the usual practice for these patients. At the discretion of the attending physician or per protocol, 9 of the 31 (29%) CD34-selected patients received posttransplant growth factor until engraftment, either G-CSF at 5 μg/kg/d subcutaneously (SC; n = 7), G-CSF at 10 μg/kg/d SC (n = 1), or GM-CSF at 500 μg/m2/d SC until 14 days posttransplant, followed by G-CSF at 5 μg/kg/d SC (n = 1). Twelve of the 237 (5.1%) patients transplanted with unselected PBSC product received G-CSF at 5 μg/kg/d SC until engraftment.
CMV screening.
Per institutional policy, patients treated at the FHCRC and the Veteran's Affairs Medical Center had weekly CMV screening studies performed, including the CMV pp65 antigenemia assay (CMV Brite; Biotest Diagnostic Corp, Denville, NJ) and viral blood culture testing weekly from day 10 posttransplant until day 100 or discharge home, as previously described.28 The CMV antigenemia kit was used according to the manufacturer's instructions. Patients with a quantitative antigenemia test (≥5 cells/slide) received antiviral therapy with ganciclovir.28 No screening was used for patients transplanted at the other 2 institutions.
Definitions.
CMV infection was defined as either evidence of any level of quantitative pp65 antigenemia or a positive blood or mouth culture. CMV disease was defined as a positive CMV shell vial or conventional culture of bronchoalveolar lavage fluid, lung biopsy, or gastric/duodenal biopsy in association with symptoms.31CMV-associated death was defined as death that occurred within 6 weeks of documented CMV disease, other than death due to progression of underlying disease.31 The day of onset of CMV disease was defined as the date of performance of a diagnostic procedure (bronchoscopy, open lung biopsy, or esophagogastroduodenoscopic biopsy) to evaluate symptoms suggestive of disease. Neutrophil engraftment was defined as the first of 3 consecutive days on which the ANC exceeded 0.5 × 109/L after the nadir. Platelet transfusion independence was defined as the first of 3 consecutive days on which the platelet count exceeded 20 × 109/L without transfusion.
Statistical methods.
Summary statistics such as median and range values of continuous valued and tabulation of categorical valued patient characteristics were reported. Comparisons between the group of patients receiving CD34-selected PBSC and those receiving unselected PBSC were made using χ2 statistics or the Wilcoxan rank sum test. Cumulative incidence curves32 for CMV disease were generated. Univariate and multivariate logistic regression analyses were performed to evaluate the impact of various factors on the risk of CMV disease and on CMV infection. The odds ratio (OR) and their associated 95% confidence intervals (CI) were reported. Because we were primarily interested in determining whether the inclusion of additional covariates modified the effect of CD34 selection on the outcome, any factors that changed the coefficient estimate by more than 10% were included in multivariate models.33
RESULTS
Engraftment.
Neutrophil engraftment was reached at a median of 10 days (range, 8 to 21 days) for those patients receiving CD34-selected stem cells and 11 days (range, 8 to 72 days) for those treated with unselected PBSC (P = .20, Wilcoxan rank sum test). Platelet transfusion independence occurred a median of 11 days (range, 7 to 47 days) for selected patients and 11 days (range, 4 to 96 days) for those treated with unselected PBSC (P = .12, Wilcoxan rank sum test).
The absolute number of peripheral blood lymphocytes at 30 days after stem cell infusion were markedly different between the 2 groups. In patients alive at day 30 after transplant, lymphocyte counts were available in 228 unselected and in 29 CD34-selected patients. Lymphocyte counts were significantly higher in the unselected group (P = .03).
Incidence of CMV disease.
