Mutations in the human RAG genes that impair, but do not abolish, recombination activity lead to Omenn syndrome, a severe primary immune deficiency that is associated with clinical and pathological features of graft-versus-host disease and oligoclonal expansion of activated, autologous T cells. We have analyzed the mechanisms accounting for peripheral oligoclonality of the T-cell repertoire. Predominance of few T-cell receptor clonotypes (both within TCRAB- and within TCRGD-expressing lymphocytes) is already detectable in the thymus and is further selected for in the periphery, with a different distribution of clonotypes in different tissues. These data indicate that oligoclonality of the T-cell repertoire in Omenn syndrome is due both to intrathymic restriction and to peripheral expansion. Moreover, the RAG genes defect that causes Omenn syndrome directly affects early stages of V(D)J recombination, but does not alter the process of double-strand-break DNA repair, including N and P nucleotide insertion.
OMENN SYNDROME (OS) is a rare, autosomal recessive combined immune deficiency characterized by diffuse erythrodermia, lymphadenopathy, hepato-splenomegaly, protracted diarrhea, failure to thrive, hypereosinophilia, and hypogammaglobulinemia, but elevated serum IgE.1-3 Unless treated with bone marrow transplantation, OS patients die of overwhelming infections and severe metabolic disturbances within the first months of life.4
A peculiar immunological phenotype has been demonstrated in OS. B cells are usually absent both in peripheral blood and in lymphoid tissues from these patients.2-9 In contrast, although lymph nodes and thymus show depletion of lymphoid cells, a variable number of autologous, activated, poorly functional and oligoclonal T cells, with a skewed Th2 profile,7,10-12 are present in peripheral blood and infiltrate the skin, gut, liver, and spleen.6,9 13-15
We have recently demonstrated that the disease is due to mutations in the genes that encode for RAG1 and RAG2, two key lymphoid-specific proteins that are essential for V(D)J recombination.16Whereas null mutations that completely eliminate the recombination activity of these proteins cause a complete block of T- and B-cell development and lead to severe combined immune deficiency with absence of mature T and B lymphocytes (T−B−SCID),17 the RAG gene mutations identified in OS patients allow the recombination machinery to rearrange, albeit with reduced efficiency, T-cell receptor (TCR) gene segments.16
Although the identification of RAG1 and RAG2 mutations represents a first step in understanding the pathogenesis of OS, several questions remain. In particular, we and others have shown that the peripheral T-cell repertoire in OS is highly restricted,6 13-16 but the mechanisms that account for this phenomenon are still poorly defined. The selective accumulation of a few T-cell clones in the periphery might reflect the preferential expression of only a few TCR specificities already in the thymus (due to the RAGs defect); alternatively, it might be the consequence of an (auto)antigen-driven peripheral expansion of a few clones out of an otherwise diversified pool of T cells generated in the thymus.
The availability of several tissues (including thymus) obtained from an OS patient gave us the opportunity to discriminate between these possibilities by comparing the pattern of V(D)J recombination in thymocytes and in T lymphocytes that infiltrate different peripheral target tissues. Furthermore, sequence analysis of the productively rearranged TCR gene segments in a series of patients with molecularly characterized OS allowed us to investigate if the RAG gene defects that account for OS result in peculiar constraints over the process of V(D)J recombination and generation of TCR specificities.
PATIENT AND METHODS
Patient.
The patient, a female infant, was the first child of a couple of consanguineous parents. She developed diffuse erythrodermia at 2 weeks of age, followed by persistent cough, protracted diarrhea, and failure to thrive. At 1 month of age, laboratory examinations performed during hospitalization showed hypoproteinemia and low serum IgG (205 mg/dL) and IgA (8 mg/dL), but increased serum IgE (9,100 kU/L) and moderate thrombocytopenia (92 × 109/L). Total lymphocytes were 8,600/μL, with a predominance of TCRGD (32%) versus TCRAB (11%) T cells. Other subsets were as follows: CD3, 43%; CD4, 11% (CD45RA, <1%; CD45R0, 11%); CD8, 17%; CD19, <1%; CD16, 46%; CD25, 12%; and CD3+DR+, 38%. In vitro proliferative response to mitogens (in counts per minute [cpm] × 10−3) was markedly decreased: phytohemagglutinin, 7.5 (v 108.3 in an age-matched control); anti-CD3 monoclonal antibody, 5.5 (v 85.8); and phorbol myristate acetate plus ionomycin, 6.2 (v 45.9). Based on these findings, a diagnosis of OS was established. Despite isolation in a protected environment and a supply of intravenous Igs, antibiotics, acyclovir, and albumin, the conditions of the infant worsened, and she died of heart failure and respiratory distress at 3 months of age. Postmortem examination showed myocarditis and pneumonia.
Mutation analysis at the RAG loci.
The RAG1 and RAG2 coding sequences were amplified from genomic DNA. Primers were designed for the amplification of theRAG genes based on their sequence, as previously described16 (RAG1 accession no. M29474 andRAG2 accession no. M94633). The RAG1 gene was amplified in 2 segments (nt 94-1852 and nt 1653-3309), and RAG2 was amplified in 1 segment (1201-2922). Sequencing was performed either directly on the polymerase chain reaction (PCR) product purified from the gel and sequenced using Thermosequenase Kit (Amersham, Amersham, UK) or on PCR products cloned in TA vector (Invitrogen, San Diego, CA). Sequencing was performed by the dideoxynucleotide chain termination method using the Sequenase.
Tissues and immunohistochemistry.
Tissue specimens were represented by an inguinal lymph node and a skin biopsy ob- tained at the age of 3 months and various organs that were removed at the time of postmortem examination. All specimens were fixed in formalin and embedded in paraffin; in addition, a small fragment was also frozen in liquid nitrogen and stored at −80°C.
