The pearl mouse is a model for Hermansky Pudlak Syndrome (HPS), whose symptoms include hypopigmentation, lysosomal abnormalities, and prolonged bleeding due to platelet storage pool deficiency (SPD). The gene for pearl has recently been identified as the beta3A subunit of the AP-3 adaptor complex. The objective of these experiments was to determine if the expression and subcellular distribution of the AP-3 complex were altered in pearl platelets and other tissues. The beta3A subunit was undetectable in all pearl cells and tissues. Also, expression of other subunit proteins of the AP-3 complex was decreased. The subcellular distribution of the remaining AP-3 subunits in platelets, macrophages, and a melanocyte-derived cell line of pearl mice was changed from the normal punctate, probably endosomal, pattern to a diffuse cytoplasmic pattern. Ultrastructural abnormalities in mutant lysosomes were likewise apparent in mutant kidney and a cultured mutant cell line. Genetically distinct mouse HPS models had normal expression of AP-3 subunits. These and related experiments strongly suggest that the AP-3 complex regulates the biogenesis/function of organelles of platelets and other cells and that abrogation of expression of the AP-3 complex leads to platelet SPD.
THE PEARL (pe) mutation arose spontaneously in the C3H strain.1 The pearl (pe)gene is inherited in an autosomal recessive manner and has been mapped to chromosome 13.2-4 Mutant mice have oculocutaneous pigment dilution with melanosomes both morphologically abnormal and reduced in quantity.5 Platelet storage pool deficiency (SPD) in pearl mutant platelets,6-8 like that of human SPD,9 is characterized by deficiencies of the platelet dense granule components serotonin and adenine nucleotides. In addition, secretion of a third subcellular organelle, the lysosome, is reduced in platelets and kidney.6,7 These phenotypes indicate the pearl gene is involved in the biogenesis/function of all three organelles. SPD in pearl mice leads to prolonged bleeding times, which can be corrected by marrow transplantation,10indicating that the pearl gene acts in marrow progenitor cells. The pearl mouse is one of a large group of mouse hypopigmentation mutants with accompanying inherited SPD.8 The molecular causes of SPD are little understood.
Pearl is an established model for human Hermansky Pudlak syndrome (HPS), an autosomal recessive inherited disease with the triad phenotype of hypopigmentation, prolonged bleeding times due to platelet SPD, and accumulation of ceroid pigment in lysosomal organelles.11-13 HPS is thus a disease of subcellular organelles with misregulation of the biogenesis/function of the related organelles, melanosomes, lysosomes, and platelet dense granules. The syndrome occurs in diverse populations worldwide and causes considerable morbidity and mortality due to a high incidence of fibrotic restrictive lung disease, granulomatis colitis, and prolonged bleeding.11 13
Additionally, pearl mice exhibit reduced sensitivity in the dark-adapted state,14 have altered somatostatin binding to the retina,15 and acceleration of retinal apoptosis,16 suggesting a model for human congenital stationary night blindness.
Intracellular protein sorting and trafficking are conducted by means of carrier vesicles.17 The formation of a number of these vesicles is mediated by heterotetrameric adaptor protein (AP) complexes. Two types of AP complexes mediate the formation of clathrin-coated vesicles.18 AP-1 recruits clathrin to vesicles at the trans-Golgi network (TGN), whereas AP-2 performs a similar function at the plasma membrane. A third adaptor-related coat complex, termed AP-3, probably facilitates trafficking of vesicles from the TGN and/or endosomal compartments to lysosomes and melanosomes.19-21 AP-3 is heterotetrameric containing two large subunits, delta and beta3, a medium subunit, mu3, and a small subunit, sigma3. In yeast, AP-3 is essential for cargo-selective transport to the yeast vacuole (lysosome).22,23 AP-3 is important for pigment granule biogenesis in Drosophila, as evidenced by the combined decrease in pigmentation and in expression of the AP-3 delta subunit in thegarnet mutant.19 24 Details of the function of AP-3 in mammals are less understood.
Recent positional/candidate cloning approaches have succeeded in the molecular identification of the pearl gene. Mutant mice contain nucleotide sequence changes in beta3A cDNA together with reductions of beta3A transcript expression,25 indicating that the primary gene defect in pearl mice is in this subunit of the AP-3 complex. The present and related experiments show abnormal expression and subcellular distribution of several subunits of this complex in platelets and other tissues of pearl mice, and strongly suggest that alterations of the AP-3 complex leads to SPD in these mice.
MATERIALS AND METHODS
Mice.
C57BL/6J pe/pe mutant mice, control C57BL/6J, C57BL/6Jpe/+, and C3H/HeJ mice together with other HPS mutant mice [ruby eye (ru/ru), gunmetal (gm/gm), light ear (le/le), muted (mu/mu), and sandy (sdy/sdy)] were obtained from the Jackson Laboratory (Bar Harbor, ME). Mice were subsequently bred and maintained in the animal facilities of Roswell Park Cancer Institute.
Cell culture.