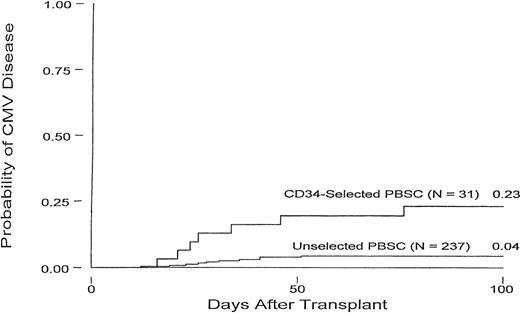
Figure 1 displays the cumulative incidence of CMV disease within 100 days of transplant for CD34-selected and unselected patients. Overall, 7 of the 31 (22.6%) CD34-selected patients developed CMV disease within 100 days posttransplant, and 4 (12.9%) died as a result of their infection. In these 7 patients, CMV disease occurred a median of 26 days (range, 16 to 76 days) after transplant.
Cumulative incidence curves for CMV disease in CD34-selected and unselected PBSC autologous transplant patients.
Cumulative incidence curves for CMV disease in CD34-selected and unselected PBSC autologous transplant patients.
Details of the characteristics of CMV disease in the CD34-selected patients are shown in Tables 2 and3. CMV screening was performed in 19 of the 31 (61.3%) CD34-selected patients. Three of these screened patients developed CMV pneumonia in the first 100 days posttransplant. Two of these 3 patients had no evidence of CMV antigenemia before the development of CMV disease. Both of these patients developed CMV disease early after transplant and began treatment on days 16 and 21, respectively. One of these patients died from their pneumonia. Only 1 of these 3 patients received a TBI-based conditioning regimen. Among the 12 patients who did not have CMV screening performed, 4 developed CMV disease. Three patients developed pneumonia and all died. One patient developed enteritis and survived. All 7 patients developing CMV disease had an underlying hematological malignancy.
Of the 237 unselected PBSC recipients, 10 patients (4.2%) developed CMV disease and 5 (2.1%) died (Tables 2 and 3). Among these 10 patients, CMV disease occurred a median of 29.5 days (range, 12 to 51 days) posttransplant. Three of these 10 unselected patients had an underlying hematological malignancy. Only 5 of the 172 (2.9%) patients screened for CMV developed disease. Two patients developed pneumonia and both died. Three patients developed enteritis, and 1 of these died. Only 2 of these patients displayed evidence of low CMV antigenemia, ie, less than 5 cells/slide before developing disease. Five of the 65 (7.7%) patients who had undergone no CMV screening studies performed developed disease. Three patients developed CMV pneumonia and 2 died. Two patients developed enteritis and recovered after antiviral therapy.
Incidence of CMV infection.
CMV infection (without disease) was detected in an additional 5 of the 19 (26.3%) CMV-screened, CD34-selected patients and in 30 of the 172 (17.4%) patients treated with unselected PBSC who were screened. These 5 CD34-selected patients developed low levels of antigenemia, with less than 5 cells/slide between days +12 to +38 posttransplant (Table 2). Three of these patients received antiviral therapy and subsequently became CMV antigenemia negative. A fifth patient was noted to have low levels of CMV antigenemia on days +31 and +38 posttransplant and had a positive upper respiratory tract culture on day +33. This latter patient had no evidence of lower tract CMV disease and antiviral therapy was not initiated.
Among the 30 patients receiving unselected PBSC who developed CMV infection, 20 (11.6% overall) of these patients had a low level of CMV antigenemia (<5 cells/slide) and did not receive antiviral therapy. Ten patients (5.8%) had significant antigenemia (>5 cells/slide), and 9 were treated with antiviral therapy. Four additional patients were noted to have either a positive blood culture (n = 2) or oral culture (n = 2) and were not treated.
Risk for developing CMV infection or disease.
Univariate and multivariate logistic regression analyses were performed to assess risk factors for the development of CMV infection or CMV disease.
As can be seen in Table 4A, CD34 selection alone was significant for the development of CMV disease with an OR of 6.62 (CI, 2.3 to 19.0; P < .001). Other variables, including age at transplant, posttransplant steroid therapy, underlying disease, number of CD34 cells infused, and treatment with TBI-based conditioning regimen, were not significant.