Immunohistochemistry was performed with an indirect streptavidin-biotin complex immunoperoxidase technique on cryostat sections that were air-dried for 18 hours after cutting and fixed in acetone for 10 minutes before staining. The following monoclonal antibodies have been applied: CD1a (Ortho Diagnostic, Milan, Italy); CD2, CD3, and CD5 (Becton Dickinson, San Jose, CA); CD19, CD20, CD30, and CD45RO (Dako, Carpinteria, CA); CD25 (IL2R; Boehringer Mannheim, Mannheim, Germany); CD45RA (Biotest, Milan, Italy); and TCRAB and TCRGD (T-cell Sciences, Cambridge, UK). Thymic epithelium was identified with anticytokeratin monoclonal antibody (clone MNF 116; Dako).
Preparation of RNA and cDNA and amplification of TCR segments.
Peripheral blood mononuclear cells were obtained after Ficoll Hypaque gradient centrifugation. Total cytoplasmic RNA and cDNA for the analysis of TCRBV and TCRDV chains were prepared from thymus and peripheral blood mononuclear cells (PBMC) and tissue-infiltrating lymphocytes, as previously described.18 Briefly, 1 to 2 μg of total RNA, prepared by the guanidium thiocyanate-phenol-chloroform method, were used to synthesize the first strand of the B and D chain-specific cDNA using a primer specific forTCRBC1 and TCRBC2 genes (βcDNA: 5′ GGG CTG CTC CTT GAG GGG CTG CGG 3′) and a primer specific for the TCRDC region (δcDNA: 5′ CAC TGG GAG AGA GAT GAC AAT AGC AG 3′). TCRBV-specific cDNA was then subjected to enzymatic amplification using a second TCRBC primer (βAI: 5′ CCC ACT GTG CAC CTC CTT CC 3′) and a TCRBV degenerated primer [Vβd: 5′ ACG TGA ATT CT(GT) T(ACT)(CT) TGG TA(CT) (AC) (AG)(AT) CA 3′] that was designed to amplify B-chain rearrangements containing virtually all of the known human TCRBV genes.19 After PCR amplifications, the specificity of the total TCRBV amplified products was analyzed using a colorimetric method and biotinylated TCRBV-specific probes.20 Subsequently, the TCRBV chains of interest were amplified by 35 cycles of PCR, using TCRBV-specific oligonucleotides (TCRBV1: 5′ GCA CAA CAG TTC CCT GAC TTG CAC 3′; TCRBV2: 5′ TCA TCA ACC ATG CAA GCC TGA CCT 3′; TCRBV3: 5′ GTC TCT AGA GAG AAG AAG GAG CGC 3′; TCRBV4: 5′ GCC CAA ACC TAA CAT TCT CAA CTC 3′; TCRBV5S1: 5′ ATA CTT CAG TGA GAC ACA GAG AAA 3′; TCRBV5S2: 5′ TTC CCT AAC TAT AGC TCT GAG CTG 3′; TCRBV6: 5′ AGG CCT GAG GGA TCC GTC TC 3′; TCRBV8: 5′ ATT TAC TTT AAC AAC AAC GTT CCG 3′; TCRBV13S1: 5′ CAA GGA GAA GTC CCC AAT 3′; TCRBV13S2: 5′ GGT GAG GGT ACA ACT GCC 3′; TCRBV14: 5′ GTC TCT CGA AAA GAG AAG AGG AAT 3′; TCRBV15: 5′ AGT GTC TCT CGA CAG GCA CAG GCT 3′; TCRBV17: 5′ AGA TAT AGC TGA AGG GTA CAG CGT 3′; TCRBV20: 5′ AGC TCT GAG GTG CCC CAG AAT CTC 3′) and the TCRBC primer (βAI).
Aliquots of the TCRDV reverse transcription reaction were amplified with primers specific for TCRDV1 (official designation TCRDV101S1: 5′ TCG CCA GGG TTC TGA TGA ACA GAA 3′), TCRDV2 (official designation TCRDV102S1A1T: 5′ AGG AAG ACC CAA GGT AAC ACA A 3′), TCRDV3 (official designation TCRDV103S1A1T: 5′ GGT ACT GCT CTG CAC TTA CGA CAC 3′), TCRDV4 (official designation ADV6: 5′ AGC CCA GCA GTG GGG AAA TCG A 3′), TCRDV5 (official designation ADV21: 5′ ACC CTG CTG AAG GTC CTA CAC ATT CC 3′), and a second primer recognizing the TCRDC region (cδ: 5′ AAT TCC TTC ACC AGA CAA GCG ACA 3′). After an initial hot start, amplification consisted of 35 to 40 cycles of 1 minute at 94°C, 1.5 minutes at 58°C, and 1.5 minutes at 72°C for TCRDV1 and TCRDV3; 1 minute at 94°C, 1.5 minutes at 60°C, and 1.5 minutes at 72°C for TCRVD2 and TCRDV5; and 1 minute at 94°C, 1.5 minutes at 64°C, and 1.5 minutes at 72°C for TCRVD4, followed by a final extension for 7 minutes at 72°C. The specificity of these primers was assessed by the use of cDNA from TCRGD+ leukemia cell lines and lymphocytes of patients with lymphoproliferative diseases. All primer combinations failed to amplify a product when cDNA was omitted from the reaction or when irrelevant cDNA was used as substrate.
Heteroduplex and TCR sequence analysis.
