Costimulatory signals supplied by genetically modified tumor cells can enable T-cell recognition of tumor-associated antigens that were previously silent when presented by unmodified tumor cells. Although the mechanism of the CD80/CD28 costimulation has been studied extensively in the normal T-cell/antigen-presenting cell (APC) interactions, it is unclear how expression of CD80 by tumor cells mediates its effect. We demonstrate here that optimal CD80 expression on a leukemic cell enhances T-cell recognition of alloantigen primarily by lowering the level of T-cell receptor (TCR) stimulation required for activation. CD80 expression by leukemic cells leads to increased survival of activated T cells by inducing upregulation of the antiapoptotic protein BCL-2, but not BCL-XL. The cytokine microenvironment in which T cells are activated is crucial in determining their differentiation and consequently the nature of the immune response generated. Many tumor cells produce immunosuppressive cytokines that may not favor the induction of cell-mediated immunity. In this study, the presence of CD80 on leukemic cells increased T-cell activation in vitro, but this did not result in the production of Th1 cytokines. We show that this is due to a leukemia-derived soluble factor that inhibits the production of Th1 cytokines. Optimal expression of a costimulatory molecule, therefore, enhances the ability of leukemic cells to present antigen by amplifying TCR signals, but the microenvironment generated by leukemic cells may suppress the immune response required for their eradication. Thus, strategies aimed at inducing antileukemic immunity by providing leukemic cells with costimulatory functions must ensure the presence of an appropriate microenvironment.
ANTIGEN-SPECIFIC T-CELL activation is a multistep process requiring at least 3 steps for optimal response: (1) contact with antigen-presenting cells (APCs) through cell adhesion molecules (CAM); (2) antigen recognition by the T-cell receptor (TCR); and (3) costimulation (eg, CD80:CD28). In the early stages of disease, most malignant cells express major histocompatibility complex (MHC) and CAMs; thus, the absence of costimulation may be one mechanism by which malignant cells bearing tumor-associated antigens (TAA) can escape immune surveillance. Consequently, provision of costimulatory molecules on malignant cells by a variety of techniques1-3 has been explored as a means of inducing antitumor immunity. Several studies, both in vivo and in vitro, have demonstrated that expression of members of the B7 family of costimulatory molecules (CD80 [B7.1] and CD86 [B7.2]) can induce immune responses to both the modified cells and, subsequently on rechallenge, the parental tumor cell.4,5 Gene transfer of CD80 into human and murine tumor cells,6,7 including leukemias,1,8,9 has been shown in vitro to increase T-cell activation, enable generation of tumor-specific cytotoxic T lymphocytes (CTLs),10 and increase the repertoire of tumor-specific CTLs.11 Although the role of CD80 in the context of normal APC:T-cell interaction has been extensively studied, the mechanism for its activity in the tumor setting has not been investigated in detail.
The precise role of CD80:CD28 costimulation in T-cell activation has not been fully established, although it is recognized as playing an important role in the outcome of T-cell activation. CD28 stimulation augments production of interleukin-2 (IL-2),12 enabling activated T cells to progress through the cell cycle,13 and also upregulates expression of anti-apoptotic proteins14-16protecting T cells from activation-induced cell death. It is unclear from these studies if CD28 produces its anti-apoptotic effect via a direct mechanism or via IL-2. Activity of CD28 is balanced by the other CD80/CD86 receptor, CTLA4, as evidenced by the lymphoproliferative disorder that develops in CTLA4-deficient mice.17 CTLA4 has a higher affinity for both CD80 and CD8618,19 and induces an opposing signal.20 However, unlike CD28, CTLA4 is only expressed on the surface by activated T cells. Therefore, the consequence of costimulation through CD80 is dependent on its level of expression and also on the relative expression of CD28 and CTLA4 on the T cell, ie, the activation status.
Although the decision to initiate an immune response is dependent on suitably primed APCs, the cytokine microenvironment plays an important role in determining the nature of any response generated. Cytokines mediate their effect by influencing the Th1/Th2 decision during T-cell differentiation. In addition, the pattern of cytokines secreted by primed T cells determines which effector arms are recruited in response to a given antigen. An immune response is only beneficial if the appropriate effectors are induced, eg, antitumor immunity is generally considered to require a cell-mediated Th1 response. Many tumor cells produce cytokines (eg, IL-10,21-23 transforming growth factor-β [TGF-β],24-30 and vascular endothelial growth factor [VEGF]31) and other soluble factors (soluble IL-2 receptor [sIL-2R],32 soluble tumor necrosis factor receptor [sTNF-R],33 and soluble Fas ligand [sFas-L]34,35) that may influence the nature of immune responses. It is possible, therefore, that the presence of costimulatory molecules may not have the desired effect on antitumor immunity by initiating T-cell activation in an immunosuppressive environment, which may inadvertently induce the generation of tolerance.31 36-38
The aim of this study has been to address the mechanisms underlying the enhanced T-cell recognition and proliferation when encountering alloantigen presented by CD80-expressing leukemic cells. We have used here an acute leukemia model to demonstrate that one of the major roles of the CD80:CD28 interaction is through the amplification of TCR signals. In addition, we have assessed the effect of CD80 expression on enabling clonal expansion by prevention of activation-induced cell death. To establish the nature of the immune response in this model, we have analyzed the cytokine profile produced in response to tumor cells expressing CD80 and compared it with that induced in response to professional APCs, ie, dendritic cells (DCs). The results of this study show a clear effect of CD80 on enhancing the initiation of T-cell activation and survival, but in a tumor microenvironment, the secretion of soluble factors may inhibit the production of the desired cell-mediated immune response.
MATERIALS AND METHODS
Cells.
U937 cells (a human monocytic leukemia cell line)39 were obtained from the American Type Culture Collection (ATCC; Rockville, MD) and grown in RPMI/10% fetal calf serum (FCS; Sigma, Dorset, UK). Peripheral blood mononuclear cells (PBMNCs) and T cells were obtained from normal donors. T cells were purified by positive selection of CD4 and CD8 cells using immunomagnetic beads (Dynal, Oslo, Norway) and beads were detached using the detachment reagent (Dynal) according to the manufacturer’s instructions. CTLL-2 cells were maintained in RPMI/10% FCS and 10 U/mL recombinant human IL-2 (rhIL-2; Chiron, Harefield, UK). For the generation of peripheral blood-derived DCs, PBMNCs were incubated in RPMI/10% FCS at 37°C and 5% CO2 for 2 to 4 hours, and nonadherent cells were removed. Adherent cells were then cultured for 7 to 10 days in the presence of 50 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF; Schering Plough, Suffolk, UK) and 1,000 U/mL IL-4 (Schering Plough). DC phenotype was confirmed by flow cytometry (see below), with purities ranging from 70% to 85%.