Evaluating combinations of factors together in multivariate logistic regression models showed that inclusion of conditioning with a TBI-based regimen and dose of CD34 cells infused amplified the effect of CD34 selection and increased the OR to 11.76 (CI, 3.1 to 44.8;P = .0004) for the development of CMV disease. In the multivariate model, conditioning with a TBI-based regimen was not significantly associated with CMV disease, but the dose of CD34 cells as well as CD34 selection were significant. In patients receiving less than 8.56 × 106 cells/kg CD34 cells/kg, the OR was 4.13 (CI, 1.1 to 15.5; P = .04), compared with an OR of 0.68 (CI, 0.1 to 4.1; P = .67) for ≥8.56 × 106CD34 cells/kg infused. Other factors did not modify the effect of CD34 selection on CMV disease and were not included in this model.
Table 4B describes the evaluation of risk factors for CMV infection (as opposed to disease) in univariate logistic regression. Steroid use posttransplant was highly significant for the development of CMV infection, with an OR of 3.00 (P = .003). CD34 selection was also noted to be significant, with an OR of 2.69 (P = .04). There was a trend for the development of CMV infection in patients who carried a diagnosis for either an autoimmune disorder or hematological malignancy relative to patients with a solid tumor, with an OR of 1.98 (P = .06). Other variables, including the age at transplant, number of CD34 cells infused, or treatment with a TBI-based regimen, did not sig- nificantly contribute to the risk of developing CMV infection.
In multivariate logistic regression analyses for the risk of CMV infection, the final model included CD34 selection, steroid use, and underlying disease. With the inclusion of these additional covariates, the OR for CD34 selection was slightly decreased over that in the univariate model (OR, 1.81; CI, 0.7 to 15; P = .27), and it was not statistically significantly associated with CMV infection. In this model, steroid use was still highly significant (OR, 3.00; CI, 1.5 to 6.3; P = .003), and underlying disease was no longer significantly associated with the risk of CMV infection (OR, 1.85; CI, 0.8 to 4.1; P = .12).
Incidence of CMV disease and infection in non-Hodgkin's lymphoma (NHL), Hodgkin's disease (HD), and MM patients.
Because all patients in the CD34-selected group and 3 of 10 patients in the unselected group who developed CMV disease had received an autologous transplant as treatment for NHL, HD, or MM (Table 3), an additional subset analysis of these patients was performed to compare the risk for developing CMV disease and CMV infection (Table 5). As seen in Table 5A, CD34-selected MM, HD, and NHL patients had a significant chance of developing CMV disease, with an OR of 17.0 (CI, 3.8 to 76.7; P< .001). In this subset of patients, the median day to CMV disease among CD34-selected and unselected patients was 26 days (range, 19 to 41 days) and 26 days (range, 16 to 76 days), respectively. This subset analysis, although significant in univariate analysis, contained too few events in the unselected group to perform multivariate analysis.
DISCUSSION
In this study, recipients of autologous, CD34-selected PBSC had an increased incidence of CMV disease. Seven of 31 (22.6%) CMV-seropositive patients who received CD34-selected PBSC developed CMV disease, and 4 patients (12.9%) died from complications of their infection. In contrast, among 237 CMV-seropositive patients undergoing an autologous transplant during the same time period who received unselected PBSC, only 4.2% developed CMV disease, and 2.1% died from complications of their CMV infection. Using univariate analysis, only CD34 selection was significant for the development of CMV disease, with an OR of 6.62 (P < .001). Multivariate adjustment for other risk factors, such as TBI-based conditioning regimen and cell dose (5.0 to 8.55 × 106 cells/kg), amplified this effect (OR, 11.8; P < .001).