Amplification products obtained from PCR performed with TCRBV- and TCRDV-specific primers were heated to 95°C for 5 minutes and then cooled to 50°C for 1 hour. The annealed samples, which were kept on ice until used, were run for 5 to 6 hours at 200 V at room temperature on a 12% nondenaturating polyacrylamide gel (PAGE; 29:1 acrylamide/bisacrylamide) performed in 1× TBE buffer (0.089 mol/L Tris-borate and 0.002 mol/L EDTA, pH 8.0). The gels were stained for 30 to 60 minutes at room temperature in the dark in a solution containing 0.75 μg/mL ethidium bromide in 200 mL of 1× TBE and were then photographed under UV light. Amplified TCRBV8 products from the T-cell line J77 and from lymphocytes stimulated with an anti-TCRBV8 segment monoclonal antibody were used as monoclonal and polyclonal TCRBV controls.21 TCRBV1-, TCRBV13S1-, TCRBV15-, and TCRDV3-amplified products from both PBMC and thymus were purified, cloned, and sequenced as described.22 Sequences were compared with published data relative to TCRBV, TCRBD, TCRBJ, TCRBC, TCRDV, TCRDD, TCRDJ, and TCRDC segments.23 D elements were assigned with the requirement for a minimum of three contiguous matches to the germline sequence.
RESULTS
Characterization of the molecular defect.
The analysis of RAG1 and RAG2 genes was performed on the genomic DNA of the patient and her parents, taking advantage of the absence of introns in the coding region of both genes. Sequence analysis showed homozygosity for a C to T transition at nucleotide 579 of the RAG1 gene, causing a missense mutation (alanine to valine) at codon 156. However, in view of the analysis of this mutant by Schwarz et al,17 this mutation can be considered as a polymorphism.
The RAG2 gene was amplified in 1 fragment and the product was directly sequenced. The analysis of the sequence showed the presence on both alleles of a missense mutation (G to A at the nucleotide 1887), leading to arginine to glutamine amino acid change at codon 229 (R229Q; data not shown). To further confirm the presence of this mutation, we cloned the PCR fragment into the TA vector and sequenced 10 independent clones. All of the examined clones bore the altered nucleotide. Both of these mutations were traced back to the phenotypically normal parents, who were heterozygous for both the RAG1 polymorphism and theRAG2 mutation. The latter was not identified in more than 100 independent alleles from control subjects of the same ethnic origin. A compound heterozygote patient, bearing a deletion of both RAG1 and RAG2 genes in one allele and theRAG2 R229Q mutation on the other allele, has been previously reported by Schwarz et al.17 The R229Q mutant had a severely decreased, albeit not abolished, V(D)J recombination activity17 and is therefore likely to be responsible for the Omenn phenotype in our patient.
Histopathology.
The histopathological changes of the lymph node and the skin were similar to those previously described in OS.12 Briefly, the lymph node parenchyma showed a moderate depletion of lymphocytes that were represented exclusively by T cells and were associated with numerous eosinophils and CD1a+ interdigitating cells. The T lymphocytes showed a mature peripheral phenotype (CD2+, CD3+, CD5+), with predominance of CD45R0+ over naive CD45RA+ cells; scattered blasts expressed CD25 and CD30. The analysis of the TCR expression showed that approximately half of the T cells were TCRAB+and the remaining were TCRGD+.
The spleen, the skin, and the mucosa-associated lymphoid tissues contained only a few scattered CD3+ T lymphocytes; also, B cells were totally absent from these tissues.
The thymic parenchyma showed lack of cortico-medullary demarcation and was largely represented by epithelial cells (stained with anticytokeratin antibody; Fig 1A), with depletion of CD3+ T cells (Fig 1B). The number of CD45R0+ lymphocytes largely exceeded that of CD45RA+ cells (Fig 1C and D); furthermore, most of T cells expressed the TCRGD (Fig 1E). The intrathymic nature of T lymphocytes was proven by the expression of the CD1a antigen (Fig 1F).
Subserial sections of the thymus stained with antibodies against cytokeratin (A), CD3 (B), CD45R0 (C), CD45RA (D), TCRGD (E), and CD1a (F). In a thymic lobule, which is largely composed of epithelial cells, the CD3+ lymphocytes predominantly express CD45R0 and TCRGD. Many lymphoid cells show delicate membrane reactivity for CD1a (streptavidin-biotin complex immunoperoxidase technique, counterstained with Mayer’s hematoxylin; original magnifications: [A] through [E], ×160; [F], ×400).
Subserial sections of the thymus stained with antibodies against cytokeratin (A), CD3 (B), CD45R0 (C), CD45RA (D), TCRGD (E), and CD1a (F). In a thymic lobule, which is largely composed of epithelial cells, the CD3+ lymphocytes predominantly express CD45R0 and TCRGD. Many lymphoid cells show delicate membrane reactivity for CD1a (streptavidin-biotin complex immunoperoxidase technique, counterstained with Mayer’s hematoxylin; original magnifications: [A] through [E], ×160; [F], ×400).
Analysis of TCRBV and TCRDV repertoires in PBMC and thymus.
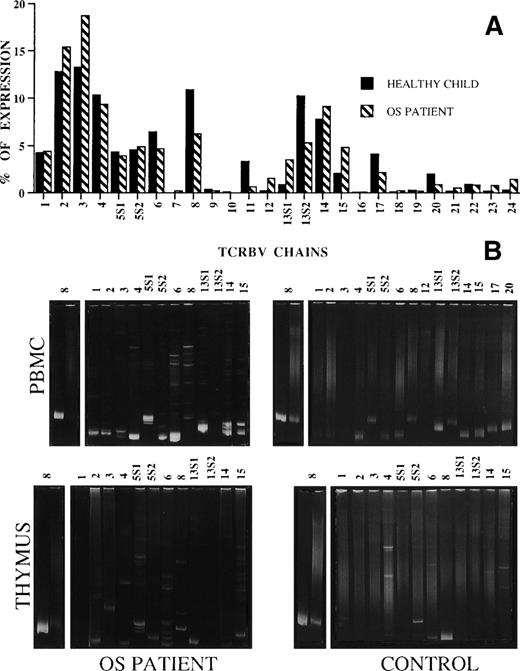
The general pattern of TCRBV usage by PBMC obtained from the patient did not significantly differ from that of unrelated age-matched healthy infants of the same ethnic origin16 and from that of the healthy infant used as a control in this study (Fig 2A). However, heteroduplex analysis of highly expressed TCRBV chains showed a truly polyclonal repertoire in the healthy control infant, but not in the patient. In fact, as shown in Fig 2B, all TCRBV products obtained from the healthy control’s PBMC migrated as smears in the polyacrylamide gel, indicating the presence of heterogeneous molecular species, whereas those from OS patients, with the only exception of TCRBV13S2 segment, resulted in single or double homoduplex bands in the context of a background of heteroduplex bands, thus suggesting the existence of mono/oligoclonal T-cell subsets within all TCRBV segments analyzed. This result is consistent with previous observations suggesting that the TCR repertoire is highly restricted in OS and that, at variance with what was found in elderly individuals,24 25 each TCRBV cell population is fully polyclonal in healthy infants.
(A) Expression of TCRBV segments by OS (▧) and a healthy age-matched infant (▪) PBMC. The data are expressed as the percentage of the color- imetric signal obtained with the individual TCRBV-specific probes. (B) Heteroduplex analysis of the indicated TCRBV chains’ PCR products of PBMC and thymocytes from the OS patient and the healthy control. On the right of each gel, monoclonal and polyclonal controls, prepared by loading in the gel the products obtained from TCRBV8-specific PCR amplification of the RNA of monoclonal (J77) and polyclonal (C1.632) cell populations,21 are shown.
(A) Expression of TCRBV segments by OS (▧) and a healthy age-matched infant (▪) PBMC. The data are expressed as the percentage of the color- imetric signal obtained with the individual TCRBV-specific probes. (B) Heteroduplex analysis of the indicated TCRBV chains’ PCR products of PBMC and thymocytes from the OS patient and the healthy control. On the right of each gel, monoclonal and polyclonal controls, prepared by loading in the gel the products obtained from TCRBV8-specific PCR amplification of the RNA of monoclonal (J77) and polyclonal (C1.632) cell populations,21 are shown.
Similarly, whereas in the control thymus most of the TCRBV chains were polyclonal, a restricted pattern of homo/heteroduplex bands was observed (although not as clearly as in PBMC) in thymocytes prepared from the patient.
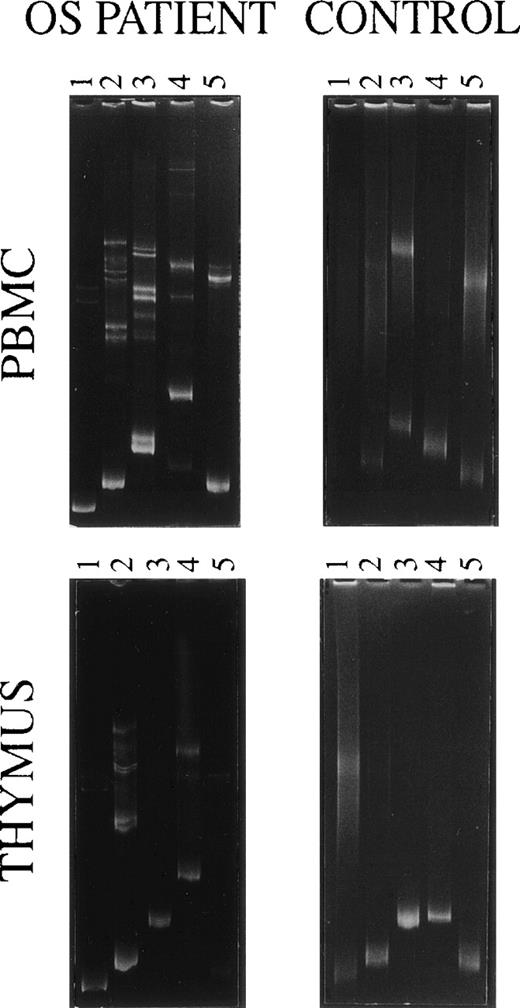
Using PCRs performed with TCRDV-specific primers followed by the heteroduplex analysis, a limited diversity was also demonstrated within TCRDV gene families from the patient’s PBMC and thymocytes (Fig 3, left). However, there were some differences between the 2 compartments: for instance, 2 homoduplex and several heteroduplex bands were obtained by loading in the polyacrylamide gel the TCRDV3 amplification product from PBMC, whereas a single band appeared when starting from thymocytes. Furthermore, all 5 TCRDV segments were amplified from PBMC, whereas thymocytes appeared to lack the message for the TCRDV5 chain. Smears only, indicative of a fully polyclonal TCRDV repertoire, were generated using PBMC and thymocytes from a healthy control, with the only exception being the TCRDV4 segment from the thymus that induced the formation of a faint homoduplex band (Fig 3, right).
Heteroduplex analysis of the indicated TCRDV chains’ PCR products of PBMC and thymocytes from the OS patient and an age-matched control.
Heteroduplex analysis of the indicated TCRDV chains’ PCR products of PBMC and thymocytes from the OS patient and an age-matched control.
Taken together, these results suggest that both peripheral and thymic TCRBV and TCRDV repertoires are highly restricted in the OS patient. This prompted us to examine the TCR repertoire in more detail. Therefore, to confirm the mono/oligoclonal nature of T-cell populations from the OS patient and to establish whether the clones observed in the peripheral blood were representative of those identified in the thymus, the nucleotide sequences of some TCRBV and TCRDV segments were determined in both samples. All the sequenced cDNA clones resulted from in-frame rearrangements and, therefore, were likely to correspond to productive TCR transcripts.
We chose to sequence the TCRBV13S1 chain because it appeared to be clonal in both peripheral and thymic lymphocytes and the TCRBV15 segment because it resulted in 2 very distinct homoduplex bands in the periphery and in a single band in the thymocytes. We also sequenced the TCRBV1 PCR product that was detectable only in the periphery and not in the thymus to ensure that it was not the result of a technical artifact.
The global analysis of the sequences derived from patients’ PBMC and thymocytes showed a predominance of 2 or a few clones within each of the TCRBV transcripts analyzed (Fig 4). In particular, the TCRBV13S1 and TCRBV15 clones, both bearing TCRBJ1S5 region, that were predominant in peripheral blood were also found in the thymus, where, however, they did not represent the prevalent clones. On the other hand, the TCRBV13S1 clones that were predominantly expressed in the thymus were not represented in the periphery. As expected, cDNA TCRBV clones generated from thymocytes from a control infant were free of predominant sequences.
Junctional nucleotide and amino acid TCRBV1, TCRBV13S1, and TCRBV15 sequences obtained from PBMC and thymocytes from the OS patient and from the age-matched control (C). Amino acid sequences are shown on the right side and displayed using the standard 1-letter code. Only the last amino acids of the TCRBV chains and the first 5′ amino acids of TCRBJ segments are shown. N/P, nontemplated and palindromic nucleotides; freq, number of each clone in the total cDNA clones characterized; °, junctions in which D regions cannot be assigned; *, clone with the following N nucleotides: CTGTCTCCCCGCATGTTAGCTCCTC.
Junctional nucleotide and amino acid TCRBV1, TCRBV13S1, and TCRBV15 sequences obtained from PBMC and thymocytes from the OS patient and from the age-matched control (C). Amino acid sequences are shown on the right side and displayed using the standard 1-letter code. Only the last amino acids of the TCRBV chains and the first 5′ amino acids of TCRBJ segments are shown. N/P, nontemplated and palindromic nucleotides; freq, number of each clone in the total cDNA clones characterized; °, junctions in which D regions cannot be assigned; *, clone with the following N nucleotides: CTGTCTCCCCGCATGTTAGCTCCTC.
Sequence analysis of TCRDV chains demonstrated that the degree of diversity of the TCRDV repertoire in the OS patient appeared to be slightly different from that of TCRBV. Indeed, despite the presence of dominant clones, a residual polyclonal background could be clearly documented in the thymus; moreover, in peripheral blood, of the 10 clones sequenced, 3 different sequences were identified (Fig 5). However, as in the case of TCRBV sequences, 2 of the clones found in peripheral blood were also detected in the thymus, whereas the dominant sequences observed in thymocytes were not detectable in the periphery.
Junctional nucleotide and amino acid TCRDV3 sequences obtained from PBMC and thymocytes from the OS patient and the control (C). Germline sequences are indicated at the top and are double underlined. Amino acid sequences are shown on the right side and displayed using the standard 1-letter code. Only the last amino acids of the TCRDV chains and the first 5′ amino acids of TCRBJ segments are shown. N/P, nontemplated and palindrome nucleotides; freq, number of each clone in the total cDNA clones characterized.
Junctional nucleotide and amino acid TCRDV3 sequences obtained from PBMC and thymocytes from the OS patient and the control (C). Germline sequences are indicated at the top and are double underlined. Amino acid sequences are shown on the right side and displayed using the standard 1-letter code. Only the last amino acids of the TCRDV chains and the first 5′ amino acids of TCRBJ segments are shown. N/P, nontemplated and palindrome nucleotides; freq, number of each clone in the total cDNA clones characterized.
Finally, all of the TCRDV3 sequences from the OS patient were joined in frame with the TCRDJ1S1 region, but this feature appeared to be a characteristic of this V segment, because it can be observed also in all the sequences obtained from the control thymocytes and in other published sequences.26
TCRBV and TCRDV repertoire diversity of tissue-infiltrating lymphocytes.
We next investigated the TCRBV and TCRDV repertoires in several other tissues (skin, lymph node, spleen, bone marrow, and intestine) to determine whether an oligoclonal pattern was also detected in these tissues and, if so, to assess whether it recapitulates the pattern observed in peripheral blood or in the thymus (indicating nonspecific lymphocytic infiltrates) or whether further compartmentalization of TCR specificities occurs in different organs and tissues.
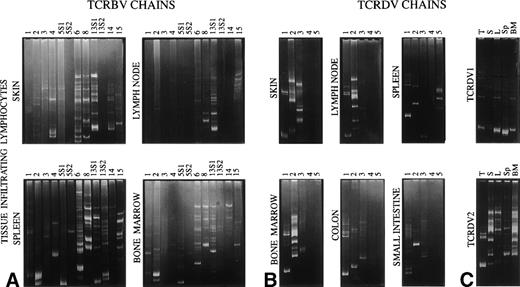
Only oligoclonal TCRBV6 and TCRBV13S1 segments were detected in the colon (data not shown); the other TCRBV-specific primers, which reproducibly amplified other samples, did not show the presence of additional TCRBV families in the colon and in the small intestine. All of the 12 TCRBV families studied could be amplified from the spleen and bone marrow, and 9 were expressed in the skin. Only 6 transcripts were detected in the lymph node. The TCRBV13S2 amplicon that was undetectable in the other samples prepared from the OS patient, including PBMC and thymus, was identified in the splenic tissue and in the bone marrow. The heteroduplex analysis demonstrated variable patterns of homoduplex/heteroduplex TCRBV bands in the different tissues (Fig 6A), with TCRBV1, TCRBV2, TCRBV3, TCRBV4, TCRBV5S1, and TCRBV5S2 segments being more heterogeneous than the other TCRBVs. With regard to TCRGD lymphocytes, TCRDV1, TCRDV2, and TCRDV3 chain amplified products were obtained from all tissues analyzed, whereas TCRDV4 and TCRDV5 segments were detected only in the spleen. Although with different patterns, most of these transcripts migrated in the gel of polyacrylamide as discrete bands of homo/heteroduplex, thus confirming the restriction of the TCRDV repertoire also in target organs (Fig 6B). When run in parallel, the TCRDV1 and TCRDV2 amplification products, which were obtained from different tissues, showed both common and tissue-specific bands, indicating that some TCR clonotypes overlap, but that other dominant clonotypes are preferentially expanded at specific sites (Fig 6C).