A melanocyte-derived cell line was obtained from epidermis of 1-day-old pearl mice as described.26 Immortalized pearl cells have been maintained in culture for more than 12 months. A control mouse melanocyte line, melan-a, was kindly provided by Dr Dorothy Bennett (St George’s Hospital Medical School, London, UK). Thioglycollate-stimulated macrophages were isolated and cultured as described.27
Probes and antibodies.
The mu3A probe for Northern blotting was obtained from J. Pevsner (The Johns Hopkins University, Baltimore, MD).28Polyclonal affinity-purified rabbit antibodies to the delta, beta3A, beta3B, mu3A/B, and sigma3A subunits of the human AP-3 complex were described by Simpson et al.19,29 The rabbit polyclonal anti-rat lgp110 antiserum was previously described.30Protein A conjugated to 15 nm colloidal gold was purchased from the Department of Cell Biology, University of Utrecht (Utrecht, The Netherlands).
Immunoblots.
Fresh tissue or tissues snap frozen in liquid nitrogen were homogenized in a proteinase inhibitor cocktail.31 The homogenized tissues were boiled in sample buffer and 40 μg protein was electrophoresed on sodium dodecyl sulfate (SDS) polyacrylamide gels and transferred to nitrocellulose in Western blotting. Blots were probed with indicated primary antibodies prepared in rabbits, followed by peroxidase-labeled goat anti-rabbit IgG secondary antibody (Kirkegaard and Perry, Gaithersburg, MD), visualization with ECL reagent (Amersham, Piscataway, NJ), and exposure to x-ray film.
Immunofluorescence.
Cells were fixed in methanol followed by acetone at −20°C, then labeled with an affinity-purified rabbit antiserum raised against the delta subunit of the AP-3 complex expressed as a fusion protein19 followed by fluorescein-conjugated donkey anti-rabbit IgG.
Endocytosis of bovine serum albumin (BSA)-gold.
Ten nanometer colloidal gold was prepared by tannic acid/tri-sodium citrate reduction of gold chloride.32 The colloid was adjusted to pH 5.5 with NaOH and conjugated to sufficient BSA to afford protection from NaCl-induced flocculation. BSA-gold was harvested using ultracentrifugation protocols which yielded monodisperse preparations free of aggregates and unbound protein.33,34 The preparations were dialyzed against phosphate-buffered saline (PBS) and adjusted to an A520 of 1.4 with PBS. One milliliter of BSA-gold was added to 4 mL Dulbecco’s Modified Eagle Medium (DMEM) + 10% fetal calf serum, and cells grown to ≈80% confluence were incubated with the conjugate-containing medium for 4 hours at 37°C followed by incubation in conjugate-free medium for 20 hours as previously described.35
Transmission electron microscopy (TEM).
For TEM, cells were removed from tissue-culture flasks by trypsinization and pelleted in a bench-top microfuge at 500gfor 2 minutes. Cells or tissue sections were fixed with 2.5% glutaraldehyde/2% paraformaldehyde in 0.1 mol/L Na cacodylate buffer, pH 7.2, for 3 hours at room temperature and processed as described previously.35 The sections were observed in a Philips CM 100 transmission electron microscope (Philips Electron Optics, Cambridge, UK) at an operating voltage of 80 kV.
Immunoelectron microscopy (immuno-EM).
Cells were prepared for immuno-EM as described by Griffiths.36 Cells were washed with PBS and fixed with 4% paraformaldehyde/0.1% glutaraldehyde in 250 mmol/L HEPES, pH 7.2, at room temperature for 1 hour. The cells were processed for immuno-EM as described previously35 and ultrathin frozen sections were collected from the knife edge using a mixture of sucrose and methylcellulose. Immunolabeling of lgp110 was performed using the protein A-gold technique37 as described previously.35
RESULTS
The beta3A protein is greatly reduced in tissues of pearl mice.
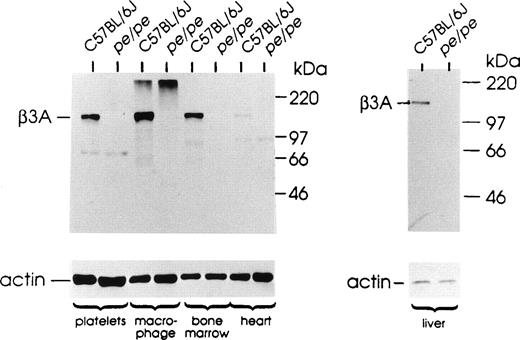
Our recent studies25 showed that beta3A transcripts of pearl mice are significantly decreased in quantity and are predicted to produce a beta3A protein with a truncation of 130 amino acids of the C-terminus of the 1,105-amino acid subunit. These results suggested that expression and function of the beta3A protein would be significantly affected in pearl tissues. This was confirmed by immunoblotting of five tissues of pearl and control C57BL/6J mice (Fig 1) using a polyclonal antibody against human beta3A.19 The 130-kD beta3A protein subunit is expressed in all control tissues examined, although quantitative variation was observed. Concentrations were relatively high in normal macrophages and platelets and intermediate in bone marrow and liver and low in heart. Very low levels were detected in normal kidney (not shown). In contrast, the beta3A subunit was not detected in any tissue of pearl mice. Further, forms of beta3A of altered size/mobility were not apparent.
The beta3A protein is expressed at very low/undetectable levels in platelets, macrophages and other tissues of pearl mice. Extracts (40 μg protein) from indicated tissues of mutant pearl mice and control C57BL/6J mice were analyzed by Western blotting. Sizes of MW standard proteins are indicated at right. Blots were reprobed with antibody to mouse actin (below) as a loading control. The high MW material observed near the gel origin in this experiment in macrophage extracts of normal and pearl mice was not reproducible.
The beta3A protein is expressed at very low/undetectable levels in platelets, macrophages and other tissues of pearl mice. Extracts (40 μg protein) from indicated tissues of mutant pearl mice and control C57BL/6J mice were analyzed by Western blotting. Sizes of MW standard proteins are indicated at right. Blots were reprobed with antibody to mouse actin (below) as a loading control. The high MW material observed near the gel origin in this experiment in macrophage extracts of normal and pearl mice was not reproducible.
Levels of other subunits of the AP-3 heterotetramer are affected in certain tissues of pearl mice.
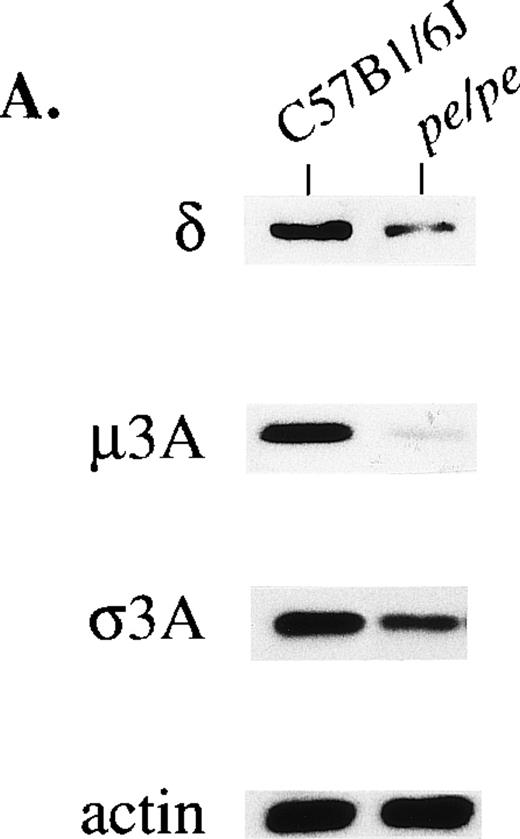
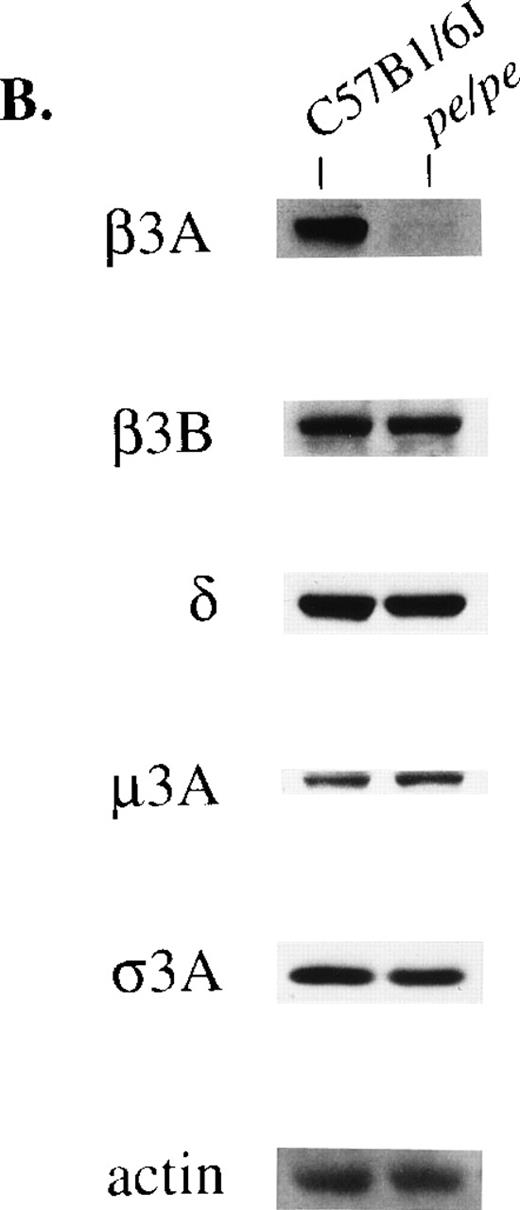
The loss of one subunit of a protein complex can lead to decreased stability of other subunit proteins. Expression of the other three subunits of AP-3 was accordingly assessed in pearl by immunoblotting (Fig 2). Levels of the 160-kD delta subunit were consistently reduced to about half normal levels in platelets from the pearl mutant (Fig 2A). Levels of the delta subunit were also significantly diminished in bone marrow and eye of pearl mice (not shown). The mu3A subunit was more drastically affected. Very small amounts of mu3A subunits (molecular weight [MW], 45 kD) and significantly reduced levels of sigma3A subunits (MW, 26 kD) were visible in pearl platelets. Similar results were observed in mutant bone marrow, eye, liver, and macrophages (mu3A) and bone marrow and eye (sigma3A) (not shown). In control experiments, no alterations in platelet levels of the gamma and sigma 1 subunits of another adaptor complex, AP-1, were observed in pearl mice (not shown).
Concentrations of other subunits of the AP-3 complex in platelets (A) and brain (B) from the pearl mutant mouse. Samples (40 μg protein) were blotted and treated with antibodies to beta3A, beta3B, delta, mu3A, sigma3A, and control actin.
Concentrations of other subunits of the AP-3 complex in platelets (A) and brain (B) from the pearl mutant mouse. Samples (40 μg protein) were blotted and treated with antibodies to beta3A, beta3B, delta, mu3A, sigma3A, and control actin.
Although loss of one subunit of a protein complex can destabilize other proteins of the complex, no effects on transcript levels of other subunit components are expected. In fact, no alterations in levels of the mu3A transcript were observed in several pearl tissues (kidney, heart, lung, bone marrow, eye, and macrophages) by Northern blotting (not shown). Thus, as expected, the observed reductions in levels of the AP-3 subunits in pearl tissues are apparently restricted to subunit proteins rather than transcripts.
Two forms of the beta3 subunit of the AP-3 complex have been described. One is the beta3A subunit,19,20 and the second is betaNAP or beta3B.38 In humans the two subunits are 61% identical at the amino acid level.19 Beta3A is ubiquitously expressed while expression of beta3B is restricted to neural tissue. Brain is unique among all tissues in that both beta3A and beta3B subunits are expressed therein. Therefore, it was predicted that, unlike other tissues, the remaining subunits of the AP-3 tetramer would occur at normal or near-normal levels in pearl brain since these subunits would form active heterotetramers with the beta3B subunit, despite the absence of beta3A. Immunoblotting (Fig 2B) confirmed this prediction. Beta3A subunit levels were nondetectable; however, there were normal levels of the beta3B, mu3A, delta, and sigma3A subunits in pearl brain.
Subcellular distribution of the AP-3 complex is altered in pearl cells.
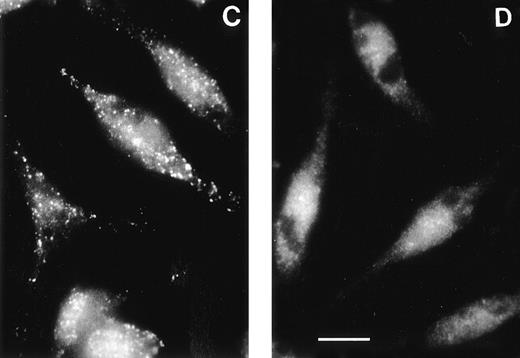
The subcellular distribution of the AP-3 complex was measured by indirect immunofluorescence, using a polyclonal antibody19to the AP-3 delta subunit (Fig 3). An intense punctate distribution was apparent in normal C57BL/6J platelets. In contrast, a weaker and more disseminated signal was apparent in platelets of pearl mice. Likewise, a punctate distribution of AP-3 in peripheral and perinuclear regions of macrophages and melan-a cells, a mouse melanocyte line, was observed, similar to its localization in other types of cells.19 20 The peripheral pattern is consistent with an endosomal distribution. However, in pearl primary macrophages and in a melanocyte-derived cell line from pearl mice, this punctate distribution was abolished and only a weak, diffuse cytoplasmic staining appeared. Thus, even though the delta subunit is expressed in pearl cells, it appears to be unable to be recruited onto membranes as seen in normal cells. Therefore, the AP-3 complex in these cells is both missing the beta3A subunit and is mislocalized, likely resulting in a nonfunctional complex.
The AP-3 delta subunit has a weak, diffuse localization pattern in pearl cells. Immunofluorescence was conducted on melan-a cells, a normal melanocyte cell line (A), on pearl melanocyte-derived cells (B), on macrophages of normal (C) and pearl (D) mice, and on platelets of normal (E) and pearl (F) mice. Scale bar = 10 μm.
The AP-3 delta subunit has a weak, diffuse localization pattern in pearl cells. Immunofluorescence was conducted on melan-a cells, a normal melanocyte cell line (A), on pearl melanocyte-derived cells (B), on macrophages of normal (C) and pearl (D) mice, and on platelets of normal (E) and pearl (F) mice. Scale bar = 10 μm.
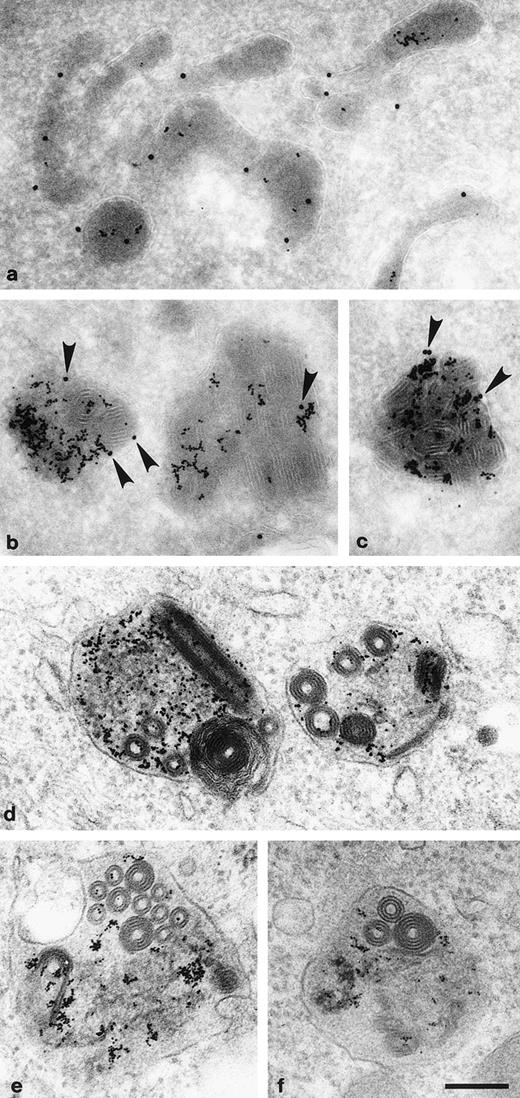
Genetic studies in yeast have shown that alkaline phosphatase, a membrane protein normally residing in the vacuole, is missorted in AP-3–deficient cells.22,23 To determine whether lysosomal membrane proteins are missorted in pearl cells, the cells were allowed to endocytose BSA coupled to 10 nm gold for 4 hours, which was chased for 20 hours to concentrate the probe in lysosomes. Frozen thin sections of such cells were then labeled with an antibody against the lysosomal membrane protein lgp11030 indirectly coupled to 15 nm gold. Figure 4a shows that the two labels are associated with the same organelles, indicating that the steady-state distribution of lgp110 in pearl cells is normal, although it is possible that it arrives at its destination via a different trafficking pathway.
Endocytosed BSA-gold accumulates in lgp110-positive electron-dense lysosomes. (a through c) Pearl cells were allowed to endocytose BSA-10 nm gold for 4 hours followed by a chase in conjugate-free medium for 20 hours. Gold accumulated in electron dense lysosomes which could be immunolabeled with antibodies to the lysosomal marker lgp110 (15 nm gold). Although most of the lysosomes were of normal appearance (a), some were filled with rolled-up membranous inclusions (b and c; arrowheads indicate the 15 nm gold-labeled lgp110). (d through f) Plastic sections of BSA-10 nm gold-containing lysosomes, showing the internal membranes. Scale bar = 200 nm.
Endocytosed BSA-gold accumulates in lgp110-positive electron-dense lysosomes. (a through c) Pearl cells were allowed to endocytose BSA-10 nm gold for 4 hours followed by a chase in conjugate-free medium for 20 hours. Gold accumulated in electron dense lysosomes which could be immunolabeled with antibodies to the lysosomal marker lgp110 (15 nm gold). Although most of the lysosomes were of normal appearance (a), some were filled with rolled-up membranous inclusions (b and c; arrowheads indicate the 15 nm gold-labeled lgp110). (d through f) Plastic sections of BSA-10 nm gold-containing lysosomes, showing the internal membranes. Scale bar = 200 nm.
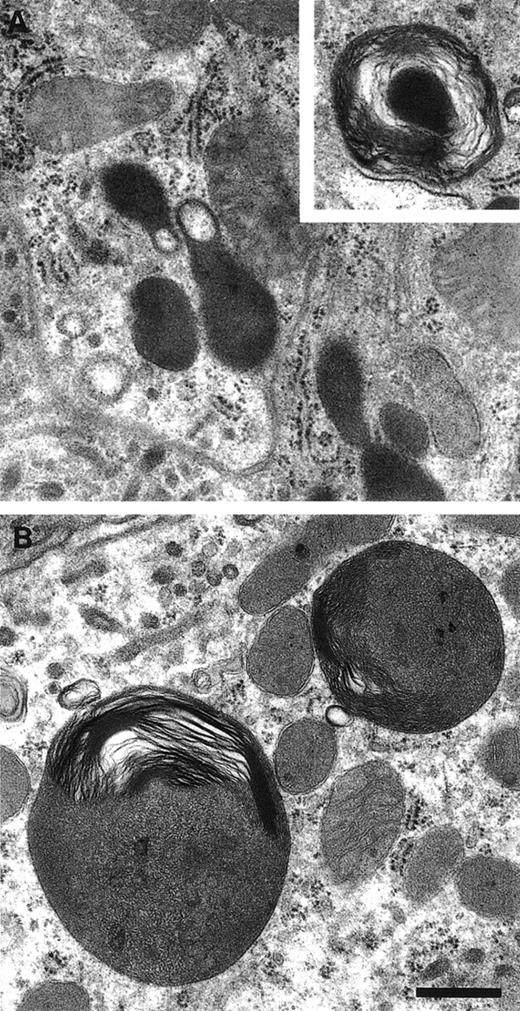
Intriguingly, some of the lysosomes in the pearl cells were found to have an unusual appearance, filled with what appear to be rolled-up membranous inclusions forming hollow cylinders (Fig 4b through f). We have observed similar structures in primary cultures of fibroblasts from mocha mice in which AP-3 function is likewise lost,39although at a lower frequency (data not shown). Thus, they may reflect abnormalities in lysosome function resulting from the AP-3 deficiency. Morphologically abnormal subcellular granules were likewise observed in kidney proximal tubule cells of pearl mice (Fig 5). Although similar structures were occasionally observed in kidney proximal cells of normal mice (Fig 5, inset) they were smaller and less numerous than those of pearl kidney. Depressed rates of secretion of kidney lysosomal enzymes have been observed in the pearl mutant.7,8 Likewise, kidney lysosomes with related morphological abnormalities similar to those of pearl (Fig5) have been observed in other mouse HPS mutants related to pearl.8 40 Thus, the abnormal granules in pearl kidney likely represent engorged lysosomes that accumulate in pearl kidney to a greater extent than in normal kidney as a result of depressed secretory rates.
Abnormal lysosomal morphology in kidney proximal tubules of the pearl mutant. Kidneys from normal (A) and pearl (B) mice were examined by standard TEM. Inset shows a rare small multilamellar lysosome in normal tissue. Scale bar = 500 nm.
Abnormal lysosomal morphology in kidney proximal tubules of the pearl mutant. Kidneys from normal (A) and pearl (B) mice were examined by standard TEM. Inset shows a rare small multilamellar lysosome in normal tissue. Scale bar = 500 nm.
Morphologically abnormal dense granules, typical of those observed in SPD, have been described in platelets of pearl mice. Whole-mount electron microscopy of air-dried unfixed platelets6combined with studies of mepacrine-labeled platelet dense granules,41 indicated that dense granules of pearl platelets are present but are essentially “empty” with very low contents of dense granule components such as serotonin and adenosine diphosphate (ADP). However, no additional subcellular or other morphological abnormalities of pearl platelets were apparent upon standard transmission electron microscopic analyses (not shown).
Other mouse SPD/HPS models have normal concentrations of beta3A.
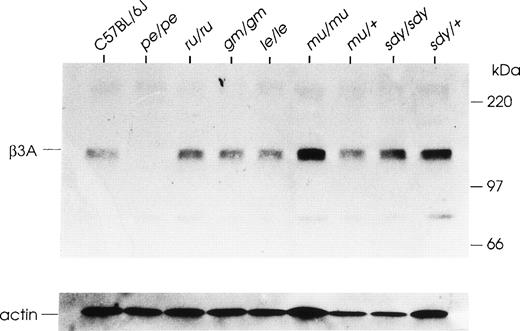
A significant number of mouse hypopigmentation mutants have organellar phenotypes similar to that of the pearl mutant and are likewise models for platelet SPD.8 Platelets, which are probably the most intensively characterized cell in all these mutants, typically have abnormalities in contents and/or secretion of dense granules and lysosomes. Acccordingly, platelet extracts of five of these mutants [ruby eye (ru/ru), gunmetal (gm/gm), light ear (le/le), muted (mu/mu), and sandy (sdy/sdy)] were analyzed by immunoblotting, using an antibody specific for the beta3A subunit (Fig 6). It is apparent that all of these mutants have levels of beta3A approximately equivalent to that of their respective control strains. In other experiments (not shown), concentrations of the delta, mu3A, and sigma3A AP-3 subunits were likewise determined to be normal in platelet extracts of the same mutants. Therefore, despite phenotypic similarities among these mouse HPS-like mutants, altered expression of proteins of the AP-3 complex appears to be specific to pearl. This result corroborates a large body of evidence that these mutants are genetically unique.8 In contrast, another mouse HPS-like mutant, mocha, has recently been found to contain mutations in the delta subunit of the AP-3 complex together with decreased expression of other AP-3 complex proteins.39
The beta3A subunit is present at normal levels in other mouse HPS mutants. Platelet extracts (40 μg protein) were Western blotted and probed with antibody to beta3A. The C57BL/6J strain is the control strain for congenic mutants pearl (pe/pe), ruby eye (ru/ru), gunmetal (gm/gm), and light ear (le/le). The muted (mu/mu) and sandy (sdy/sdy) mutants are maintained on other inbred backgrounds and therefore the appropriate genetic control strains are mu/+ andsdy/+, respectively. Blots were reprobed with an antibody to mouse actin (below) as a loading control.
The beta3A subunit is present at normal levels in other mouse HPS mutants. Platelet extracts (40 μg protein) were Western blotted and probed with antibody to beta3A. The C57BL/6J strain is the control strain for congenic mutants pearl (pe/pe), ruby eye (ru/ru), gunmetal (gm/gm), and light ear (le/le). The muted (mu/mu) and sandy (sdy/sdy) mutants are maintained on other inbred backgrounds and therefore the appropriate genetic control strains are mu/+ andsdy/+, respectively. Blots were reprobed with an antibody to mouse actin (below) as a loading control.
DISCUSSION
These experiments indicate that alterations in the AP-3 complex cause platelet SPD. The primary mutation in pearl, a mouse model for platelet SPD,6,8 occurs in the gene for the beta3A subunit of the AP-3 complex.25 The present studies show that expression of the beta3A subunit protein is completely or nearly completely eliminated in several tissues, including platelets, of pearl mice. Loss of beta3A occurs at both the transcript25 and protein levels. Further, by immunofluorescent microscopy a remaining subunit, delta, of the AP-3 complex is mislocalized subcellularly in several pearl tissues including platelets. Such a substantial loss of beta3A, combined with altered AP-3 subcellular distribution, would be expected to abrogate normal AP-3 function in pearl mice and lead to platelet SPD.
The absence of beta3A in all tissues of pearl mice analyzed by Western blotting suggests that the loss is systemic. This is consistent with other evidence for multitissue involvement in both mouse8and human HPS/SPD.12
The likelihood that AP-3 function is lost in pearl tissues is supported by immunoblotting evidence for a nearly complete loss of the mu3A subunit. The beta and mu subunits of the AP-1 and AP-2 complexes have been shown to interact using the yeast two-hybrid system,42and the loss of the mu3 subunit in the pearl tissues indicates that without such an interaction, the mu subunit is unstable and gets degraded. The gamma and sigma1 subunits of the AP-1 complex and the alpha and sigma2 subunits of the AP-2 complex were also found to interact in the two-hybrid system,42 and the delta and sigma3 subunits of the AP-3 complex appear to interact as well because they can be coimmunoprecipitated from pearl cell cytosol (A.A.P. and M.S.R., unpublished observations). However, immunofluorescence microscopy (Fig 3) shows that the delta subunit in pearl is mislocalized and fails to associate with membranes, indicating that the partial complexes formed from the delta and sigma subunits are not functional. An exceptional tissue is brain where normal levels of other AP-3 subunits occur in pearl mice. The probable explanation is that the brain specific beta3B subunit effectively substitutes for the loss of the beta3A subunit (Fig 2), thus stabilizing the complex in mutant mice.
The phenotypes of the pearl mutant suggest novel roles for the AP-3 complex in SPD and in regulation of granule structure, biogenesis, and function of organelles in mammalian tissues. Pearl mice have abnormalities in structure and/or function of at least three related organelles: lysosomes, melanosomes, and platelet dense granules. Lysosomes from several tissues of pearl mice are significantly altered in function. Constitutive secretion of lysosomes from kidney proximal tubule cells of pearl mice is reduced to one third the normal rate7 and thrombin-mediated secretion of lysosomal enzymes from platelets occurs at half the normal rate.6 The abnormal lysosomal structures detected in melanocyte-derived cells and kidney of pearl mice in these experiments suggest a morphological basis for the lysosomal secretion defect. Structurally related organelles containing unusual membranous multilamellar-like material have been observed in melanocytes derived from patients with HPS.43Also, related abnormal structures have been detected in kidney of several other mouse HPS/SPD mutants,40 suggesting these structures may be a general feature of genes causing HPS/SPD. Further, these structures may be relevant to the kidney failure noted in a subgroup of HPS patients.11 Increased rates of synthesis of lysosomal enzymes have been observed in kidney of the pearl mutant.7 Retinal pigment epithelium of pearl mice contains fewer melanosomes that are morphologically larger and contain irregular boundaries with clumps of melanin.5 Pearl melanosomes are mislocalized, being absent within the apical processes of retinal pigment epithelial cells. Also, the basal membrane of this tissue lacks infoldings.5 The dense granules of pearl platelets are grossly abnormal in content6 (see below). Together, these phenotypes indicate that the AP-3 complex regulates all three organelles. Such a role for AP-3 is consistent with converging genetic and cell biological evidence of similarities in biosynthesis, composition, and regulation of these organelles.8 44
The importance of the AP-3 complex in the biosynthesis of melanosomes is supported by the fact that mutations in the delta subunit ofDrosophila AP-3 produce the garnet eye pigment phenotype.19-24Garnet flies, like pearl mice,5 have reduced eye pigmentation leading to aberrant eye pigment color.
The detailed mechanism(s) by which reductions in AP-3 cause the granule abnormalities characteristic of the pearl phenotype are undefined. However, recent studies in both yeast and mammals suggest possible scenarios. Selective deletions of yeast AP-3 subunits abolish transport of alkaline phosphatase and the vacuolar t-SNARE, Vam3p, to the yeast lysosome or vacuole.22,23 In mammals, the AP-3 complex similarly regulates targetting to the lysosomal membrane of the lysosomal integral membrane proteins LAMPI and LIMPII45 and is necessary for in vitro formation of synaptic vesicles from endosomes.46 Alterations in these vesicle budding and cargo selection functions of AP-3 may cause the abnormalities of lysosomes, melanosomes, and platelet dense granules of pearl mice. Further, the AP-3 complex is located in the trans-Golgi network and in more peripheral endosomal regions of the cell,19,20 sites within the generally agreed subcellular pathway for biogenesis/trafficking of lysosomal proteins. These locations are also consistent with observed defects in constitutive and stimulated secretion of lysosomal contents from kidney7 and platelets6 of pearl mice, because lysosomal enzyme secretion may involve retrograde transport from lysosomes through the prelysosomal/late endosomal compartment.35,47 Detailed molecular studies indicate that the AP-3 complex recognizes both tyrosine20,48 and dileucine22,49 50 signals on lysosomal proteins during vesicle budding and cargo selection.
Normal platelet activation releases at the site of a wound massive quantities of serotonin and ADP, stored in dense granules, a process critical for normal clotting. The near absence of these components in dense granules leads to platelet SPD and prolonged bleeding times.8,9 Therefore, it is important to understand the relationship of AP-3 abnormalities and the diagnostic “empty” platelet dense granules of SPD observed in pearl mice.6 It may be reasonably speculated that this relationship is similar to that observed between AP-3 deficiencies and lysosomal mistargetting. That is, critical receptors/transporters of serotonin and adenine nucleotides may not be properly captured by a defective AP-3 complex during biosynthesis of the platelet dense granule membrane. This mutant platelet phenotype may mimic the observed mistargetting of Vamp-3 and alkaline phosphatase to the vacuole in yeast AP-3 mutants.22,23 It is likewise consistent with the finding45 that proper targetting of the lysosomal membrane proteins to lysosomes requires the AP-3 complex. Receptor-deficient dense granules would ultimately lead to the “empty” dense granules of SPD. It is now possible to directly test whether mistargetting of important granule membrane proteins occurs in pearl platelets and other cells.
The importance of the AP-3 complex in SPD and in the biosynthesis/function of lysosomes, melanosomes, and platelet dense granules is further documented by results of recent studies on the mouse mocha hypopigmentation mutant. Mocha mice, arising from a mutation on mouse chromosome 10, have abnormalities in and greatly reduced expression of the delta subunit of the AP-3 complex and accompanying reduced expression of other AP-3 subunits.39Like pearl mice, mocha mice present with platelet SPD, and the effects of the mocha mutant on intracellular organelles are similar to those of pearl.8 In addition, mocha mice display an interesting hyperactive phenotype.39,51 It is likely that the hyperactive phenotype does not occur in pearl because the presence of the beta3B subunit retains normal levels of the AP-3 tetramer (Fig 2) in pearl brain. In contrast, no alternative delta subunit is expressed in brain. Therefore, AP-3 subunits and function are absent in mocha brain.39 The availability of both pearl and mocha mutants, defective in expression of the beta3A or delta subunits, respectively, of AP-3 should allow definitive tests of the role of AP-3 in SPD, subcellular trafficking, and related processes in mammals.
Recently the gene responsible for the major form of human HPS common in Puerto Rico has been cloned.52,53 This gene is ubiquitously expressed and encodes a novel protein predicted to be membrane-associated and 700 amino acids in size. The pale-ear mouse is the appropriate mouse homologue for this form of human HPS.53,54 The pale-ear, pearl, and mocha HPS/SPD genes have related phenotypic effects on subcellular organelles8 as does the recently cloned beige gene,55 and their protein products may interact with each other or with the same targetting signals. Therefore, it is possible that all four genes may regulate the same or closely related pathways of organelle biogenesis/function.
The phenotypic heterogeneity of HPS in humans suggested that the disease is caused by multiple primary gene defects. Genetic heterogeneity is supported by the fact that a large number (14) of separate mouse hypopigmentation genes cause HPS-like phenotypes8 and, more directly, by reports of HPS patients who have no discernible mutations in the recently identified human HPS gene.56,57 In fact, two brothers with HPS and with molecular alterations in the beta3A subunit have recently been identified.58 Therefore, the pearl mouse is an appropriate animal model for these patients. Additional and corresponding mouse HPS mutants will be required to model other HPS patients accurately.
HPS is often a very debilitating disease due to SPD with associated prolonged bleeding, colitis, and fibrotic lung disease, which leads to increased mortality13 in the third to fifth decades of life. Because no curative therapies exist, improved diagnostic and therapeutic approaches would be welcome. The beta3A gene is now an obvious target for these studies.
ACKNOWLEDGMENT
Dr Dorothy Bennett kindly provided the melan-a cell line. We thank Joanne Pazik for aid in culturing of melanocyte lines and Michael E. Rusiniak and Edward P. O’Brien for helpful suggestions. We are grateful to Dr Lawrence Pinto (Northwestern University, Evanston, IL) and Dr Stephen Hardies (University of Texas, San Antonio) for their help in earlier characterizations of the pearl mutant.
L.Z., S.J., and L.F. contributed equally to this study.
Supported by National Institutes of Health Grants No. HL31698, HL51480, and EY12104 (R.T.S.), by grants from the Medical Research Council and the Wellcome Trust (M.S.R.), Grants No. EY09192 and EY0898 and grants from The Eye and Ear Foundation of Pittsburgh (M.B.G.), Grant No. HD28623 (R.E.), and by shared resources of the Roswell Park Cancer Center Support Grant (P30 CA16056).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Richard T. Swank, PhD, Roswell Park Cancer Institute, Elm and Carlton St, Buffalo, NY; e-mail:rswank@mcbio.med.buffalo.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal