Factor IXR94S is a naturally occurring hemophilia B defect, which results from an Arg 94 to Ser mutation in the second epidermal growth factor (EGF)-like module of factor IX. Recombinant factor IXR94S was activated by factor XIa/calcium with an ≈50-fold reduced rate and by factor VIIa/tissue factor/phospholipid/calcium with an ≈20-fold reduced rate compared with wild-type factor IX. The apparent molecular mass of the light chain of factor IXaR94S was ≈6 kD higher than that of plasma or wild-type factor IX, which was not corrected by N-glycosidase F digestion. This result indicated the presence of additional O-linked carbohydrate in the mutant light chain, probably at new Ser 94. The initial rate of activation of factor X by factor IXaR94S in the presence of polylysine was 7% ± 1% of the initial rate of activation of factor X by plasma factor IXa, and the kc/Km for activation of factor X by factor IXaR94S/factor VIIIa/phospholipid/calcium was 4% ± 1% of the kc/Km for activation of factor X by plasma factor IXa/factor VIIIa/phospholipid/calcium. The reduced efficiency of activation of factor X by factor IXaR94S in the tenase enzyme complex was due to a 58-fold ± 12-fold decrease in kcat with little effect on Km. In conclusion, the R94S mutation had introduced an O-linked carbohydrate, which markedly impaired both activation by factor XIa and turnover of factor X in the tenase enzyme complex.
FACTOR IX is a vitamin K–dependent coagulation protein whose deficiency results in hemophilia B.1 Factor IX circulates in plasma as an inactive single chain glycoprotein of 415 amino acids and participates in both the intrinsic and extrinsic coagulation pathways. After initiation of coagulation, factor IX can be activated to a two-chain serine proteinase factor IXa by either factor XIa or factor VIIa/tissue factor.2 Activation of factor IX by either pathway occurs by cleavage of the Arg145-Ala146 and Arg180-Val181 peptide bonds to yield a light and heavy chain linked by two disulfide bonds. Subsequently, the enzyme factor IXa forms a macromolecular complex with its cofactor factor VIIIa, to activate factor X to factor Xa in the presence of calcium and a negatively charged phospholipid surface.3 4
Factor IX is synthesized in the liver as a precursor molecule of 461 amino acids containing a 28-residue signal peptide and an 18-residue propeptide, both of which are proteolytically cleaved before secretion.5,6 The mature molecule consists of an N-terminal γ-carboxyglutamic acid-rich domain (Gla domain) followed by a short hydrophobic amino acid stack domain, two epidermal growth factor (EGF)-like modules, an activation peptide region, and a serine proteinase module.5,6 The first EGF module participates in factor VIIIa and factor X binding and is required for activation of factor IX by factor VIIa/tissue factor.7-13The second EGF module and the serine proteinase module have also been proposed to participate in factor VIIIa and factor X binding,11,14,15 although studies using proteolytic fragments of factor IX have argued against a direct binding function for the second EGF module.16 17
Approximately 35% of individuals with hemophilia B possess immunologically normal levels of factor IX antigen, but reduced levels of factor IX coagulant activity.18 These variants are designated cross-reacting material positive (CRM+). The analysis of naturally occurring CRM+ mutants provides valuable information concerning structure-function relationships of factor IX. While a number of CRM+ mutants have been characterized to date, few have been reported to occur in the second EGF module.19 One of these, Factor IX Fukuoka, contains an Asp92 to His substitution and was shown to result in defective binding to factor X.20 This mutation occurred at a residue, which is highly conserved among all vitamin K–dependent coagulation proteins.
We now report functional characterization of the first identified naturally occurring mutation involving a factor IX–specific residue within the second EGF module, in which Arg94 is replaced by Ser (factor IXR94S), resulting in a moderate form of hemophilia B. We show that the R94S mutation markedly impaired both activation by factor XIa and factor VIIa/tissue factor and turnover of factor X in the tenase enzyme complex and present evidence that the mutation introduced a site for O-linked glycosylation in the second EGF module.
MATERIALS AND METHODS
Materials.
Plasma-derived human coagulation factors VIIa, IX, X, XIa, thrombin, as well as coagulation protein from Russell’s viper venom (RVV-X) and human tissue factor were purchased from Enzyme Research Laboratories (South Bend, IN). Dialyzed fetal calf serum and L-polylysine were obtained from Sigma-Aldrich (Castle Hill, NSW, Australia). The chromogenic substrate, MeO-CO-D-CHG-Gly-Arg-p-nitroanilide (CHG-GR-pNA, Spectrozyme FXa), was obtained from American Diagnostica (Greenwich, CT), human factor IX–deficient plasma and activated partial thromboplastin time (APTT) reagent from Organon Teknika (Durham, NC), and Lipofectin from GIBCO-BRL (Gaithersburg, MD). Rabbit polyclonal anti–factor IX antibodies and peroxidase-conjugated goat polyclonal anti–factor IX antibodies were purchased from Dako (Carpinteria, CA). Kind gifts used in this study were: Factor VIII from Dr William Drohan (American Red Cross, Rockville, MD); Sepharose-coupled calcium-dependent monoclonal antibody (A-7) directed against the light chain of factor IX from Dr Kenneth Smith (University of New Mexico School of Medicine, Albuquerque); factor IX/factor X–binding protein from the venom of Trimesesurus flavoviridis(T flavoviridis) (IX/X-bp) and rabbit anti-IX/X-bp polyclonal antibody from Dr T. Morita (Meiji College of Pharmacy, Tanashi-Shi, Tokyo, Japan); calcium-dependent monoclonal antibody (JK-IX) directed against the light chain of factor IX from Dr Teriuko Sugo (Jichi Medical School, Tochigi, Japan); monoclonal antibody C10D directed against the heavy chain of factor IX from Dr Arthur Thompson (University of Washington, Seattle). pED mammalian expression vector was from Genetics Institute (Cambridge, MA). Phospholipid vesicles (85:15, PC:PS) were prepared as described previously.14
Construction of mutant cDNA.
The cDNA for human factor IX in pUC12, mutated to provide convenient cloning sites at the boundaries of the aromatic amino acid stack and first EGF module, the first EGF and second EGF modules, and the second EGF and serine proteinase modules was prepared previously.14 The mutated factor IX cDNA, inserted into thePstI site of pUC12, has an SalI site at the boundary between the aromatic amino acid stack and first EGF modules, and aKpnI site between the second EGF and serine proteinase modules. Using polymerase chain reaction (PCR) mutagenesis by overlap extension,21 Arg 94 (AGA) was mutated to a Ser (AGT), such that the final PCR-derived product could be cloned into theSalI and KpnI sites of the factor IX cDNA. The mutant was characterized by DNA sequencing, and the cDNA encoding factor IXR94S was subcloned into the PstI site of the mammalian expression vector, pED.22
Cell culture and expression.