Heteroduplex analysis of TCRBV (A) and TCRDV (B) chains in lymphocytes infiltrating the indicated tissues. In (C), the amplification products of TCRDV1 and TCRDV2 obtained from different tissues (T, thymus; S, skin; L, lymph node; Sp, spleen; BM, bone marrow) are run in parallel.
Heteroduplex analysis of TCRBV (A) and TCRDV (B) chains in lymphocytes infiltrating the indicated tissues. In (C), the amplification products of TCRDV1 and TCRDV2 obtained from different tissues (T, thymus; S, skin; L, lymph node; Sp, spleen; BM, bone marrow) are run in parallel.
Effect of RAG mutations on the V(D)J recombination process in OS.
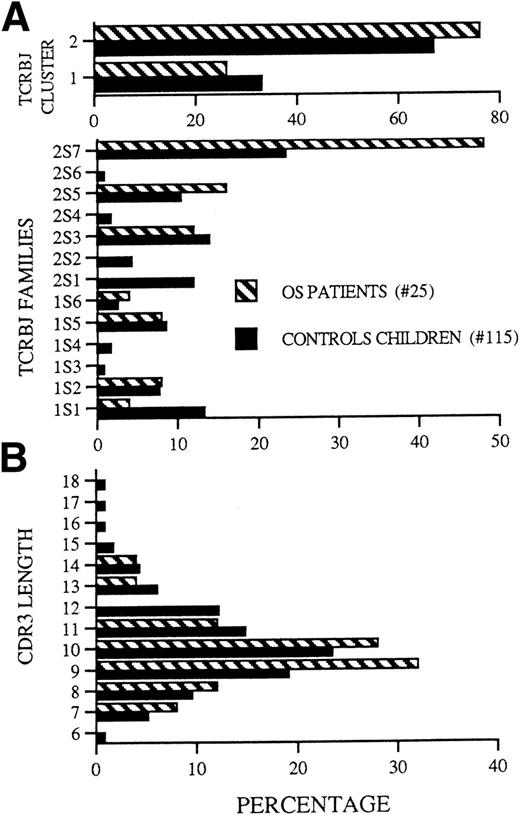
Among 130 sequences obtained from PBMC and thymocytes from this and 2 other molecularly defined OS patients observed at our institution (OS patients no. 1 and 4 from Villa et al16), 25 were different from each other. As compared with a group of 115 sequences prepared from age-matched children and used as control, these sequences showed a statistically significant bias (P = .0002) in the TCRBJ2 versus TCRBJ1 cluster use (Fig 7A, top panel). Furthermore, when compared with controls, lymphocytes from OS patients predominantly use the TCRBJ2S7 segment (P = .031); in contrast, the TCRBJ2S1 and TCRBJ1S1 segments are much less expressed in patients with OS than in controls (Fig 7A, bottom panel).
(A) (Top) Frequency of TCRBJ1 and TCRBJ2 cluster usage. TCRJ regions frequencies were calculated from the data set and are shown here as a histogram. (Bottom) Frequency of individual TCRBJ usage. (B) Lengths of the CDR3 loops. The number of OS sequences is 25. The number of sequences from age-matched children is 115.
(A) (Top) Frequency of TCRBJ1 and TCRBJ2 cluster usage. TCRJ regions frequencies were calculated from the data set and are shown here as a histogram. (Bottom) Frequency of individual TCRBJ usage. (B) Lengths of the CDR3 loops. The number of OS sequences is 25. The number of sequences from age-matched children is 115.
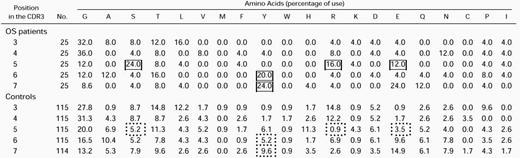
Because the length and amino acid composition of the antigen-combining site (CDR3) of the TCR may provide information about the factors involved in the rearrangement process or about the nature of ligand(s) that select previously rearranged segments, we compared the length and amino acid composition of the CDR3 elements of the TCR in the 3 OS patients reported above and in sequences from age-matched controls. In both groups, most of CDR3 elements were 9 or 10 amino acids long (Fig7B), with a broader length range in the control than in the OS group (6 to 18 v 7 to 14). Furthermore, the mean CDR3 length was shorter in the OS group than in controls (Table1a); this difference became significant only when the analysis of OS patients was extended to include all the TCR sequences obtained from OS patients published so far (this work and previous studies13-16). However, detailed analysis of the molecular mechanisms that may modify the structure of the TCR transcripts at the V-D and D-J junctions failed to show significant abnormalities in patients with OS versus controls (Table 1c through f), with the possible exception of a higher frequency of complete V and J elements (Table 1b). Although common amino acid stretches were not identified within the CDR3 of individual patients or among different patients, there was a biased usage of particular amino acids in certain positions of the CDR3 region (Table 2). As already reported for murine and human adults’ TCR sequences,27 28glycine (G) is by far the most frequently used residue in the TCR CDR3 sequences from both OS patients and control children. Moreover, the amino acids serine (S), tyrosine (Y), arginine (R), and asparagine (N) were significantly more represented at specific positions of the CDR3 in OS patients as compared with sequences from age-matched controls.