Flow cytometric analysis.
U937 cells were stained using the following antibodies; anti-CD80, anti-HLA-DR (Becton Dickinson, Oxford, UK), anti-CD86 (Serotec, Oxford, UK), and anti-MHC class I (Cymbus Biosciences, Southampton, UK). Biotinylated-CTLA4.Ig (Ancell Corp, Bayport, MN) was used to confirm the ability of CD80 to bind to one of its receptors and was also used in functional blocking studies. Confirmation of DC phenotype was by dual-labeling using anti-CD1a and anti-CD14 (Serotec; CD1a+, CD14−). Flow cytometric analysis was performed using a FACS Vantage (Becton Dickinson) and Cellquest software (Becton Dickinson).
Generation of CD80+ U937 cells.
Initially, CD86− U937 cells were obtained by fluorescence-activated cell sorting (FACS) cells previously cultured for 48 hours in 20 μg/mL phorbol myristate acetate (PMA; Sigma), which increases CD86 expression (data not shown). The sorted CD86− negative cells failed to express CD86 on further exposure to PMA. U937 cells were transduced using a myeloproliferative sarcoma virus (MPSV)-based retroviral vector encoding human CD80 cDNA with Hygromycin-resistance gene as a selectable marker.1 40Hygromycin-resistant cells were then further sorted by FACS for high CD80 expression (U937-CD80 cells). Control empty vector infected cells were selected for Hygromycin resistance and subsequently sorted for absence of CTLA4.Ig binding (U937-M3P).
Proliferation assays.
PBMNCs or T cells (>98% purity) were cocultured for 5 days at 105/well in triplicate with irradiated control U937-M3Ps, U937-CD80 cells, or third-party DCs at the stated concentrations. U937 cells were irradiated with 100 Gy and third-party DCs with 35 Gy. On day 5,3H-thymidine (0.5 μCi/well; Amersham International, Little Chalfont, UK) was added for the final 20 hours. The cells were then harvested and 3H-thymidine incorporation was measured by liquid scintillation. Where indicated, CTLA4.Ig (10 ng/mL) was added to block CD80 interaction with its cognate receptors.
Helper T lymphocyte precursor cells (HTLp) frequency analysis.
The frequency of responding HTLp cells was assessed by limiting dilution analysis as previously described.41 Briefly, PBMNCs were added to irradiated stimulator cells (control U937-M3P or U937-CD80) at serial dilutions. Each dilution was performed in replicates of 24, as were wells containing stimulators or responders alone. After 4 days, 100 μL of supernatant was transferred to a new plate. CTLL-2 cells, previously starved of IL-2 for 24 hours, were then added at 104/well. 3H-thymidine (0.5 μCi/well) was added after 8 to 10 hours and incorporation was measured after a further 20 hours. Wells were scored as positive if the reading exceeded 3 standard deviations above the mean for the stimulator alone wells. Frequency analysis was calculated by maximum likelihood statistical analysis, using software kindly provided by Dr Peter Brookes (Hammersmith Hospital, London, UK).41
Calcium mobilization.
PBMNCs were resuspended to 107/mL in RPMI and labeled with 3 μmol/L Indo-1 (Molecular Probes, Eugene, OR) for 30 minutes at 37°C, washed, and resuspended in RPMI at 106/mL. Cells were then mixed with either U937-M3P or U937-CD80 cells at a 1:1 ratio. Kinetic studies of intracellular calcium concentration were performed on a FACS Vantage equipped with 488 nm and UV lines (60 mW). The calcium concentration was determined by the bound:free Indo-1 ratio (ie, fluorescence at 480 nm:530 nm). By gating on lymphocytes, leukemic cells were excluded, on the grounds of both scatter properties and Indo-1 labeling. A concentration of anti-CD3 (clone OKT-3; Janssen-Cilag, Bucks, UK) was established (ie, 0.1 ng/mL) that, with cross-linking using a final dilution of 1/175 RAM (Rabbit antimouse Ig; Dako A/S, Glostrup, Denmark), gave suboptimal activation of lymphocytes in the presence of U937-M3Ps. This experiment was then repeated either with the addition of 1 μg/mL of anti-CD28 (clone CD28.2; PharMingen, San Diego, CA) or in the presence of U937-CD80 cells. Control experiments were performed using anti-CD28 or U937-CD80 without anti-CD3. Adequate Indo-1 labeling was confirmed by stimulation with ionomycin (2 μg/mL).
Viability of activated T cells.
Mixed lymphocyte:leukemic cell reactions (MLLRs) were set up using PBMNCs (2 × 106/mL in RPMI with 10% autologous serum) mixed at a responder:stimulator ratio of 5:1 with either irradiated U937-M3Ps or irradiated U937-CD80s and then incubated at 37°C and 5% CO2 for 4 days. Cells were then labeled using fluorescein isothiocyanate (FITC)-anti-CD69, FITC-anti-CD25 (Bioproducts, Heidelberg, Germany), phycoerythrin (PE)-anti-CD4, and PE-anti-CD8 (Sigma). Dual-labeled cells were sorted by FACS and then plated at a concentration of 1 × 105/mL in complete medium containing 30 U/mL rhIL-2. Sorted T cells from the U937-M3P MLLRs were also plated in conditioned media from the original U937-CD80 MLLR containing 30 U/mL IL-2. Viabilities were established using trypan blue exclusion.
Measurement of BCL-2 and BCL-XL.
MLLRs were set up as described above, and a positive control was incorporated using PBMNCs stimulated with anti-CD3 and anti-CD28. After 4 days, PBMNCs (106) were dual-labeled for surface CD25 (Bioproducts) and intracellular BCL-2 (Dako) or BCL-XL(Zymed Laboratories Inc, San Francisco, CA) using a commercially available fix and permeabilization kit (Fix and Perm; Caltag Laboratories, Burlingame, CA). Cells were initially fixed and labeled with the unconjugated intracellular antibodies according to the manufacturer’s instructions. Cells were washed in ice-cold phosphate-buffered saline containing 2.5% FCS (PBSF), and PE-labeled secondary antibody (rabbit antimouse 1:30 dilution; Dako) was added for 20 minutes at room temperature. After a further wash in PBSF, cells were incubated for 10 minutes at room temperature with pure mouse Ig (Sigma) followed by FITC-anti-CD25 for 20 minutes at 4°C. Cells were then washed in PBSF and resuspended in PBS. Flow cytometric analysis was performed using a FACS Vantage. Gates were set on the lymphocyte region. For each experiment, an appropriate isotype-matched control antibody (IMC) was used to determine the level of nonspecific binding. The level of protein expression in CD25+ cells was calculated by subtracting the mean fluorescence intensity (MFI) for the IMC from that with the specific antibodies. A control assay was set up to confirm the specificity of the BCL-XL antibody in a flow cytometric-based assay. Normal PBMNCs were either left unstimulated or activated for 48 hours with anti-CD3 alone or anti-CD3 and anti-CD28. Intracellular staining and flow cytometric analysis was as described above.