Dihydrofolate reductase (DHFR)-deficient Chinese hamster ovary cells (CHO-DUKX-B11)23 were grown in a modified Eagle’s medium supplemented with 10% fetal calf serum, 2 mmol/L L-glutamine, 100 U/mL of penicillin and streptomycin, and 5 μg/mL vitamin K. A total of 20 μg of plasmid DNA containing the cDNA encoding wild-type factor IX or mutant factor IXR94S was transfected into the cells using Lipofectin. Three days after transfection, cells were selected for DHFR expression using medium deficient in ribonucleosides. Surviving foci were isolated using cloning rings and allowed to grow to confluence in 24-well plates. The presence of factor IX protein secreted into tissue culture medium was screened by a sandwich enzyme-linked immunosorbent assay (ELISA) procedure. Briefly, plates were coated with rabbit polyclonal antifactor IX antibodies, blocked with 5% bovine serum albumin (BSA), and incubated with medium derived from isolated transfected cells. The presence of human factor IX was confirmed using peroxidase-conjugated goat polyclonal anti-human factor IX antibody. Those clones expressing the highest concentration of wild-type factor IX or factor IXR94S were isolated and used for large-scale tissue culture.
Purification of recombinant wild-type factor IX and factor IXR94S.
Supernatant from CHO cells expressing the recombinant wild-type factor IX or factor IXR94S was collected, made 2 mmol/L in benzamidine, and concentrated eightfold to 10-fold using an Amicon 0.22-μm spiral ultrafiltration cartridge (Amicon, Inc, Beverly, MA). The concentrated supernatant was made 10 mmol/L in CaCl2 and applied to a column containing Sepharose-coupled calcium-dependent A7 monoclonal antibody, which has been used previously to purify γ-carboxylated factor IX.24 The column was washed with 0.02 mol/L Tris/HCl, pH 7.4, 0.15 mol/L NaCl (TBS) containing 10 mmol/L CaCl2 and then with 0.02 mol/L Tris/HCl, pH 7.4, 1 mol/L NaCl containing 10 mmol/L CaCl2. After equilibration of the column in TBS containing 10 mmol/L CaCl2, protein was eluted with TBS containing 20 mmol/L EDTA. Fractions containing eluted protein, as determined by absorbance at 280 nm, were pooled, dialyzed against TBS, and concentrated using an Amicon Centriprep 30 microconcentrator. Protein concentration was measured by BCA assay (Pierce, Rockford, IL) using purified plasma factor IX as a standard. Concentrated proteins were stored at −70°C.
Clotting assays.
Wild-type factor IX and factor IXR94S were characterized functionally for activity in plasma-based clotting assays. A one-stage APTT was performed using human factor IX–deficient plasma, rabbit brain phospholipid, and micronized silica.25 Clotting activity of the recombinant proteins was calculated from a standard curve generated using purified plasma human factor IX and activity expressed as a percentage of plasma factor IX coagulant activity.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting.
Samples were separated on 12.5% or 5% to 15% SDS-PAGE under reducing conditions.26 Samples were reduced by boiling with 20 mmol/L dithiothreitol for 5 minutes and the cysteines blocked using 40 mmol/L iodoacetamide. The proteins were either stained with Coomassie Brilliant Blue or transferred to Immobilon-P membranes (Millipore, Bedford, MA) and developed and visualized using chemiluminescence according to the manufacturer’s instructions (DuPont, Boston, MA). Primary murine monoclonal antibodies were used at 5 μg/mL and secondary rabbit antimouse IgG horseradish peroxidase-conjugated antibodies (Dako) were used at 1:2,000 dilution.
Binding of factor IX to IX/X-bp.
Binding of factor IX to phospholipid vesicles.
Binding of factor IX to phospholipid vesicles was measured by separating bound from free factor IX in an air-fuge.28Factor IX (10 μg/mL) and 75:25 DOPC:DOPS vesicles (15 μmol/L) was added to polyallomer centrifuge tubes containing 0.05 mol/L HEPES, 0.125 mol/L NaCl, 10 mmol/L CaCl2 or 10 mmol/L EDTA, 5 mg/mL BSA, pH 7.4 buffer. The reactions were incubated for 20 minutes and the phospholipid vesicles sedimented in an air-fuge (Beckman, Sydney, Australia) for 20 minutes. The centrifugation sedimented ≥95% of the phospholipid vesicles. The supernatant was aspirated and the free factor IX was determined by converting factor IX to IXa with factor XIa. Factor IX and XIa were incubated at a weight ratio of 10:1 in binding buffer for 3 hours at 37°C. Nonspecific binding of factor IX to control tubes not containing phospholipid vesicles represented ≤10% of the binding to phospholipid vesicles. The moles of factor IX bound to phospholipid vesicles was calculated from the total factor IX and the ratio of bound versus total factor IX.
Activation of factor IX by factor XIa.
Purified human factor XIa (5 or 18 nmol/L) was incubated with plasma factor IX or factor IXR94S (2.7 μmol/L) in TBS containing 10 mmol/L CaCl2 at 37°C. At discrete time intervals, aliquots were quenched with 20 mmol/L EDTA and resolved on SDS-PAGE under reducing conditions. Proteins were visualized by staining with Coomassie Brilliant Blue or transferred to Immobilon-P membranes and Western blotted using monoclonal antibodies directed against either the light chain (JKIX) or heavy chain (C10D) of factor IX.
Activation of factor IX by factor VIIa/tissue factor.
Purified human factor VIIa (2 nmol/L), tissue factor (4 nmol/L), and 75:25 DOPC:DOPS vesicles (1 mmol/L) was incubated with plasma factor IX or factor IXR94S (0.5 μmol/L) in TBS containing 5 mmol/L CaCl2 at 37°C. The factor VIIa/tissue factor/DOPC:DOPS/Ca2+ enzyme complex was established by incubation for 10 minutes before addition of factor IX to start the reaction. At discrete time intervals, aliquots were quenched with 20 mmol/L EDTA and resolved on SDS-PAGE under reducing conditions. Proteins were transferred to Immobilon-P membranes and blotted with monoclonal antibodies directed against the heavy chain (C10D) of factor IX.
Hydrolysis of CHG-GR-pNA by factor IXa.
Plasma factor IXa or factor IXaR94S (0.05 to 1 μmol/L) was incubated with CHG-GR-pNA (2.5 mmol/L) in TBS containing 0.1% BSA. The increase in absorbance at 405 nm was continuously monitored using a Thermomax Kinetic Microplate Reader (Molecular Devices, Palo Alto, CA). The initial rates were calculated at substrate consumption of less than 10%.
Activation of factor X by factor IXa or factor IXaR94S in the presence of polylysine.