DISCUSSION
OS is a rare, severe immune deficiency with well-defined clinical and immunological features that is most often due to mutations in theRAG genes. In our series, among 8 patients in which a diagnosis of OS was based on typical clinical and immunological features, 6 showed mutations in either RAG1 or RAG2 (Villa et al16 and data not shown). The residual expression and function of the RAG mutants identified in most OS patients allow a partial V(D)J recombination activity16 and account for the peculiar leakiness of OS as compared with its allelic SCID variant with undetectable T and B lymphocytes (T−B− SCID) that is due to severeRAG gene mutations.17 We have reported a patient with OS who carried a homozygous mutation in the RAG2 gene leading to the R229Q amino acid substitution. The same patient was also homozygous for the alanine to valine substitution at codon 156 of theRAG1 gene. Both changes have been previously reported by Schwarz et al17; in particular, the A156V change in theRAG1 gene was shown not to affect the efficiency of the recombination process using an extrachromosomal V(D)J assay and is therefore considered a polymorphism. In contrast, the R229Q substitution was reported in 1 allele of the RAG2 gene from an SCID patient who carried a complete deletion encompassing bothRAG1 and RAG2 genes on the second allele. When transfected into a human fibroblast cell line, the RAG2 R229Q mutant was found to induce RAG2 protein expression at comparable levels as the wild-type RAG2 construct; however, when assayed for in vitro V(D)J recombination, the R229Q mutant showed profound defects in both coding and signal joint formation.17 Interestingly, the immunological phenotype of the patient with the R229Q RAG2mutation reported by Schwarz et al17 differed from typical T−B− SCID because of a large proportion (59%) of autologous CD3+ lymphocytes. The possibility of gene reversion was ruled out by mutation analysis. Moreover, the T lymphocytes from this patient were found to express an oligoclonal TCRAV and TCRBV repertoire.17 As a whole, our data and those reported by Schwarz et al17 indicate that the R229Q RAG2 mutant allows residual V(D)J recombination activity. Although no data are available on the clinical features in the patient described by Schwarz et al,17 our patient had typical clinical and immunological features of OS. Whether the same leaky mutation in RAG genes may result in a different clinical and immunological phenotype (depending on background genetic or environmental factors) awaits the investigation of a larger series of patients.
It is known for several years that OS is characterized by an intrinsic defect of the lymphocyte lineage. In fact, in OS patients, B lymphocytes are usually absent and the T-cell phenotype and repertoire are profoundly abnormal. In most cases, T lymphocytes from OS patients belong to the TCRAB lineage and show an unbalanced CD4/CD8 ratio that may, however, vary from patient to patient.2,6,7 In a minority of OS patients, an increased or even predominant proportion of T cells that express the TCRGD is observed.6,7Occasionally, OS patients present a predominant TCRAB+CD4−CD8− population.29Therefore, it appears that CD4/CD8 homeostasis that is under genetic control in healthy individuals30 may be lost in OS patients.
There are several pieces of evidence suggesting that, independently from the type of TCR expressed, the T-cell repertoire of OS patients is highly restricted.6,13-16 This peculiarity has been recently ascribed to mutations that impair, but do not completely abolish, the function of RAG1 and RAG2genes.16 Apparently, all of the TCRBV gene elements could be used by OS patients to create functional TCR, but the diversity within each TCRBV family is very limited. The resulting TCR repertoire strongly differs from the fully polyclonal pattern of healthy children, but rather resembles that of patients with acute (Epstein-Barr virus) or chronic (human immunodeficiency virus) infections.31-34 On the other hand, the possibility that restriction of the T-cell repertoire in OS is mainly due to chronic infections occurring in infants with combined immune deficiency is unlikely, in view of the fact that a polyclonal T-cell repertoire was identified in patients with major histocompatibility complex class II deficiency,35 even if chronically infected (our own data; data not shown).
The demonstration of restricted TCRAB and TCRGD repertoires in the thymus from the OS patient might either reflect an intrinsic developmental problem within this organ or recirculation and immigration of a few clonotypes that have undergone peripheral expansion. To discriminate between the 2 possibilities, we have compared the T-cell repertoire in the thymus with that from peripheral blood and tissue-infiltrating T lymphocytes. We found that only a few TCRBV and TCRDV specificities were expressed in thymocytes as well as in circulating and in tissue-infiltrating lymphocytes. In particular, the observation that the sequence of the predominantly expressed specificities are different in the thymus versus the periphery and that some specificities are uniquely found in the thymus argues against the possibility that the repertoire identified in the thymus simply reflects that of circulating T lymphocytes that have re-entered the thymus. To further prove that the restriction of the T-cell repertoire identified in the thymus reflects an intrinsic developmental problem within this organ, rather than recirculation and immigration from the periphery, we have performed a detailed immunohistochemical analysis to identify the origin of thymic T cells. These lymphocytes were predominantly TCRGD+ and CD45R0+; coexpression of the CD1a antigen indicated their thymic-cortical origin. It should be noted that, even in normal thymuses, cortical thymocytes are CD45R0+.36
As a whole, our data indicate that the defect in the RAG2 gene in this patient resulted in a restricted T-cell repertoire already in the thymus, but our data do not allow us to distinguish between the possibility of a primary repertoire restriction event versus selective activation and expansion (possibly in response to autoantigens) of individual clonotypes within this organ. The latter hypothesis is supported by the demonstration that in our patient both thymocytes and peripheral blood T cells express a similar proportion of different V-gene families, suggesting that patients with OS have the potentiality to generate multiple TCR specificities. The residual background demonstrated in the heteroduplex analysis, particularly within thymic TCRDV segments, and the occurrence of clonotypes that, although predominantly expressed in the thymus, are not detectable in the periphery further indicate active, ongoing intrathymic lymphopoiesis in OS.
We also found that the diversity of some TCR segments appeared to be different in the various peripheral tissues, with some TCR families, such TCRBV1 and TCRBV13S2 or TCRDV4 and TCRDV5, being detectable in some samples, but not in others. Furthermore, comparison of TCRDV1 and TCRDV2 amplification products obtained from different tissues, showed that, whereas some TCR clonotypes are widely distributed, others appear to be tissue-specific. These features argue against a simple compartmentalization of T-cell clones that are preferentially rearranged and/or expanded in the thymus, but rather suggest that the poorly functional and diverse T cells of OS patients may nonetheless be capable of undergoing antigen-mediated expansion in peripheral tissues. The higher representation of serine, tyrosine, arginine, and asparagine at specific CDR3 positions of the 25 distinct clones derived from this patient and from 2 previously reported patients (patients no. 1 and 4 from Villa et al16) further suggests the possibility of antigen-driven selection of T lymphocytes in OS. In view of the clinical features of OS that to some extent resemble what has been observed in graft-versus-host disease, it has been suggested that the process of peripheral T-cell expansion is driven by autoantigens,13 although this has not been formally proven.
The process of V(D)J recombination involves several proteins in addition to RAGs. In particular, the addition of exogenous nucleotides (N diversity) mediated by the terminal deoxynucleotidyl transferase (TdT) or of palindromic sequences (P nucleotides) due to asymmetrical opening of the hairpin or deletional events results in modification of the sequence at the border between V-D and D-J segments, thus contributing to variability of the CDR3 element that plays an important role in antigen-binding specificity.37,38 The possibility that RAG genes mutations affect the structure of CDR3 has been implied by previous observations and assessed in this study. Harville et al14 found a lack of N nucleotides in TCR sequences from OS patients, but this finding has not been confirmed in other recent studies.13,15 However, the OS patients reported in these studies were not characterized for RAG genes defects, making it difficult to draw conclusions on the possible effects of RAGgenes on the efficiency of N nucleotides insertions and CDR3 modification. In the series of 25 different TCR sequences from our 3 patients with OS due to RAG defects, we have conclusively shown that the addition of N nucleotide is intact in OS. A global evaluation of the structure and length of the CDR3 showed a shorter mean length in infants with OS as compared with age-matched controls; however, this difference was statistically significant only when the sequences from other OS patients (reported by other groups13-15) were included. Apart from the limit of including patients with unknown genetic defects, it appears that the difference of the mean CDR3 length between OS patients and controls is marginal (9.42 v 10.16), with obvious overlap in the range between the 2 groups. Its real relevance in antigen-binding may therefore be questioned. Moreover, we did not find evidence for abnormalities in P nucleotide insertion or in trimming of the coding elements. Similar observations were made by Schwarz et al17 in patients with T−B− SCID due to RAGmutations that severely affect RAG protein expression and/or function. Although the number of coding joints formed by the RAG mutants in an extrachromosomal V(D)J assay was dramatically reduced as compared with wild-type RAG proteins, the quality of the rare coding joints, including nucleotide loss and addition, was indistinguishable from normals.17 Similarly, although B− SCID patients exhibit an irregular recombination pattern at the JH locus, it has been shown that their recombination machinery is competent for the addition of P and N nucleotides.39
The preferential usage of the TCRBJ2 cluster in 25 distinct sequences from 3 patients with OS (ie, the patient described here and patients no. 1 and 4 from Villa et al16), rather than indicating preferential association of the mutant RAG proteins with recombination signal sequences (RSS) in this region, is due to the fact that rearrangements occur only through a mechanism that involves deletion of the intervening sequences; therefore, the TCRBD2S1 segment can only join to TCRBJ2, whereas TCRBD1S1 may recombine with either the TCRBJ1 or the TCRBJ2 clusters.
In conclusion, we have shown that the generation of an altered T-cell repertoire in OS reflects both an intrathymic restriction and peripheral, (auto)antigen-driven expansion. Moreover, the RAGgenes defect that accounts for the disease directly affects early stages of V(D)J recombination, but does not alter the process of double-strand-break DNA repair, including N and P nucleotide insertion.
ACKNOWLEDGMENT
The authors thank Olga Alebardi for her technical assistance.
Supported by Istituto Superiore di Sanità Grant No. 30A.0.26 (to L.I.), Telethon Grants No. E.668 (to L.D.N.) and E.495 (to A.V.), Biomed2 concerted action CT 983007, MURST (co-finanziamento 1997 to L.D.N.), CNR P.F. Biotecnologie, and Cariplo (paper no. 29).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Luigi D. Notarangelo, MD, Department of Pediatrics, University of Brescia, Spedali Civili, 25123 Brescia, Italy; e-mail: notarang@master.cci.unibs.it.

![Fig. 1. Subserial sections of the thymus stained with antibodies against cytokeratin (A), CD3 (B), CD45R0 (C), CD45RA (D), TCRGD (E), and CD1a (F). In a thymic lobule, which is largely composed of epithelial cells, the CD3+ lymphocytes predominantly express CD45R0 and TCRGD. Many lymphoid cells show delicate membrane reactivity for CD1a (streptavidin-biotin complex immunoperoxidase technique, counterstained with Mayer’s hematoxylin; original magnifications: [A] through [E], ×160; [F], ×400).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/10/10.1182_blood.v94.10.3468.422k34_3468_3478/6/m_blod422sig01z.jpeg?Expires=1769209024&Signature=oOKy-rz7eeuvjpYKwFFFqlSA9M1v1~Z1Tb2toCpi~71MEDY26fqLTZ~qm9YJhPG8aJdvR-o3lDV6pYjgHNygKqtGs2dKpXCGvPWI6KfYMXqA7rT1m1qbHoZoN1i9f3bxukeGv2miSce6PByeNrguHwCi0mUkXv0Z2z3HTbyRKAIH5DnxsBg9zR4gc4K8jdPu~AdSbCVSB6Z7QNHB2fc68vF3Nhh~BLIBpUn8mKIiN9l9vQSudkelKy9tghQjShaK2CnP1CpY9u-N9Ts8j6Or6HPjm97duga0CLemlmZ2oUUnr2qersSO94SrKo2p31vdg7IFI-wNiUU3nsHVj~zXRQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)