Cytokine analysis.
Cytokine analysis was performed on 5-day MLLRs using enzyme-linked immunosorbent assay (ELISA) kits, IL-2, IL-4, IL-10, and γ-interferon (γ-IFN; Genzyme, Cambridge, MA), as per the manufacturer’s instructions. The detection limits were 10, 6, 5, and 6 pg/mL for IL-4, IL-2, IL-10, and γ-IFN, respectively.
For intracellular cytokine analysis, PBMNCs were cultured at 106/mL in the presence of a transwell insert (pore size, 0.4 μm; Falcon, Becton Dickinson) containing U937 cells at 105/mL for 24 hours. Cells were then activated with PMA (10 ng/mL) and Ionomycin (1 μg/mL) overnight in the presence of monensin (1.4 μg/mL; all from Sigma). For neutralizing assays, agents were used at a concentration of 5 μg/mL anti–TGF-β, 30 ng/mL anti–IL-10, 10 μmol/L Indomethacin, 100 U/mL IL-2, and 50 pmol IL-12. Labeling with conjugated antibodies to cytokines (IL-2-FITC, IL-4-PE, IL-10-PE, and γ-IFN-FITC; all from Serotec) was performed using a commercially available permeabilization kit (Fix and Perm; Caltag Laboratories). Both irrelevant conjugated antibodies and unstimulated cells were used as controls to determine the level of positivity.
RESULTS
Phenotype of U937 cells: Expression of MHC class I and II and absence of CD80/CD86 on control cells.
In this study, we have used the human acute monocytic leukemia cell line U937 that has a phenotype similar to many primary acute myeloid leukemias (AMLs) and, importantly, expresses high levels of MHC class I and class II (Fig 1A). Wild-type U937 cells express low levels of CD86, which is upregulated by PMA. Both CD80 and CD86 have a higher affinity for the negative regulator CTLA4 than they do for CD28.18 Therefore, low-level expression of these costimulators will preferentially activate CTLA4. To eliminate any effect of CD86, U937 cells were stimulated with PMA for 48 hours, and then CD86−cells were sorted by FACS. These cells remained CD86−and failed to bind CTLA4.Ig (Fig 1B) even with further stimulation with PMA (data not shown). Using retroviral vectors, we generated 2 cell lines, 1 expressing CD80 (U937-CD80) and control cells infected with the control vector (U937-M3P). Costimulation through CD28 is optimal when CD80 molecules are expressed at high levels.42 43Therefore, U937-CD80 cells were further sorted for high CD80 expression determined by both anti-CD80 antibody and CTLA4.Ig binding. In addition to MHC, both U937 cell lines express cell adhesion molecules: ICAM-1 (CD54), LFA-1 (CD11a), and Mac-1 (CD11b) (data not shown).
MHC class I and II and CD80 expression by U937 cells. (A) Single-color labeling demonstrated that U937 cells have similar levels of MHC class I and II as DCs. Control U937-M3P cells were CD80−, and U937-CD80 cells had a high level of CD80 expression that was greater than that of DC. (B) Dual-labeling using anti-CD80 antibody and CTLA4.Ig demonstrated no CD80/CD86 expression on the control U937-M3P population and high anti-CD80 as well as CTLA4.Ig binding on the U937-CD80 population.
MHC class I and II and CD80 expression by U937 cells. (A) Single-color labeling demonstrated that U937 cells have similar levels of MHC class I and II as DCs. Control U937-M3P cells were CD80−, and U937-CD80 cells had a high level of CD80 expression that was greater than that of DC. (B) Dual-labeling using anti-CD80 antibody and CTLA4.Ig demonstrated no CD80/CD86 expression on the control U937-M3P population and high anti-CD80 as well as CTLA4.Ig binding on the U937-CD80 population.
CD80 expression increases proliferative responses of allogeneic T cells to U937 cells.
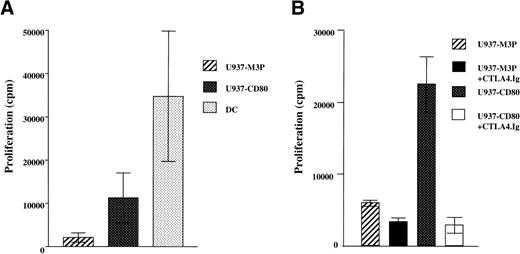
To compare the responses to CD80+ and control U937 cells, purified allogeneic T cells (Fig 2A) were used in 5-day MLLRs. T cells from 5 normal donors demonstrated up to a 16-fold increase (range, 1.7- to 16-fold; mean, 7-fold; median, 3.6-fold) in their response to CD80+ U937 cells compared with the control (Fig 2A). Despite high levels of CD80 and MHC expression, responses to U937-CD80 cells were still less than third-party DCs at a lower stimulator:responder ratio. The specificity of the enhanced response to CD80 was demonstrated by the ability of its solubilized high-affinity receptor, CTLA4.Ig, to inhibit T-cell proliferation (Fig 2B).
CD80 expression increases proliferative responses of allogeneic T cells to U937 cells. (A) T cells were cocultured with irradiated U937-M3P, U937-CD80, or third-party DCs. Cumulative data of proliferative responses from 8 experiments with 5 different donors with mean ±SE are shown. Stimulator:responder ratios were 1:1 and 1:10 for the leukemic and DCs, respectively. T cells demonstrated up to a 16-fold increase in their proliferative response to U937-CD80 cells compared with the control cells. (B) The increased proliferative response of T cells to CD80+ cells seen in (A) could be inhibited by soluble CTLA4.Ig (10 ng/mL), demonstrating this is a CD80-mediated effect. These data are representative of 3 independent experiments.
CD80 expression increases proliferative responses of allogeneic T cells to U937 cells. (A) T cells were cocultured with irradiated U937-M3P, U937-CD80, or third-party DCs. Cumulative data of proliferative responses from 8 experiments with 5 different donors with mean ±SE are shown. Stimulator:responder ratios were 1:1 and 1:10 for the leukemic and DCs, respectively. T cells demonstrated up to a 16-fold increase in their proliferative response to U937-CD80 cells compared with the control cells. (B) The increased proliferative response of T cells to CD80+ cells seen in (A) could be inhibited by soluble CTLA4.Ig (10 ng/mL), demonstrating this is a CD80-mediated effect. These data are representative of 3 independent experiments.
CD80 expression increases the frequency of precursor T cells activated.