The catalytic efficiency of activation of factor X by plasma factor IXa or factor IXaR94S was measured in the presence of polylysine. Factor IXa (50 nmol/L) was incubated with 60 nmol/L polylysine in 0.1 mol/L triethanolamine, 0.1 mol/L NaCl, 0.1% polyethylene glycol (PEG) 6000, pH 9.0 buffer29 30 at 37°C. Factor X was added to a final concentration of 0.6 μmol/L to start the reaction. At discrete time intervals, samples were removed and diluted 1 in 25 into a solution of 300 μmol/L CHG-GR-pNA in the triethanolamine buffer. This solution was incubated for 5 minutes and the reaction was stopped by adding an equal volume of 50% acetic acid. The absorbance at 405 nm was a function of the amount of factor Xa generated and increased linearly with time. A standard curve of known factor Xa concentrations was used to determine factor Xa activity generated, which was then plotted as a function of time.
Activation of factor X by plasma factor IXa or factor IXaR94S in the tenase enzyme complex.
The ability of plasma factor IXa or factor IXaR94S to assemble with factor VIIIa on a phospholipid surface and to catalyze factor X activation was studied using the purified proteins. Plasma factor IXa (0.17 nmol/L) or factor IXaR94S (1.5 nmol/L) was incubated with human factor X (0 to 100 nmol/L), human factor VIIIa (14 U/mL), phospholipid vesicles (35 μmol/L) and Ca2+ (5 mmol/L) in TBS containing 0.1% BSA at 37°C. At discrete time intervals, 25 μL-aliquots were taken and added to microtiter wells containing 25 μL of TBS containing 0.1% BSA and 0.5 mol/L EDTA. Factor VIII had been preactivated with 2 nmol/L thrombin for 2 minutes and the thrombin neutralized by addition of 50 nmol/L hirudin.31 The initial velocities of factor Xa generation were measured using CHG-GR-pNA as described above. The apparent kinetic parameters for activation of factor X by plasma factor IXa or factor IXaR94S in the tenase enzyme complex were determined by fitting of the data to the Michaelis-Menten equation using nonlinear regression analysis (Scientist, Salt Lake City, UT).
RESULTS
Expression and purification of recombinant wild-type factor IX and factor IXR94S.
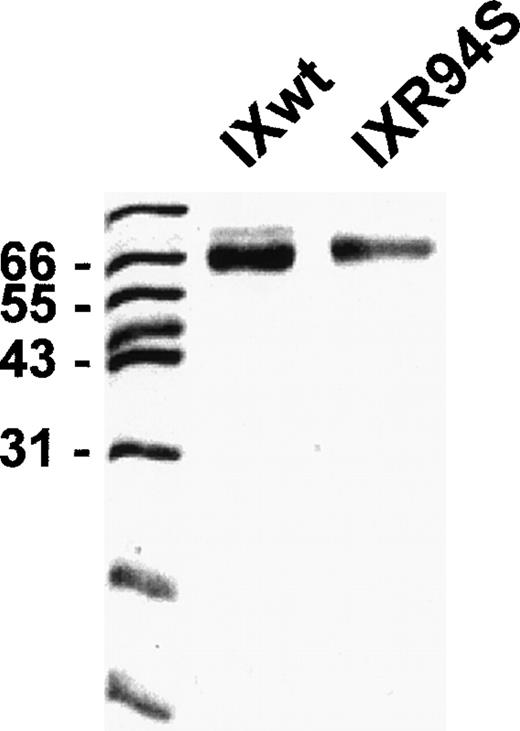
Recombinant wild-type factor IX and mutant factor IXR94S were stably expressed in CHO cells and purified by affinity chromatography using a calcium-dependent monoclonal antibody (A7) directed against the Gla domain of the protein. Purified recombinant proteins were resolved on SDS-PAGE and visualized by staining with Coomassie Brilliant Blue (Fig 1). Both proteins migrated as a single band under reducing conditions, although factor IXR94S migrated marginally slower than recombinant wild-type factor IX. This was probably due to the presence of additional carbohydrate in the mutant protein (see below).
SDS-PAGE analysis of purified recombinant wild-type factor IX and factor IXR94S. Recombinant proteins (3 μg) were resolved on 12.5% SDS-PAGE under reducing conditions and stained with Coomassie Blue.
SDS-PAGE analysis of purified recombinant wild-type factor IX and factor IXR94S. Recombinant proteins (3 μg) were resolved on 12.5% SDS-PAGE under reducing conditions and stained with Coomassie Blue.
Coagulant activity of plasma and wild-type factor IX and factor IXR94S.
In a plasma-based APTT assay, the coagulant activity of wild-type recombinant factor IX was 96% ± 8% that of purified plasma factor IX. In contrast, factor IXR94S exhibited coagulant activity, which was 6% ± 1% that of plasma factor IX.
Comparison of the Gla-domains of plasma and wild-type factor IX and factor IXR94S.
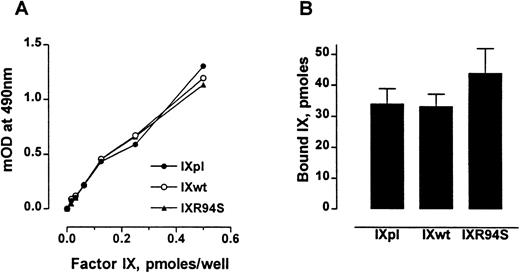
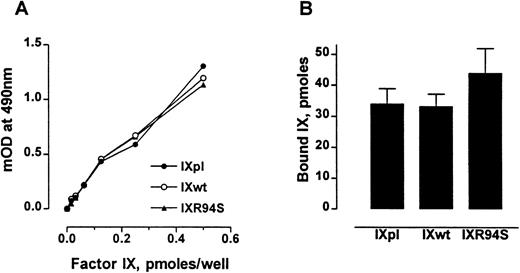
The anticoagulant protein IX/X-bp has been previously shown to be a probe for monitoring the conformational state of the Gla domain of factor IX.27 Factor IXR94S bound IX/X-bp with the same affinity as plasma or wild-type factor IX (Fig 2A). Also, factor IXpl, IXwt, and IXR94S bound to 75:25 DOPC:DOPS vesicles to the same extent (Fig 2B). No specific binding to phospholipid vesicles was observed in buffer containing 10 mmol/L EDTA (not shown). These results implied that the Gla-domains of the recombinant factor IX proteins were intact and functional.
Comparison of the Gla-domains of plasma factor IX, wild-type factor IX, and factor IXR94S. (A) Increasing moles of factor IXpl (○), factor IXwt (•), or factor IXR94S (▴) were coated onto microtiter wells and then incubated with 15 μg/mL of IX/X-bp in the presence of 5 mmol/L Ca2+ in TBS. The binding of IX/X-bp to factor IX was measured by ELISA. The three factor IX proteins bound IX/X-bp equally well. (B) Binding of factor IX to 75:25 DOPC:DOPS vesicles. The three factor IX proteins bound phospholipid vesicles equally well. The bars and errors represent the mean and standard deviation (SD) of three experiments.