A subset analysis of patients diagnosed with HD, NHL, and MM and transplanted with either CD34-selected PBSC and unselected PBSC showed a significant increased risk for developing CMV disease in those patients who were treated with CD34-selected PBSC (OR, 17; P< .001; Table 5A). Seven of the 17 (41%) MM, HD, and NHL patients in the CD34-selected PBSC group developed CMV disease. In comparison, 76 of the 237 CMV-seropositive patients who received unselected PBSC had MM, NHL, and HD. Of these 76 patients, 3 patients (3.9%) developed CMV disease and 2 patients died. This incidence of CMV disease was not significantly increased from the incidence seen in the whole unselected group (4.2%) or in the patients without hematological malignancies (4.6%).
CD34-selected PBSC are increasingly used with the hope of reducing relapses by decreasing the number of tumor cells infused with the autograft. Data supporting an actual improvement in disease-free survival for these patients are not yet available.10,13 To date, CD34-selected autologous PBSC have been administered mainly to patients with breast cancer, lymphoma, or MM. The published data with CD34-selected autografts do not report an increased incidence of CMV disease among CD34-selected autograft patients. The initial pilot studies using CD34-selected autologous PBSC in patients with MM described 8 episodes of interstitial pneumonia resulting in 4 deaths in 74 transplanted patients.6-9 Unfortunately, the number of CMV-seropositive patients that were treated is not documented. If one assumes a 50% incidence of CMV-seropositive patients and that all of the cases of interstitial pneumonia occurred in CMV-seropositive patients, then the number of pulmonary complications due to CMV would be similar to the present report. Recently, Vescio et al13reported the outcome of a multicenter phase III trial evaluating patients with MM who received CD34-selected or unselected autologous PBSC. There were approximately 60 patients in each arm. They reported no significant difference in terms of infections between the 2 groups. However, a high number of patients in each arm (56%) developed an infection in the first 100 days. Data were not provided on the type of infections that developed, the use of prophylactic Ig therapy, or the CMV serostatus of the patients. In 1 phase III study conducted in patients with breast cancer, patients were randomized to receive either CD34-selected marrow grafts (n = 42) or standard buffy coat bone marrow grafts (n = 47).10 There was no significant difference in the incidence of infection in either arm: 52% infection in the CD34-selected patients and 47% infection in the standard marrow graft patients. The actual etiology of these reported infections was not described.
Whether there are real differences between the present report and other studies evaluating CD34 selection on the incidence of CMV disease is uncertain. Because CMV disease is uncommon after conventional autografting with unselected PBSC,25-28 it may not have been suspected in the patients who received a CD34-selected autograft and developed symptoms of CMV disease. In addition, incomplete virology studies of bronchoscopy samples, biopsy, and autopsy samples may have been performed and could result in an underestimation of the true incidence of CMV disease.34 Finally, patients with hematological malignancies may be more immunocompromised not only because of their underlying disease, but also because they are more heavily treated with chemotherapy before transplant and are thus at increased risk for CMV disease when CD34-selected PBSC are administered (Table 5A).
CD34 selection results in a 2 to 3 log depletion of lymphocytes. The impact of this depletion on immune reconstitution and susceptibility to infectious complications such as CMV has not been described in detail after autologous transplantation. In the phase III study evaluating CD34 selected marrow grafts in breast cancer patients, immunological reconstitution for both arms of the study was assessed at 100 days, 6 months, and 1 year after transplant.10 By immunophenotyping, the number of NK and suppressor cells was normal by day 100 in both groups. However, the number of CD4 cells in the CD34-selected group did not reach normal levels until 1 year posttransplant. These data were confirmed in the phase III study evaluating CD34-selected PBSC autografts in patients with MM.13 Vescio et al13 report a significantly lower CD4 lymphocyte count at day 100 posttransplant in patients receiving CD34-selected autografts that persisted until 1 year posttransplant. Additionally, Lemoli et al35 studied immune reconstitution in 13 patients with MM who received a CD34-selected autologous product. They noted that the posttransplant absolute CD4 count in these patients was significantly lower than pretreatment levels, with rapid recovery of total number of lymphocyte and NK cells. Finally, Bomberger et al36 reported that the restoration of normal numbers of both T and B cells were delayed during the first 2 months after infusion of CD34-selected PBSC. Mainly CD4−CD8+, α/β TCR+, CD45RO+, and CD45 RA− cells were noted to be circulating. Thus, there appears to be a loss of the diversity after infusion of CD34-selected cells that is normally seen in the T-cell repertoire after infusion of unselected cells. In our study, the patients who received CD34-selected PBSC and survived beyond 30 days had significantly lower numbers of circulating lymphocytes at 30 days posttransplant (P = .03) as compared with patients treated with unselected PBSC. This may indicate delayed recovery of CMV-specific cytotoxic and helper T cells.
Nothing is yet known about immune reconstitution to CMV after infusion of autologous CD34-selected PBSC. Indeed, little is known about immune reconstitution to CMV after an unselected autologous transplant. Recently, Reusser et al37 studied the reconstitution of CMV-specific CD8+ cytotoxic T lymphocytes (CTL) and CD4+ T-helper (Th) cells in 15 CMV-seropositive autologous transplant patients who received autologous bone marrow or unselected PBSC. They found that CMV-specific CD8+ CTL and CD4+ Th responses were restored in the majority of patients within the first 3 months and that the presence of CD8+ CTL activity afforded protection from CMV infection. Brugger et al38 have speculated that a higher incidence of viral infections within the first 3 months after autologous transplant may be a result of these T-cell defects. Preliminary data in our laboratory show that the patients who developed CMV disease after infusion of CD34-selected PBSC had the lowest number of CD3, CD4, and CD8 cells in their stem cell product. All of these studies point to the existence of more profound immunodeficiency early after the infusion of CD34-selected PBSC as compared with unselected autologous PBSC. Unfortunately, this study was a retrospective analysis and data were not available on the immune reconstitution of these patients after transplant.
Studies in patients receiving an allogeneic transplant have shown that administration of ganciclovir or foscarnet after documentation of CMV excretion in blood, urine, or throat can prevent the occurrence of CMV disease. More recently, earlier detection in blood by CMV antigenemia or polymerase chain reaction (PCR) assays may allow prompt intervention to prevent disease in recipients of allogeneic transplants.28,31,39-41 In this situation, plasma PCR of CMV DNA appears to be both sensitive and specific.42 In the present study, 2 patients who received CD34-selected autologous PBSC developed CMV disease despite weekly testing for CMV antigenemia. This may indicate that, in patients receiving CD34-selected PBSC, the standard CMV screening test for infection is too insensitive just before or immediately after engraftment, perhaps due to low numbers of circulating leukocytes during this period. We believe that, given this increased incidence of CMV disease among CD34-selected autologous stem cell recipients, close monitoring and anti-CMV preventive therapy are needed in seropositive patients receiving autologous CD34-selected PBSC. We have instituted a policy at the FHCRC that incorporates early monitoring by plasma PCR followed by antigenemia testing after engraftment and antiviral treatment for those patients with positive results. Because the definitive benefits of CD34 selection on survival have yet to be demonstrated, future studies using CD34-selected autologous PBSC should require close patient monitoring for the development of infections, including CMV. In some patients, risks from pronounced immunodeficiency could reduce any potential therapeutic gains from the infusion of CD34-selected cells.
ACKNOWLEDGMENT
The technical support of Elizabeth Soll, Sue Tracy-Waisanen, and Chris Davis is greatly appreciated. We express our gratitude to all members and staff of the Puget Sound Oncology Consortium who enrolled their patients on study and provided us with follow-up.
Supported by Grants No. CA18029, CA47748, CA18221, CA15704, and HL35444 and the Jose Carreras Foundation Against Leukemia.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Leona A. Holmberg, PhD, MD, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, PO Box 19024, MS D5-390, Seattle, WA 98109-1024.