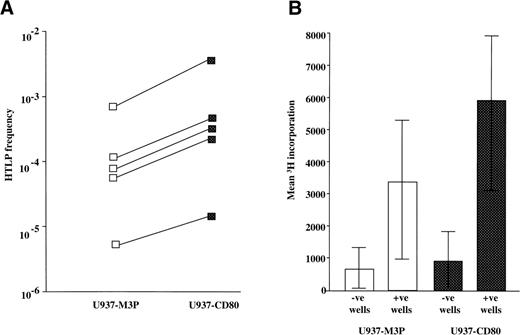
The increased T-cell proliferation induced by CD80 may be explained by either an increased frequency of precursor T cells activated or activation of the same number of precursors receiving a greater proliferative stimulus (ie, effects on the initiation of T-cell activation or later events). To test this, HTLp frequencies (measured using the IL-2–dependent CTLL-2 cell line) to CD80+ and CD80− U937 cells were measured by limiting dilution analysis. CD80 expression consistently increased the precursor frequency (mean, 4.1-fold; range, 2.8- to 5.5-fold; Fig 3A). Calculated HTLp frequencies correlated with the proliferation observed in bulk cultures. The possibility that CD28 stimulation by CD80 induced a nonspecific proliferation was excluded by analysis of the mean values of the negative wells. This showed similar background levels for CD80+ and CD80− stimulators (Fig 3B). At the lowest dilution (2.5 × 103 cells/well), the majority of wells were negative, so any positive wells were likely to have contained single precursors. Because the readout for the HTLp frequency assay is a sensitive IL-2 bioassay, a comparison of the values of the positive wells at the lowest dilution also indicates the amount of IL-2 secreted after activation of a single precursor. This analysis showed only a 78% increase in IL-2 production in the presence of CD80+ stimulators (Fig 3B). We can conclude therefore that CD28 stimulation may increase IL-2 secretion, but that, in this model of antigen presentation by leukemic cells, this is of less significance than the effect on precursor activation.
CD80 expression by tumor cells increases the frequency of precursor T cells activated. (A) The data show calculated HTLp frequencies (log10 scale) to U937-M3P and U937-CD80 cells for 5 individuals as described in Materials and Methods. Precursor frequencies were measured by a limiting dilution assay. The frequencies were increased up to 5-fold by CD80 expression and correlated with the proliferation observed in bulk cultures. (B) This bar chart represents the mean ± SE 3H-thymidine incorporation by the IL-2–dependent cell line, CTLL-2, for the wells of the bottom dilution from the 5 experiments shown in (A). The values from wells scored negative or positive are shown, when either U937-M3P (□) or U937-CD80 (▪) cells were used. 3H-thymidine incorporation of the negative wells was similar in both U937-M3P and U937-CD80 assays, demonstrating a comparable baseline level. Mean CTLL-2 proliferation in the positive wells was 78% greater in the presence of U937-CD80 cells. This suggests that, after activation, precursors stimulated by U937-CD80 cells produce more IL-2.
CD80 expression by tumor cells increases the frequency of precursor T cells activated. (A) The data show calculated HTLp frequencies (log10 scale) to U937-M3P and U937-CD80 cells for 5 individuals as described in Materials and Methods. Precursor frequencies were measured by a limiting dilution assay. The frequencies were increased up to 5-fold by CD80 expression and correlated with the proliferation observed in bulk cultures. (B) This bar chart represents the mean ± SE 3H-thymidine incorporation by the IL-2–dependent cell line, CTLL-2, for the wells of the bottom dilution from the 5 experiments shown in (A). The values from wells scored negative or positive are shown, when either U937-M3P (□) or U937-CD80 (▪) cells were used. 3H-thymidine incorporation of the negative wells was similar in both U937-M3P and U937-CD80 assays, demonstrating a comparable baseline level. Mean CTLL-2 proliferation in the positive wells was 78% greater in the presence of U937-CD80 cells. This suggests that, after activation, precursors stimulated by U937-CD80 cells produce more IL-2.
CD28 stimulation lowers the threshold for TCR triggering.
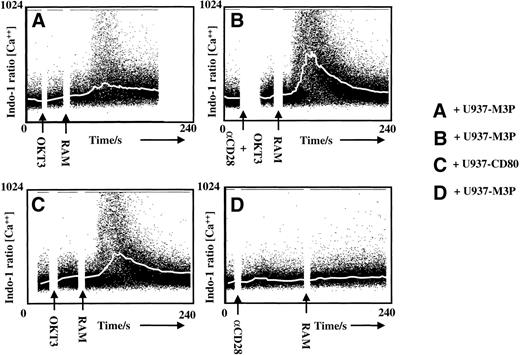
The observed increase in precursor activation suggests that CD80 stimulation of CD28 acts in part by regulating TCR signaling. To test this, the effect of CD28 stimulation on calcium mobilization after suboptimal TCR stimulation was studied. Using the defined suboptimal concentration of cross-linked anti-CD3, TCR stimulation alone in the presence of U937-M3P cells induces a minimal increase in intracellular calcium (Fig 4A). However, simultaneous TCR and CD28 stimulation either by anti-CD28 (Fig 4B) or by the presence of CD80-expressing tumor cells (Fig 4C) enhanced calcium mobilization. Neither CD28 cross-linking (Fig 4D) nor U937-CD80 cells (data not shown) induced calcium mobilization in the absence of TCR stimulation. Therefore, CD28 stimulation through CD80 expression by tumor cells lowers the threshold of TCR triggering, ie, enables the initiation of T-cell activation at a lower level of TCR stimulation.
CD28 stimulation lowers the threshold for TCR triggering. Calcium mobilization in normal Indo-1–loaded T cells, before and after TCR stimulation with anti-CD3 followed by cross-linking with polyclonal rabbit antimouse Ig antibody (RAM). The plots show intracellular calcium concentrations (ratio of bound:free Indo-1) over a 240-second time period. The white line represents the median calcium concentration at each 10-second time slice. In (A), a suboptimal level of anti-CD3 was determined that, with cross-linking in the presence of U937-M3P cells, gave poor median calcium mobilization. Costimulation through CD28 by addition of a specific activating antibody at the same time as anti-CD3 markedly increased the number of activated T cells (B) not seen using anti-CD28 alone (D). Enhanced T-cell activation was also seen when CD28 stimulation was provided by CD80+U937-CD80 cells instead of U937-M3Ps (C). U937-CD80 cells, in the absence of anti-CD3, also failed to trigger calcium mobilization (data not shown).