Comparison of the Gla-domains of plasma factor IX, wild-type factor IX, and factor IXR94S. (A) Increasing moles of factor IXpl (○), factor IXwt (•), or factor IXR94S (▴) were coated onto microtiter wells and then incubated with 15 μg/mL of IX/X-bp in the presence of 5 mmol/L Ca2+ in TBS. The binding of IX/X-bp to factor IX was measured by ELISA. The three factor IX proteins bound IX/X-bp equally well. (B) Binding of factor IX to 75:25 DOPC:DOPS vesicles. The three factor IX proteins bound phospholipid vesicles equally well. The bars and errors represent the mean and standard deviation (SD) of three experiments.
Activation of plasma and wild-type factor IX and factor IXR94S by factor XIa.
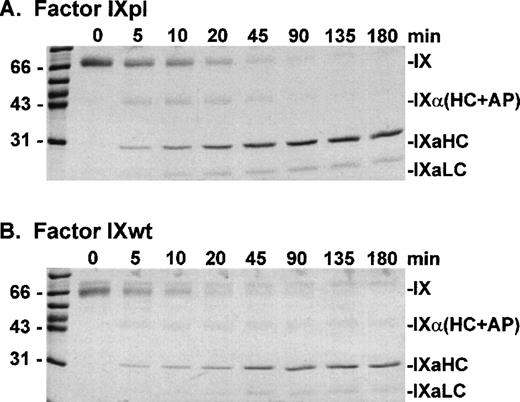
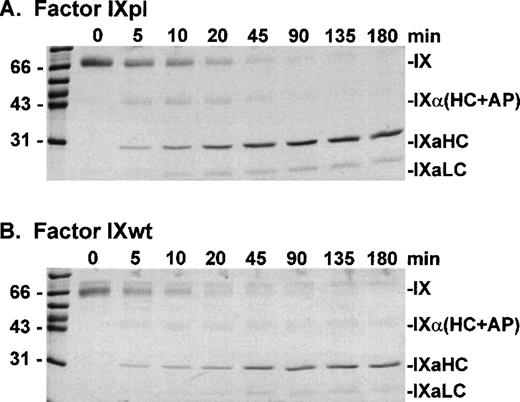
Factor IXpl, IXwt, and IXR94S were activated by factor XIa at a molar enzyme:substrate ratio of 1:500 (Fig 3). Samples of the reactions were resolved on reducing SDS-PAGE and stained with Coomassie Brilliant Blue. Plasma and wild-type factor IX were activated at equivalent rates producing identical heavy chain and light chain cleavage products. Factor IXR94S, however, was activated at a substantially reduced rate and the light chain was not apparent using Coomassie Brilliant Blue staining (not shown).
SDS-PAGE analysis of factor XIa activation of plasma factor IX and wild-type factor IX. Factor IXpl (A) or IXwt (B) (2.7 μmol/L) was incubated with factor XIa (5 nmol/L) in TBS containing 10 mmol/L Ca2+ at 37°C. At discrete time intervals, aliquots (3 μg) of the reactions were resolved on 12.5% SDS-PAGE under reducing conditions and stained with Coomassie Blue.
SDS-PAGE analysis of factor XIa activation of plasma factor IX and wild-type factor IX. Factor IXpl (A) or IXwt (B) (2.7 μmol/L) was incubated with factor XIa (5 nmol/L) in TBS containing 10 mmol/L Ca2+ at 37°C. At discrete time intervals, aliquots (3 μg) of the reactions were resolved on 12.5% SDS-PAGE under reducing conditions and stained with Coomassie Blue.
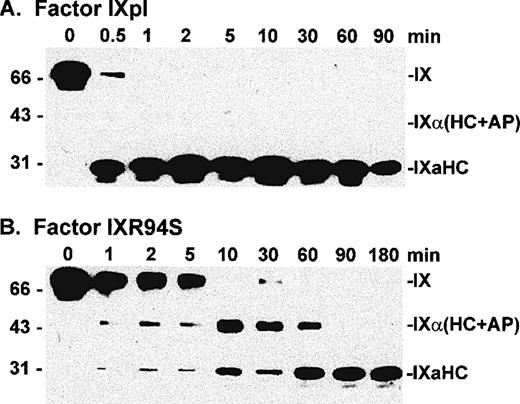
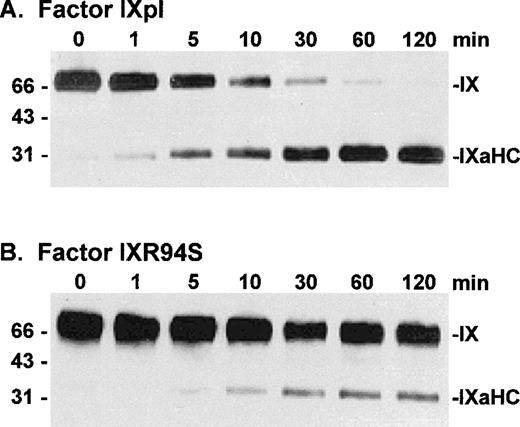
To investigate the fate of the light chain of IXR94S, factor IXpl and IXR94S were activated by factor XIa at a molar enzyme:substrate ratio of 1:150 and the activation products examined by Western blot using monoclonal antibodies recognizing either the heavy (Fig 4) or light (Fig 5) chains of factor IXa.
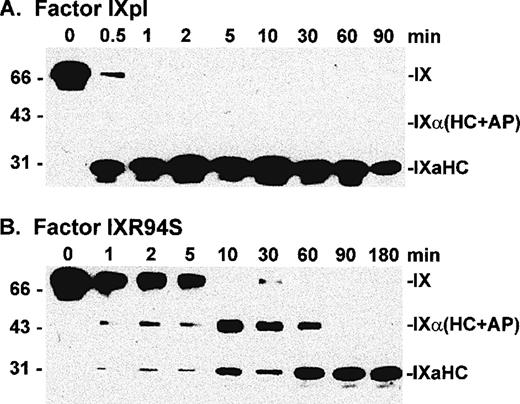
Western blot analysis of factor XIa activation of plasma factor IX and factor IXR94S using a factor IXa heavy chain antibody. Factor IXpl (A) or IXR94S (B) (2.7 μmol/L) was activated by factor XIa (18 nmol/L) in TBS containing 10 mmol/L Ca2+ at 37°C. The activation products were sampled at the indicated time intervals, resolved on reducing 12.5% SDS-PAGE, and transferred to Immobilon-P polyvinylidene fluoride (PVDF) membrane. The membrane was blotted with the C10D monoclonal antibody, which recognizes the heavy chain (HC) of factor IXa, and developed using chemiluminescence. The positions of intact factor IX, IX (HC + AP) and IXaHC are indicated. The positions of the molecular weight markers are indicated at left.
Western blot analysis of factor XIa activation of plasma factor IX and factor IXR94S using a factor IXa heavy chain antibody. Factor IXpl (A) or IXR94S (B) (2.7 μmol/L) was activated by factor XIa (18 nmol/L) in TBS containing 10 mmol/L Ca2+ at 37°C. The activation products were sampled at the indicated time intervals, resolved on reducing 12.5% SDS-PAGE, and transferred to Immobilon-P polyvinylidene fluoride (PVDF) membrane. The membrane was blotted with the C10D monoclonal antibody, which recognizes the heavy chain (HC) of factor IXa, and developed using chemiluminescence. The positions of intact factor IX, IX (HC + AP) and IXaHC are indicated. The positions of the molecular weight markers are indicated at left.
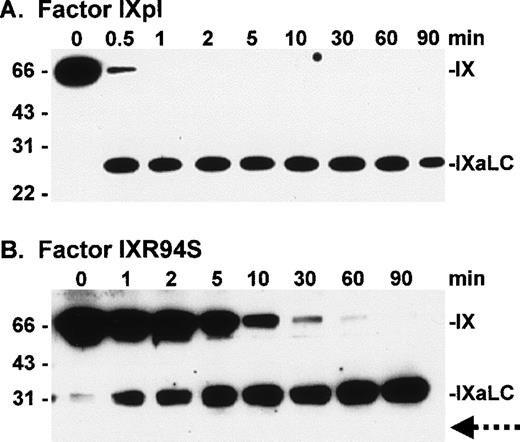
Western blot analysis of factor XIa activation of plasma factor IX and factor IXR94S using a factor IXa light chain antibody. Factor IXpl (A) or IXR94S (B) (2.7 μmol/L) was activated by factor XIa (18 nmol/L) in TBS containing 10 mmol/L Ca2+ at 37°C. The activation products were sampled at the indicated time intervals, resolved on reducing 12.5% SDS-PAGE, and transferred to PVDF membrane. The membrane was blotted with the JKIX monoclonal antibody, which recognizes the light chain (LC) of factor IXa, and developed using chemiluminescence. The positions of intact factor IX and IXaLC are indicated. The dotted arrow in (B) indicates the usual migration position of the factor IXa light chain. The positions of the molecular weight markers are indicated at left.
Western blot analysis of factor XIa activation of plasma factor IX and factor IXR94S using a factor IXa light chain antibody. Factor IXpl (A) or IXR94S (B) (2.7 μmol/L) was activated by factor XIa (18 nmol/L) in TBS containing 10 mmol/L Ca2+ at 37°C. The activation products were sampled at the indicated time intervals, resolved on reducing 12.5% SDS-PAGE, and transferred to PVDF membrane. The membrane was blotted with the JKIX monoclonal antibody, which recognizes the light chain (LC) of factor IXa, and developed using chemiluminescence. The positions of intact factor IX and IXaLC are indicated. The dotted arrow in (B) indicates the usual migration position of the factor IXa light chain. The positions of the molecular weight markers are indicated at left.
Factor IXpl was activated completely within 1 to 2 minutes (Fig 4A), while factor IXR94S was activated at a much slower rate (Fig 4B). Densitometric analysis of the 31-kD heavy chain bands indicated that factor IXR94S was activated by factor XIa at approximately 2% of the rate of activation of factor IXpl or IXwt. An intermediate heavy chain product with an approximate molecular mass of 44 kD was observed with factor XIa activation of factor IXR94S (Fig 4B). This product, termed factor IXα, consists of the heavy chain plus activation peptide (HC + AP) and its presence indicates that the light chain (LC) is cleaved from the AP at the Arg145-Ala146 peptide bond. The activation peptide is cleaved from the heavy chain at the Arg180-Val181 peptide bond.
The light chain of factor IXaR94S migrated at an apparent molecular mass of 30 kD (Fig 5B), which is significantly higher than the light chain of plasma (or wild-type) factor IX, which migrated at an apparent molecular mass of 24 kD (Fig 5A). Moreover, the light chain of factor IXaR94S migrated at a molecular mass which is virtually identical to that of the heavy chain. This would account for the apparent absence of the light chain of factor IXR94S on Coomassie-stained SDS-PAGE. The intact mutant protein also migrated at a slightly higher molecular mass than the wild-type protein (Fig 1). The increase in the observed molecular mass of the intact mutant protein and its activated light chain was probably due to the presence of additional carbohydrate in the light chain of factor IXR94S. Digestion of factor IXaR94S with N-glycosidase F did not reduce the molecular weight of the mutant light chain to that of plasma factor IXa light chain (not shown), indicating that the carbohydrate was not N-linked. This finding implied that the R94S mutation had introduced a site for O-glycosylation.
Activation of plasma and wild-type factor IX and factor VIIa/tissue factor.
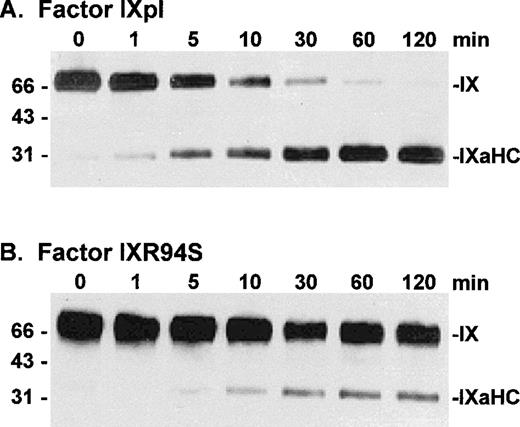
Factor IXpl, IXwt, and IXR94S were activated by factor VIIa/tissue factor/DOPC:DOPS/Ca2+ enzyme complex at a molar enzyme:substrate ratio of 1:250. Samples of the reactions were resolved on reducing SDS-PAGE and Western blotted using the factor IXa heavy chain (C10D) antibody. Plasma and wild-type factor IX were activated at equivalent rates (not shown), which were similar to that described for activation of plasma factor IX by Lawson and Mann.32 Factor IXR94S, however, was activated at a substantially reduced rate (Fig 6). Densitometric analysis of the 31-kD heavy chain bands indicated that factor IXR94S was activated by factor VIIa/tissue factor/DOPC:DOPS/Ca2+ at approximately 5% of the rate of activation of factor IXpl or IXwt.
Western blot analysis of factor VIIa/tissue factor/DOPC:DOPS/Ca2+ enzyme complex activation of plasma factor IX and factor IXR94S using a factor IXa heavy chain antibody. Factor IXpl (A) or IXR94S (B) was activated by factor VIIa/tissue factor/DOPC:DOPS/Ca2+ enzyme complex in TBS at 37°C. The reaction component concentrations were: 0.5 μmol/L factor IXpl or IXR94S, 2 nmol/L factor VIIa, 4 nmol/L tissue factor, 1 mmol/L DOPC:DOPS, and 5 mmol/L Ca2+. The activation products were sampled at the indicated time intervals, resolved on reducing 5% to 15% SDS-PAGE, and transferred to PVDF membrane. The membrane was blotted with the C10D monoclonal antibody, which recognizes the heavy chain (HC) of factor IXa, and developed using chemiluminescence. The positions of intact factor IX and IXaHC are indicated. The positions of the molecular weight markers are indicated at left.
Western blot analysis of factor VIIa/tissue factor/DOPC:DOPS/Ca2+ enzyme complex activation of plasma factor IX and factor IXR94S using a factor IXa heavy chain antibody. Factor IXpl (A) or IXR94S (B) was activated by factor VIIa/tissue factor/DOPC:DOPS/Ca2+ enzyme complex in TBS at 37°C. The reaction component concentrations were: 0.5 μmol/L factor IXpl or IXR94S, 2 nmol/L factor VIIa, 4 nmol/L tissue factor, 1 mmol/L DOPC:DOPS, and 5 mmol/L Ca2+. The activation products were sampled at the indicated time intervals, resolved on reducing 5% to 15% SDS-PAGE, and transferred to PVDF membrane. The membrane was blotted with the C10D monoclonal antibody, which recognizes the heavy chain (HC) of factor IXa, and developed using chemiluminescence. The positions of intact factor IX and IXaHC are indicated. The positions of the molecular weight markers are indicated at left.
Amidolytic activity of plasma factor IXa and factor IXaR94S.
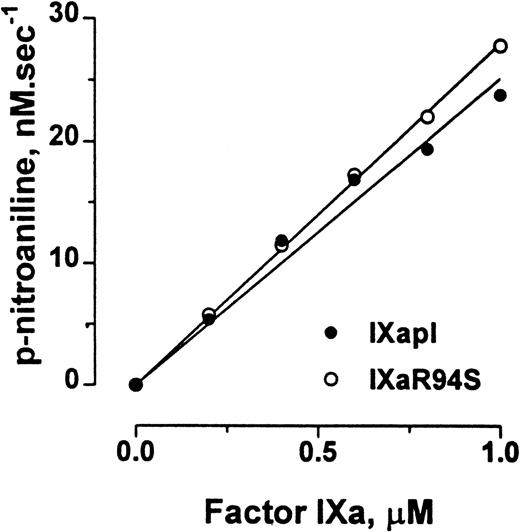
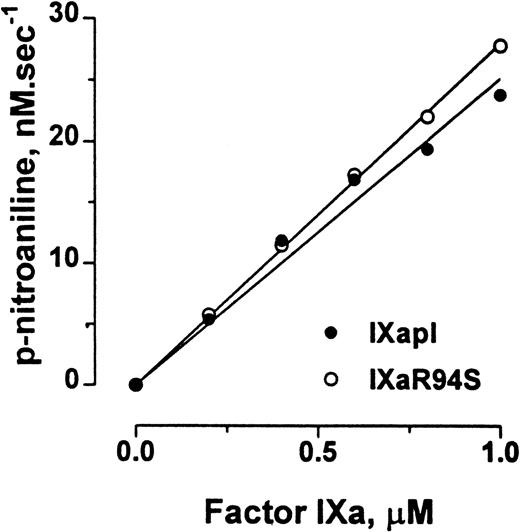
Factor IXa cleaves the chromogenic substrate CHG-GR-pNA, albeit with low catalytic efficiency. Plasma factor IXa and factor IXaR94S hydrolyzed CHG-GR-pNA with initial rates of 1.51 ± 0.06 min−1 and 1.68 ± 0.02 min−1, respectively (Fig 7).
Comparison of the amidolytic activity of plasma factor IXa and factor IXaR94S. Varying concentrations of plasma factor IXa (○) or factor IXaR94S (•) were incubated with 2.5 mmol/L CHG-GR-pNA in TBS containing 0.1% BSA, and the increase in absorbance at 405 nm with time was measured. The initial velocity of p-nitroaniline formation is expressed as a function of enzyme concentration. The solid lines represent the linear regression fit to the data. The observed rate of hydrolysis of CHG-GR-pNA by plasma factor IXa and factor IXaR94S was 1.51 ± 0.06 min−1 and 1.68 ± 0.02 min−1, respectively.
Comparison of the amidolytic activity of plasma factor IXa and factor IXaR94S. Varying concentrations of plasma factor IXa (○) or factor IXaR94S (•) were incubated with 2.5 mmol/L CHG-GR-pNA in TBS containing 0.1% BSA, and the increase in absorbance at 405 nm with time was measured. The initial velocity of p-nitroaniline formation is expressed as a function of enzyme concentration. The solid lines represent the linear regression fit to the data. The observed rate of hydrolysis of CHG-GR-pNA by plasma factor IXa and factor IXaR94S was 1.51 ± 0.06 min−1 and 1.68 ± 0.02 min−1, respectively.
Activation of factor X by plasma factor IXa or factor IXaR94S in the presence of polylysine.
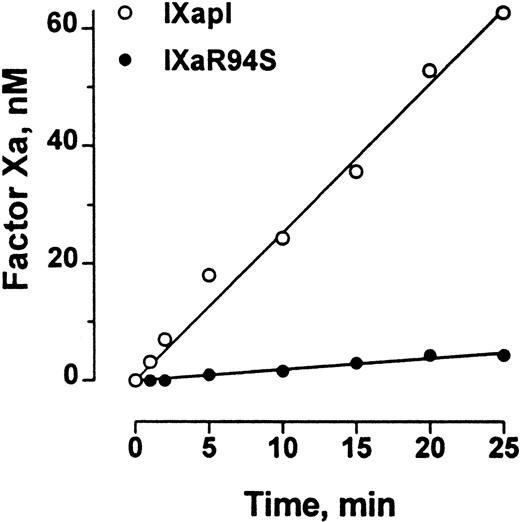
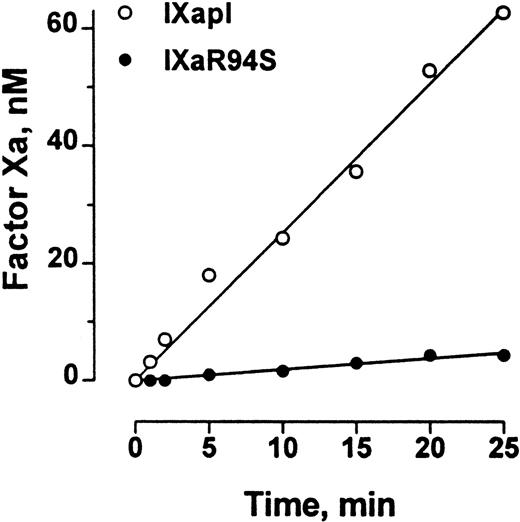
Polylysine can partially substitute for phospholipid and factor VIIIa in the tenase enzyme complex. The efficiency of factor X activation by factor IXa is enhanced at least 300-fold by polylysine.29The initial rate of activation of factor X by factor IXaR94S in the presence of polylysine was 0.19 ± 0.01 nmol/L/min−1, compared with the initial rate of activation of factor X by plasma-derived factor IXa of 2.54 ± 0.07 nmol/L/min−1 (Fig 8). Therefore, the efficiency of activation of factor X by factor IXaR94S in the presence of polylysine was 7% ± 1% of the efficiency of activation by plasma factor IXa.
Comparison of the factor X–activating activity of plasma factor IXa and factor IXaR94S in the presence of polylysine. Human factor X (600 nmol/L) was activated by 50 nmol/L plasma factor IXa (○) or factor IXaR94S (•) in the presence of polylysine (60 nmol/L) in 0.1 mol/L triethanolamine, 0.1 mol/L NaCl, 0.1% PEG 6000, pH 9.0 buffer at 37°C. At discrete time intervals, aliquots of the reactions were assayed for factor Xa concentration using CHG-GR-pNA. The results are expressed as concentration of factor Xa formed as a function of time. The solid lines represent the linear regression fit to the data. The initial rate of factor X activation by plasma factor IXa and factor IXaR94S was 2.54 ± 0.07 nmol/L/min−1 and 0.19 ± 0.01 nmol/L/min−1, respectively.
Comparison of the factor X–activating activity of plasma factor IXa and factor IXaR94S in the presence of polylysine. Human factor X (600 nmol/L) was activated by 50 nmol/L plasma factor IXa (○) or factor IXaR94S (•) in the presence of polylysine (60 nmol/L) in 0.1 mol/L triethanolamine, 0.1 mol/L NaCl, 0.1% PEG 6000, pH 9.0 buffer at 37°C. At discrete time intervals, aliquots of the reactions were assayed for factor Xa concentration using CHG-GR-pNA. The results are expressed as concentration of factor Xa formed as a function of time. The solid lines represent the linear regression fit to the data. The initial rate of factor X activation by plasma factor IXa and factor IXaR94S was 2.54 ± 0.07 nmol/L/min−1 and 0.19 ± 0.01 nmol/L/min−1, respectively.
Activation of factor X by plasma factor IXa or factor IXaR94S in the tenase enzyme complex.
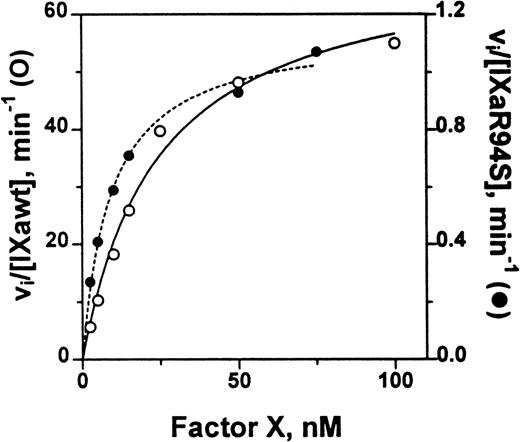
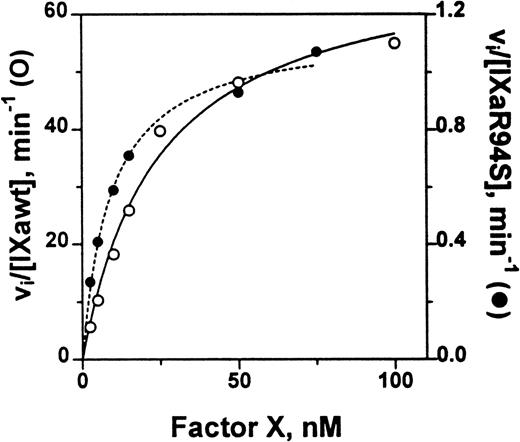
The catalytic efficiency of factor IXaR94S in the tenase enzyme complex was compared with plasma factor IXa. The factor VIIIa concentration dependence for activation of factor X by either plasma factor IXa or factor IXaR94S in the tenase enzyme complex was first determined. A factor VIIIa concentration of 2 U/mL gave half-maximal activity for activation by plasma factor IXa, while essentially maximal activity was achieved with 6 U/mL (not shown). A factor VIIIa concentration of either 6 U/mL or 14 U/mL made no change to the rate of activation of factor X by factor IXaR94S, implying that the concentration of factor VIIIa required for half-maximal activity was <6 U/mL. Therefore, to ensure that the rate of the tenase reaction was not limited by factor VIIIa, a factor VIIIa concentration of 14 U/mL was chosen for estimation of the kinetic parameters for factor X activation. The Km and kcat for activation of factor X by either plasma factor IXa or factor IXaR94S was estimated from the data shown in Fig 9.
Comparison of the catalytic activity of plasma factor IXa and factor IXaR94S in the tenase enzyme complex. Plasma factor IXa (0.17 nmol/L, ○, ___) or factor IXaR94S (1.5 nmol/L, •, —-) was incubated with factor X (0 to 100 nmol/L) in the presence of phospholipid vesicles (35 μmol/L), factor VIIIa (14 U/mL) and Ca2+ (5 mmol/L) in TBS containing 0.1% BSA at 37°C. At discrete time intervals, aliquots of the reactions were quenched with 50 mmol/L EDTA and factor Xa quantitated using CHG-GR-pNA. The data has been normalized with respect to enzyme concentration by expressing the ordinate axis as initial velocity, vi, divided by the factor IXa or factor IXaR94S concentration. The lines represent the best fit of the data to the Michaelis-Menten equation using nonlinear regression analysis. The kinetic parameters are summarized in Table 1.
Comparison of the catalytic activity of plasma factor IXa and factor IXaR94S in the tenase enzyme complex. Plasma factor IXa (0.17 nmol/L, ○, ___) or factor IXaR94S (1.5 nmol/L, •, —-) was incubated with factor X (0 to 100 nmol/L) in the presence of phospholipid vesicles (35 μmol/L), factor VIIIa (14 U/mL) and Ca2+ (5 mmol/L) in TBS containing 0.1% BSA at 37°C. At discrete time intervals, aliquots of the reactions were quenched with 50 mmol/L EDTA and factor Xa quantitated using CHG-GR-pNA. The data has been normalized with respect to enzyme concentration by expressing the ordinate axis as initial velocity, vi, divided by the factor IXa or factor IXaR94S concentration. The lines represent the best fit of the data to the Michaelis-Menten equation using nonlinear regression analysis. The kinetic parameters are summarized in Table 1.
The kinetic parameters for factor X activation are listed in Table 1. The parameters for plasma factor IXa were similar to those determined by van Diejen et al.33It is apparent that the Km for factor X is effectively the same for either plasma factor IXa or factor IXaR94S. However, the kcat for cleavage of factor X by factor IXaR94S is 58-fold ± 12-fold slower than that for plasma factor IXa.
DISCUSSION
The purpose of this study was to evaluate the structural and kinetic properties of a variant factor IX molecule in which there is a single amino acid substitution at a factor IX–specific residue (Arg94 to Ser) in the second EGF module. This mutation results in a moderately severe Hemophilia B and is characterized by a factor IX clotting activity of 1% to 2% and a factor IX antigen level of 84%.34 This factor IX mutation was significant for two reasons. First, this is only the second characterized case of a naturally occurring mutation in the second EGF module in which the antigenic level is normal, but functional clotting activity is significantly impaired. Second, it is the first mutation described at a factor IX–specific residue in the second EGF module and so has the potential to report on factor IX–specific interactions such as with factor VIII and factor X.
Factor IXR94S was activated by factor XIa/calcium at a rate, which was ≈2% that of plasma factor IX, and by factor VIIa/tissue factor/phospholipid/calcium at a rate which was ≈5% that of plasma factor IX. This is the first amino acid substitution identified in factor IX in which there is a significant reduction in the rate of cleavage by factor XIa. Factor IX New London, which is caused by a Pro50 to Glu substitution in the first EGF module, was originally thought to result in a reduced rate of activation by factor XIa,35 but has since been shown to exhibit normal activation rates.13 Activation of factor IXR94S was complete, albeit slow. This is in contrast to the virtual absence of factor XIa cleavage of the Arg145-Ala-146 peptide bond in factor IX Chapel Hill in which Arg145 is replaced by His.29 Despite this mutation, the clotting activity of factor IX Chapel Hill is 20% that of factor IXaβ and the clinical bleeding tendency is very mild.29
One other mutation in the second EGF module of factor IX has been characterized to date. Factor IX Fukuoka, which has a Asn92 to His mutation in the second EGF module, is characterized by 3% clotting activity and 64% factor IX antigen levels. In contrast to the R94S mutation, the N92H mutation results in a normal rate of activation by factor XIa, but a reduced rate of turnover of factor X in the tenase enzyme complex.
The apparent molecular mass of the light chain of factor IXaR94S was ≈6 kD higher than the light chain of plasma or wild-type factor IXa. The apparent molecular mass of the intact factor IXR94S was also higher than wild-type factor IX. This finding implied that the R94S mutation had resulted in incorporation of additional carbohydrate into the light chain. There are four sites of attachment of O-linked oligosaccharides in plasma factor IX involving Ser53 and Ser61 within the first EGF module, which are uniformly glycosylated,36 and Thr159 and Thr169 in the activation peptide (residues 145-180), which are partially glycosylated.37 The O-linked carbohydrates in the first EGF module may play a role in receptor-ligand interaction.38,39 The new carbohydrate in the light chain of factor IXR94S is probably O-linked to the new Ser. The R94S mutation creates a Gly-Ser-Cys motif, which is identical to that at Ser61 in the first EGF domain. Ser61 is O-fucosidically linked to the tetrasaccharide, NeuAcα(2 → 6)Galβ(1 → 4)GlcNAcβ(1 → 3)Fucα1 → O-Ser.36,38 Moreover, O-glyconase, which cleaves Galβ1 → 3GalNAc-Ser/Thr linkages, does not cleave the O-fucosidically linked tetrasaccharide at Ser61,36,38 and O-glyconase digestion did not correct the difference in the molecular weights between the mutant and wild-type light factor IX chains (not shown). In addition, digestion of factor IXaR94S with N-glycosidase F, which hydrolyzes all types of N-glycan chains from mammalian glycoproteins, also did not correct the difference in the molecular weights between the mutant and wild-type light chains (not shown). These results support a fucosidically linked saccharide at Ser94 in the R94S mutant. Jentoft40 has shown that a major function of O-glycosylation is to induce a specific conformation. Therefore, it is likely that the presence of an O-linked carbohydrate at Ser94 in the second EGF module will perturb the tertiary structure of this region.
The amidolytic activity of factor IXaR94S was the same as plasma factor IXa. This finding suggested that the environment of the active site of the serine proteinase module was not perturbed by the R94S mutation, although perturbation at exosites was not excluded by this result. In contrast, the initial rate of activation of factor X by factor IXaR94S in the presence of polylysine was 7% of the initial rate of activation of factor X by plasma factor IXa. It is unlikely that this result was due to an impaired Gla domain, as the plasma and recombinant proteins bound a calcium-dependent monoclonal antibody, the Gla-dependent IX/X-binding protein, and phospholipid vesicles to the same extent.
The kc/Km for activation of factor X by factor IXaR94S/factor VIIIa/phospholipid/calcium was 4% of the kc/Km for activation of factor X by plasma factor IXa/factor VIIIa/phospholipid/calcium. The reduced catalytic efficiency of factor IXaR94S in the tenase enzyme complex was due to a 58-fold decrease in kcat with little effect on Km. These results do not exclude the possibility that factor IXaR94S may have bound with slightly reduced affinity or nonproductively to factor VIIIa in the tenase complex, which could have accounted for some of the reduced catalytic efficiency of factor X activation by tenase. However, the magnitude of reduction in the initial rate of activation of factor X by factor IXaR94S compared with plasma factor IX in the absence of factor VIIIa (7% of the initial rate for plasma factor IXa) was effectively the same as the magnitude of reduction in kc/Km for activation of factor X in the presence of factor VIIIa and phospholipid (4% of the kc/Km for plasma factor IXa). Taken together, these results implied that the reduced rate of turnover of factor X was not due to impaired binding of factor IXaR94S to factor VIIIa in the tenase complex.
The findings reported herein suggest that the second EGF module of factor IXa is important for catalytically productive binding of factor X. In other words, the mutation in second EGF domain of factor IXa may have perturbed the nature, but not the overall affinity of factor X binding such that it was poorly activated by the factor IXa serine proteinase domain. Further evidence for this proposal comes from the work of Nishimura et al.20 These investigators characterized factor IX Fukuoka, which has a Asn92 to His mutation in the second EGF module. This mutation caused a moderate form of hemophilia, which was characterized by 3% clotting activity and 64% factor IX antigen levels. The N92H mutation results in a reduced rate of activation of factor X in the presence of polylysine (8% of the initial rate for plasma factor IXa) and a reduced kc/Km for activation of factor X in the tenase complex (2.3% of the kc/Km for plasma factor IXa). The reduced catalytic efficiency of factor IXa Fukuoka in the tenase enzyme complex is due to a 1,000-fold decrease in kcat and a 24-fold decrease in Km. Therefore, the major effect of the N92H mutation is a reduced rate of turnover of factor X in the tenase enzyme complex. These findings are in general agreement with the results for the R94S mutation described herein. It is also possible that the R94S mutation perturbed the catalytic activity of the serine proteinase domain of the mutant factor IXa, although this seems unlikely.
Christophe et al41 have suggested that the two EGF modules in factor IX associate electrostatically via Glu78 in the first module and Arg94 in the second module and that this interaction is important for binding of the factor VIII light chain. However, it is not known whether the second EGF module facilitates binding of factor VIII to the first module or whether factor VIII binding spans both EGF modules.
In conclusion, the R94S mutation appeared to have resulted in the incorporation of an additional O-linked carbohydrate in the light chain, probably at the new Ser in the second EGF module, which markedly impaired both activations by both factor XIa and factor VIIa/tissue factor and turnover of factor X in the tenase enzyme complex.
Supported by grants from the National Health and Medical Research Council of Australia.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Mark S. Hertzberg, MB, BS, PhD, Department of Haematology, Level 2, ICPMR, Westmead Hospital, Westmead, NSW 2145, Australia; e-mail: markh@westmed.wh.su.edu.au.