CD28 stimulation lowers the threshold for TCR triggering. Calcium mobilization in normal Indo-1–loaded T cells, before and after TCR stimulation with anti-CD3 followed by cross-linking with polyclonal rabbit antimouse Ig antibody (RAM). The plots show intracellular calcium concentrations (ratio of bound:free Indo-1) over a 240-second time period. The white line represents the median calcium concentration at each 10-second time slice. In (A), a suboptimal level of anti-CD3 was determined that, with cross-linking in the presence of U937-M3P cells, gave poor median calcium mobilization. Costimulation through CD28 by addition of a specific activating antibody at the same time as anti-CD3 markedly increased the number of activated T cells (B) not seen using anti-CD28 alone (D). Enhanced T-cell activation was also seen when CD28 stimulation was provided by CD80+U937-CD80 cells instead of U937-M3Ps (C). U937-CD80 cells, in the absence of anti-CD3, also failed to trigger calcium mobilization (data not shown).
CD80 expression enhances survival of activated T cells.
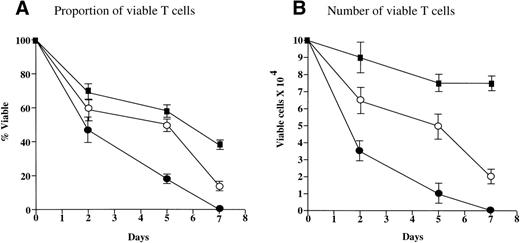
After a 4-day MLLR, activated T cells stimulated in the presence of U937-M3P cells were found to express similar levels of the high-affinity IL-2 receptor (CD25) as those stimulated in the presence of U937-CD80 cells. The percentages of CD25+ T cells were 12.5% and 12.6%, and the levels of CD25 expression given as the MFI were 105 au and 109 au for U937-M3P and U937-CD80, respectively. These activated cells were sorted and returned to culture with exogenous IL-2 (30 U/mL). Analysis of the viability and numbers of sorted CD25+ T cells demonstrated that, after 7 days in culture, viability of cells stimulated by U937-CD80 cells decreased to almost one third (Fig 5A). The cell count was only marginally reduced (Fig 5B), indicating a combination of continued proliferation and death. By day 7, T cells that had been activated in the presence of U937-M3P cells had undergone activation-induced cell death and there were no viable cells present. When T cells from the U937-M3P MLLR were cultured in the presence of conditioned medium from the U937-CD80 MLLR as well as rhIL-2, there was an improvement in both the cell count and the viability, suggesting that CD28 stimulation induces the secretion of another T-cell growth factor.
CD28 stimulation reduces death and promotes expansion of T cells. PBMNCs were activated in the presence of U937-M3Ps or U937-CD80s in a 4-day MLLR. Activated T cells were sorted and cultured in exogenous IL-2 (30 U/mL). Activated T cells from the U937-M3P MLLR were also cultured in conditioned medium from the U937-CD80 MLLR. In this experiment, T cells activated in the presence of U937-CD80 cells (▪) demonstrated 38% viability by day 7 (A), but viable cell numbers were still 80% of the original count (B), suggesting that there was an expansion of a specific population. T cells cultured in the presence of U937-M3P cells (•) demonstrated zero viability by day 7. The addition of conditioned medium from the MLLR that used U937-CD80 cells as stimulators to these cells improved their viability (○). These data are the mean of 3 independent experiments ± SE.
CD28 stimulation reduces death and promotes expansion of T cells. PBMNCs were activated in the presence of U937-M3Ps or U937-CD80s in a 4-day MLLR. Activated T cells were sorted and cultured in exogenous IL-2 (30 U/mL). Activated T cells from the U937-M3P MLLR were also cultured in conditioned medium from the U937-CD80 MLLR. In this experiment, T cells activated in the presence of U937-CD80 cells (▪) demonstrated 38% viability by day 7 (A), but viable cell numbers were still 80% of the original count (B), suggesting that there was an expansion of a specific population. T cells cultured in the presence of U937-M3P cells (•) demonstrated zero viability by day 7. The addition of conditioned medium from the MLLR that used U937-CD80 cells as stimulators to these cells improved their viability (○). These data are the mean of 3 independent experiments ± SE.
U937-CD80 cells increase BCL-2 but not BCL-XL.
Clonal expansion of T cells is partly dependent on the upregulation of antiapoptotic proteins such as BCL-2 and BCL-XL, leading to the prevention of activation-induced cell death. To determine whether observed differences in T-cell survival were due to altered expression of antiapoptotic proteins, levels of BCL-2 and BCL-XL were measured in responding T cells after 4 days of stimulation with either U937-M3P or U937-CD80 cells. Dual-color flow cytometry was used to detect expression of these antiapoptotic proteins in activated T cells (ie, CD25+). Cells stimulated by U937-CD80 cells demonstrated a 40% increase in expression of BCL-2 (measured as MFI) compared with those stimulated by U937-M3P cells (P = .023 by paired t-test; Fig 6A). Although reports suggest that the major antiapoptotic protein upregulated by CD28 ligation is BCL-XL, we were not able to detect any expression in activated T cells even when the stimulator cells expressed CD80 (Fig 6B). Activation with anti-CD3 and anti-CD28 induced BCL-XL expression, which was not seen with anti-CD3 alone. CD28 stimulation mediated by CD80 expression on tumor cells is therefore less effective in inducing antiapoptotic proteins.
U937-CD80 cells cause upregulation of BCL-2 but not BCL-XL. PBMNCs were activated in the presence of irradiated U937-M3Ps or U937-CD80s in a 4-day MLLR. BCL-2 ( ) and BCL-XL (▪) levels were measured in the activated (CD25+) population by dual surface and intracellular flow cytometry as described in Materials and Methods. U937-CD80 cells stimulated a 40% greater expression of BCL-2 in CD25+cells measured by MFI compared with stimulation with U937-M3Ps (P = .023, paired t-test). There was no significant increase in BCL-XL with either stimulator. These data are the mean ± SE of 4 experiments. Expression of BCL-XL in CD25+ cells stimulated by either (1) anti-CD3 anti-CD28 or (2) U937-M3P or U937-CD80 cells. Cells were dual-labeled for anti-CD25 and anti–BCL-XL after 48 or 96 hours of stimulation, respectively. The histograms show expression of BCL-XL in the CD25+ population. Labeling with control antibodies gave the same level of fluorescence as that with anti–BCL-XL after stimulation with anti-CD3 alone. Therefore, T-cell receptor ligation alone is insufficient to induce BCL-XL expression. The addition of CD28 cross-linking induced expression of BCL-XL, which was not observed when CD28 stimulation was through CD80 expression on U937 stimulator cells.
U937-CD80 cells cause upregulation of BCL-2 but not BCL-XL. PBMNCs were activated in the presence of irradiated U937-M3Ps or U937-CD80s in a 4-day MLLR. BCL-2 ( ) and BCL-XL (▪) levels were measured in the activated (CD25+) population by dual surface and intracellular flow cytometry as described in Materials and Methods. U937-CD80 cells stimulated a 40% greater expression of BCL-2 in CD25+cells measured by MFI compared with stimulation with U937-M3Ps (P = .023, paired t-test). There was no significant increase in BCL-XL with either stimulator. These data are the mean ± SE of 4 experiments. Expression of BCL-XL in CD25+ cells stimulated by either (1) anti-CD3 anti-CD28 or (2) U937-M3P or U937-CD80 cells. Cells were dual-labeled for anti-CD25 and anti–BCL-XL after 48 or 96 hours of stimulation, respectively. The histograms show expression of BCL-XL in the CD25+ population. Labeling with control antibodies gave the same level of fluorescence as that with anti–BCL-XL after stimulation with anti-CD3 alone. Therefore, T-cell receptor ligation alone is insufficient to induce BCL-XL expression. The addition of CD28 cross-linking induced expression of BCL-XL, which was not observed when CD28 stimulation was through CD80 expression on U937 stimulator cells.
U937-CD80 cells fail to induce a Th1 cytokine profile.
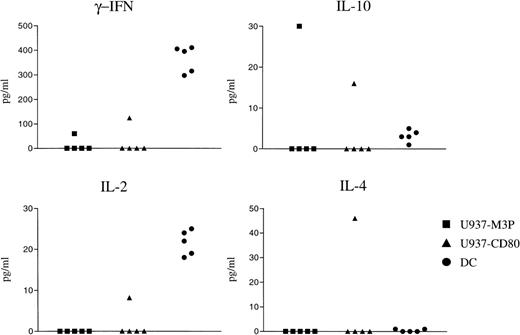
The pattern of cytokines produced by T cells plays a pivotal role in the nature of an immune response to a given antigen. Secretion of Th1 and Th2 cytokines produced in response to U937-M3P, U937-CD80, or third-party DCs were compared. As anticipated, third-party DCs produced a Th1 cytokine pattern (γ-IFN, IL-2, and no Th2 cytokines). In 4 of 5 donors, there was no detectable cytokine response induced by either CD80+ or CD80− U937 cells despite moderate proliferation. With 1 donor that had identical proliferative responses to U937-CD80 cells and third-party DCs, there was significantly less Th1 cytokine production, but also detectable secretion of Th2 cytokines (Fig 7). In this experiment, the production of IL-4 was particularly enhanced by the expression of CD80 on U937 cells. These data indicate that, although CD80 expression on U937 cells increases T-cell activation and proliferation, there is a failure to induce the secretion of appropriate Th1 cytokines in response to alloantigen.
U937-CD80 cells may induce a Th2 cytokine profile. T cells were cocultured with irradiated U937-M3P, U937-CD80, or third-party DCs in a 5-day MLLR and cytokine ELISAs were performed on the resultant supernatant. DCs produced a Th1 cytokine pattern as anticipated (high levels of γ-IFN and IL-2). Of 5 experiments using different responders, only 1 demonstrated detectable cytokine production. This was more of a Th2 cytokine pattern (IL-4 and IL-10 were detected and IL-2 and γ-IFN were much lower than induced by the DCs). There was no detectable cytokine production (IL-2, IL-4, IL-10, or γ-IFN) from 5-day irradiated U937 cells alone.
U937-CD80 cells may induce a Th2 cytokine profile. T cells were cocultured with irradiated U937-M3P, U937-CD80, or third-party DCs in a 5-day MLLR and cytokine ELISAs were performed on the resultant supernatant. DCs produced a Th1 cytokine pattern as anticipated (high levels of γ-IFN and IL-2). Of 5 experiments using different responders, only 1 demonstrated detectable cytokine production. This was more of a Th2 cytokine pattern (IL-4 and IL-10 were detected and IL-2 and γ-IFN were much lower than induced by the DCs). There was no detectable cytokine production (IL-2, IL-4, IL-10, or γ-IFN) from 5-day irradiated U937 cells alone.
Soluble factors secreted by U937 cells inhibit Th1 cytokine production.
To test the hypothesis that soluble factors derived from U937 cells directly influence cytokine secretion, cytokine production by PBMNCs stimulated by PMA/ionomycin in the absence or presence of U937 cells was compared by intracellular flow cytometric analysis. U937 cells were separated by a transwell insert enabling soluble factors produced by the leukemic cells to interact with the activated T cells, but avoiding the influence of cell:cell contact. Dual labeling of Th1 (γ-IFN and IL-2) and Th2 (IL-4 and IL-10) was performed as described in Materials and Methods. Resting PBMNCs had very low levels of cytokine production, whereas stimulated ones demonstrated increased levels of IL-2 and γ-IFN. Both of these cytokines were inhibited when cells were stimulated in the presence of U937s (Fig 8A). No significant levels of IL-4 or IL-10 were detected in any of the assays, but they were only short-term assays, ie, before peak secretion occurs. Attempts to overcome the observed inhibition by either blocking known Th1 inhibitors (TGF-β, IL-10, and prostaglandin E2[PGE2]; not shown) or overcoming it with concurrent addition of exogenous IL-2 or IL-12 were unsuccessful (Fig8B). However, preincubation of PBMNCs with exogenous IL-2 for 4 hours before exposure to U937 cells overcame the inhibitory effect (Fig 8C). This preventative action of IL-2 on cytokine inhibition was not seen with either IL-12 or GM-CSF even with preincubation times of up to 24 hours (data not shown).
Soluble factors secreted by U937 cells inhibit Th1 cytokine production. (A) FACS analysis of intracellular cytokines (γ-IFN and IL-4, IL-2, and IL-10) was performed on PBMNCs that had been stimulated with PMA/ionomycin in the absence or presence of U937 cells separated by a 0.4-μm pore membrane. PBMNCs stimulated in the absence of U937 cells were almost exclusively producing γ-IFN and IL-2. Those activated in the presence of U937 cells showed a much-reduced number producing γ-IFN and IL-2. (B) The same experiment in the presence of reagents to either block potential Th1 inhibitors (neutralizing antibodies to TGF-β, IL-10, or [not shown here] indomethacin to block PGE2) or overcome them (IL-2 and IL-12) showed all had no effect when added concurrently with U937 cells. (C) PBMNCs were incubated with IL-2 for 4 hours before the addition of U937 cells and were then analyzed for γ-IFN production by intracellular staining as described above. Preincubation with IL-2 overcame the effect of the leukemia-derived inhibitory factor on γ-IFN production.
Soluble factors secreted by U937 cells inhibit Th1 cytokine production. (A) FACS analysis of intracellular cytokines (γ-IFN and IL-4, IL-2, and IL-10) was performed on PBMNCs that had been stimulated with PMA/ionomycin in the absence or presence of U937 cells separated by a 0.4-μm pore membrane. PBMNCs stimulated in the absence of U937 cells were almost exclusively producing γ-IFN and IL-2. Those activated in the presence of U937 cells showed a much-reduced number producing γ-IFN and IL-2. (B) The same experiment in the presence of reagents to either block potential Th1 inhibitors (neutralizing antibodies to TGF-β, IL-10, or [not shown here] indomethacin to block PGE2) or overcome them (IL-2 and IL-12) showed all had no effect when added concurrently with U937 cells. (C) PBMNCs were incubated with IL-2 for 4 hours before the addition of U937 cells and were then analyzed for γ-IFN production by intracellular staining as described above. Preincubation with IL-2 overcame the effect of the leukemia-derived inhibitory factor on γ-IFN production.
DISCUSSION
In this study, we have demonstrated that CD80 expression on a leukemic cell increases T-cell recognition and proliferation primarily by lowering the threshold for TCR signal transduction and also by enhancing survival signals. However, in this model, soluble factors secreted by the leukemic cells inhibit cytokine production, thus preventing the development of the desired cell-mediated immunity.
Several studies have shown enhanced T-cell proliferative responses to CD80-expressing tumor cells by generating a costimulatory signal through ligation of CD28 on T cells.4,5 The role of CD28 ligation during T-cell activation by professional APCs has generally been considered to be primarily by promoting IL-2 secretion and, hence, growth and survival. However, we show here that, when CD80+tumor cells are used as nonprofessional APCs, the primary mechanism for enhancing T-cell recognition is through amplification of TCR signals, ie, during the initial activation phase. These results are consistent with the finding of reduced TCR occupancy required for T-cell activation in the presence of CD28 stimulation.44 A consequence of this augmentation of TCR signaling is the potential recognition of previously silent antigens, a hypothesis supported by the finding of an expanded T-cell repertoire generated against CD80+ tumor cells.11
Although initiation of T-cell activation is not dependent on CD28, CD28 plays an important role in the survival of activated T cells. It is now becoming clear that, in addition to effects on IL-2 secretion, CD28 plays a role in T-cell survival through upregulation of the antiapoptotic proteins, BCL-XL, and, to a lesser extent, BCL-2.14-16 We have been able to show here in vitro that expression of CD80 by tumor cells induces the upregulation of BCL-2, delaying the death observed in T cells activated in the absence of CD80-mediated costimulation. Although additional cross-linking of CD28 with TCR stimulation induced BCL-XL expression, no BCL-XL expression could be detected when CD28 was stimulated by CD80+ U937 cells. Several studies suggest that signaling through CD28 has a more profound effect on BCL-XL than BCL-2,45 which may be due in part to CD28-mediated IL-2 secretion, because signaling through the IL-2 receptor (high and intermediate affinity) itself may induce BCL-XL. One mechanism to explain the failure of CD80-expressing U937 cells to induce BCL-XL may therefore be a consequence of the inhibition of IL-2 secretion by a U937-derived soluble factor(s). Equally, the inhibitory factors may act on downstream components of the CD28 signaling pathway blocking both cytokine and BCL-XL synthesis.
Controversy exists regarding the role of costimulation in the Th1/Th2 decision, but cytokines appear to play a more important role in determining the nature of an immune response to a given antigen. The microenvironment generated by a malignant cell is clearly different to that induced by professional APCs. Tumor cells produce many cytokines (eg, TGF-β and IL-10), soluble cytokine receptors (eg, sIL-2R), and other biological response modifiers (eg, PGE2) that are known to inhibit cell-mediated immunity. Despite enhanced proliferative responses, T cells stimulated by leukemic cells in this model were generally unable to produce detectable levels of cytokines. In 1 exception, in which there was detectable cytokine production, the profile was more of a Th2 than a Th1 pattern. In this model, a leukemia-derived soluble factor(s) inhibits Th1 cytokine synthesis, but does not prevent proliferation; costimulation is therefore enabling T-cell activation to occur in a nonpermissive environment for the generation of cell-mediated immunity. This may explain the synergistic effect seen in immune gene therapy studies of coexpressing CD80 with T-cell growth factors (eg, IL-2,46 IL-7,47 and IL-1248). This concept is supported by the observation in this study that preincubation of PBMNCs with IL-2 overcomes the inhibitory effect of U937 cells on T-cell cytokine production. However, importantly, the inability of IL-2 to prevent this inhibition when administered concurrently with leukemic cells suggests that, to be successful in a therapeutic setting, a state of minimal residual disease would have to be created before the administration of gene-modified cells.
The findings of this study have several implications for understanding the mechanisms by which leukemic cells may evade immune rejection, eg, absence of response to donor leukocyte infusions after leukemic relapse after allogeneic bone marrow transplantation. Absence of costimulatory molecules on leukemic cells may result in either ignorance or deletion of antigen-specific T cells depending on the level of antigen expressed. If an antigen is expressed at a low level, there is an inadequate TCR signal for T-cell activation and the antigen will not be recognized. If an antigen is expressed at a high level, signaling through the TCR alone is sufficient to induce T-cell activation, but the lack of survival signals would result in activation-induced cell death and ultimately clonal deletion. Expression of costimulatory molecules by a leukemic cell enables T cells to recognize lower levels of antigen that induce the survival signals required for clonal expansion of leukemia-reactive T cells, but the microenvironment generated by the leukemia may create a further defense against immune rejection. Tumor-derived factors have been shown to have numerous effects on T cells,25-27 APCs,30,49 and effector functions.50 Primary T-cell responses in the presence of immunosuppressive cytokines, eg, IL-1051,52 or TGF-β,53 have been shown to lead to the induction of tolerance rather than productive immunity. Therefore, activation of leukemia-reactive T cells by expression of costimulatory molecules on the leukemic cell may lead to active tolerance if the leukemic cells generate an immunosuppressive microenvironment. This may partly explain variable results with CD80-modified tumor cells and the observed association between positive effect and inherent immunogenicity.54 There are known potent Th1 cytokine inhibitors (IL-10,22 TGF-β,22,50 and PGE251), but from blocking studies (IL-10, TGF-β, and PGE2) and direct assays (IL-10 and TGF-β), we have no evidence to suggest that cytokine inhibition by U937 cells is caused by any of these. Although cell lines may not be representative of their primary counterparts, in all 10 primary AML cases we have studied so far, a similar effect of leukemia-derived factor(s) on synthesis of IL-2 and γ-IFN has been observed (data not shown). Therefore, the data presented here using U937 cells may be extended to human leukemias.
Several powerful methods are now available to elicit CTL responses to tumor-associated antigens in vitro and in vivo. It remains to be addressed how developing tumors bearing such antigens fail to be rejected; absence of costimulation remains a candidate mechanism. The provision of costimulation has the potential to expose previously silent antigens to recognition by specific T cells; however, tumor-elaborated factors may prevent the generation of effective antitumor immunity.
ACKNOWLEDGMENT
The authors thank Prof D. Vergani (University College Hospital, London, UK) and Dr M. Peakman (King’s College School of Medicine and Dentistry, London, UK) for critical reading of the manuscript.
Supported by the Kay Kendall Leukaemia Fund and the Leukaemia Research Fund of the United Kingdom.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to W.J.R. Hirst, MD, Department of Haematological Medicine, King’s College School of Medicine and Dentistry, Leukaemia Sciences Laboratories, The Rayne Institute, 123 Coldharbour Lane, London, UK, SE5 9NU.




![Fig. 8. Soluble factors secreted by U937 cells inhibit Th1 cytokine production. (A) FACS analysis of intracellular cytokines (γ-IFN and IL-4, IL-2, and IL-10) was performed on PBMNCs that had been stimulated with PMA/ionomycin in the absence or presence of U937 cells separated by a 0.4-μm pore membrane. PBMNCs stimulated in the absence of U937 cells were almost exclusively producing γ-IFN and IL-2. Those activated in the presence of U937 cells showed a much-reduced number producing γ-IFN and IL-2. (B) The same experiment in the presence of reagents to either block potential Th1 inhibitors (neutralizing antibodies to TGF-β, IL-10, or [not shown here] indomethacin to block PGE2) or overcome them (IL-2 and IL-12) showed all had no effect when added concurrently with U937 cells. (C) PBMNCs were incubated with IL-2 for 4 hours before the addition of U937 cells and were then analyzed for γ-IFN production by intracellular staining as described above. Preincubation with IL-2 overcame the effect of the leukemia-derived inhibitory factor on γ-IFN production.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/10/10.1182_blood.v94.10.3479.422k29_3479_3490/6/m_blod42229008ax.jpeg?Expires=1769135593&Signature=n-bj6qphiLrAN-kHke2ja-OcuSHgDYXyQ0MSr35TblY5j4Wb1-V~RXkjyXUwQX5wY2WLYTpn7JoZ6ofPY2PgD8EhKKnts50NtfBuijDiid9BoTGRuG6gn7WfcWRzxHLrrqKNnuSUy2Lz-BdQ92SPUeFT8j4bTWwEc3FQX~8K55~BFRDShhaC6NtfVfux8Y41Su7Tuwk9HzqHdm~xNnjyPqkoVzUVbbgZmxDbVA-7Or0wmb9j-N9csrmgdc8eUDxdJYtJb5knVPuqaw6sIirs-L7GqALy9NIxoQrdHTjmui3JqnIUjtThiEfskfYup8OB7BVsfhVPp-H6f79yEsYkUQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 8. Soluble factors secreted by U937 cells inhibit Th1 cytokine production. (A) FACS analysis of intracellular cytokines (γ-IFN and IL-4, IL-2, and IL-10) was performed on PBMNCs that had been stimulated with PMA/ionomycin in the absence or presence of U937 cells separated by a 0.4-μm pore membrane. PBMNCs stimulated in the absence of U937 cells were almost exclusively producing γ-IFN and IL-2. Those activated in the presence of U937 cells showed a much-reduced number producing γ-IFN and IL-2. (B) The same experiment in the presence of reagents to either block potential Th1 inhibitors (neutralizing antibodies to TGF-β, IL-10, or [not shown here] indomethacin to block PGE2) or overcome them (IL-2 and IL-12) showed all had no effect when added concurrently with U937 cells. (C) PBMNCs were incubated with IL-2 for 4 hours before the addition of U937 cells and were then analyzed for γ-IFN production by intracellular staining as described above. Preincubation with IL-2 overcame the effect of the leukemia-derived inhibitory factor on γ-IFN production.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/10/10.1182_blood.v94.10.3479.422k29_3479_3490/6/m_blod42229008bx.jpeg?Expires=1769135593&Signature=EeSr7UzJnp0HTd6ROLXSzSAgwyWldQuZQ6ESFaDn7cb-ezmUCch5b23-Q58RbY5yCtR4SdeTl7ay~lEqrCO2uy6umUpvOzFXC4DjdONZ84MAFj36kZAJTuUvJP7vtMT5ClkI01Nv~TdXvXmqO59C3E1j-FNHqkVE7QiVXLRUYav9ew5lZsVSyn-QvwmIZgfzh7IXHjgMV08WaQ4zBOidtR6N8qjblTirW387xi6rr6BNvnE1rChaFMVnY8QSlWfGqRbZy4cbk3vD2u3eKw5BVz8-8mcrOOIjUOzA716M~a73PWArXLkJ-rIU45bW7JOZvRn2xfngfjz9jlw5UTI89A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 8. Soluble factors secreted by U937 cells inhibit Th1 cytokine production. (A) FACS analysis of intracellular cytokines (γ-IFN and IL-4, IL-2, and IL-10) was performed on PBMNCs that had been stimulated with PMA/ionomycin in the absence or presence of U937 cells separated by a 0.4-μm pore membrane. PBMNCs stimulated in the absence of U937 cells were almost exclusively producing γ-IFN and IL-2. Those activated in the presence of U937 cells showed a much-reduced number producing γ-IFN and IL-2. (B) The same experiment in the presence of reagents to either block potential Th1 inhibitors (neutralizing antibodies to TGF-β, IL-10, or [not shown here] indomethacin to block PGE2) or overcome them (IL-2 and IL-12) showed all had no effect when added concurrently with U937 cells. (C) PBMNCs were incubated with IL-2 for 4 hours before the addition of U937 cells and were then analyzed for γ-IFN production by intracellular staining as described above. Preincubation with IL-2 overcame the effect of the leukemia-derived inhibitory factor on γ-IFN production.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/10/10.1182_blood.v94.10.3479.422k29_3479_3490/6/m_blod42229008cx.jpeg?Expires=1769135593&Signature=M812IkhGdYURilu6Ugjj1cpWJv8u0sPw5yBjElIaCqkXfszCa3tWPLUxkfOkSOqWAJU8vYOcHCwd7dHcd09hB87qI1yy7Grcm3xLwLPa8DSyMcUV38Osu8yT1TZFxWuvO2URHnD5msXT0L~VS5nTEpiUEZPZPcRltNaEfDNAybVftuvTzq41WJ0HSlsCoxcdncg921UBDUydMXpyXXcZynA4nnFg4gCLbtbNMwOiXIfFRQLFnm7ietljUQl1SHlwv0VCBjvDWK3AGklHiMN5qZIpNmUk27IZXadlaKJf3dhEHiov4zj~bQCbRZ4hibl9yiWFD3D9Vawld35GRP-Rng__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal