During human blood clotting, 2-antiplasmin (2AP) becomes covalently linked to fibrin when activated blood clotting factor XIII (FXIIIa) catalyzes the formation of an isopeptide bond between glutamine at position two in 2AP and a specific ɛ-lysyl group in each of the -chains of fibrin. This causes fibrin to become resistant to plasmin-mediated lysis. We found that chemically Arg-modified 2AP, which lacked plasmin-inhibitory activity, competed effectively with native 2AP for becoming cross-linked to fibrin and as a consequence, enhanced fibrinolysis. Recombinant 2AP reported to date by other groups either lacked or possessed a low level of FXIIIa substrate activity. As a first step in the development of an engineered protein that might have potential as a localized fibrin-specific fibrinolytic enhancer, we expressed recombinant 2AP in Pichia pastoris yeast. Two forms of nonglycosylated recombinant 2AP were expressed, isolated and characterized: (1) wild-type, which was analogous to native 2AP, and (2) a mutant form, which had Ala substituted for the reactive-site Arg364. Both the wild-type and mutant forms of 2AP functioned as FXIIIa substrates with affinities and kinetic efficiencies comparable to those of native 2AP, despite each having an additional acetylated Met blocking group at their respective amino-termini. Wild-type recombinant 2AP displayed full plasmin inhibitory activity, while mutant 2AP had none. Neither the absence of glycosylation nor blockage of the amino-terminus affected plasmin-inhibitory or FXIIIa substrate activities of wild-type 2AP. When our mutant 2AP, which lacked plasmin-inhibitory function, was added to human plasma or whole blood clots, urokinase (UK)-induced clot lysis was enhanced in a dose-dependent manner, indicating that mutant 2AP augmented lysis by competing with native 2AP for FXIIIa-catalyzed incorporation into fibrin.
HUMAN α2-ANTIPLASMIN (α2AP) is the primary inhibitor of plasmin-mediated fibrinolysis.1 In its major circulating form, it is a single-chain glycoprotein of 452 amino acid residues with potential glycosylation sites, Asn87, Asn256, Asn270, Asn277, and a total carbohydrate content of 13%.2 α2AP is cross-linked to fibrin during blood coagulation by activated factor XIII (FXIIIa) via Gln2 in the α2AP molecule,3and as a consequence, fibrin becomes more resistant to fibrinolysis.4 5
α2AP belongs to the serine proteinase inhibitor family (serpins). The inhibitory function of a serpin depends on an exposed reactive-site peptide bond (P1-P1’) that functions as an ideal substrate for the cognate proteinase.6 The reactive-site P1-P1’ in α2AP for plasmin is Arg364-Met365.7 Plasmin inhibition occurs by rapid complex formation with α2AP via covalent bonding between the reactive-site Arg364 of α2AP and the active-site Ser of plasmin.7 Arg-modified α2AP, prepared in our laboratory by treatment of native α2AP with an Arg-specific chemical reagent (phenylglyoxal), lost all plasmin inhibitory activity, but retained full FXIIIa substrate activity.8 Because modified, nonactive α2AP competitively inhibited the cross-linking of native α2AP to fibrin, fibrinolysis occurred at markedly lower urokinase (UK) concentrations and with less accompanying fibrinogenolysis than when only native α2AP was present.8 These findings prompted our efforts to develop a recombinant α2AP that might also competitively inhibit FXIIIa-mediated native α2AP-fibrin cross-linking and, as a result, enhance fibrinolysis.
Two previous reports documented the expression of recombinant human α2AP, but in one report,9 the recombinant α2AP lacked the ability to become cross-linked into fibrin, and in the other work,10 the protein possessed only weak cross-linking activity. Using Chinese hamster ovary cells, Holmes et al9 expressed recombinant α2AP, which inadvertently had three additional amino-terminal amino acids that corresponded to the three carboxyl-terminal amino acids of the added signal peptide sequence used to cause secretion of α2AP. This recombinant α2AP totally lacked FXIIIa substrate activity for becoming cross-linked into fibrin, which is essential for α2AP to function as a localized inhibitor of plasmin. Sumi et al10 constructed an expression vector for recombinant α2AP, which included a cDNA fragment that coded for its carboxyl-terminal region and a gene fragment that coded for its signal peptide and N-terminal region. Baby hamster kidney cells transfected by this vector, expressed recombinant α2AP, which had 12 additional amino acids at the N-terminus and possessed only one third of the cross-linking activity of native α2AP.
We now report the expression, purification, and characterization of both a wild-type and a mutant form of α2AP. Recombinant human α2AP was expressed in Pichia pastorisyeast, using the alcohol oxidase 1 promoter.11Recombinant wild-type α2AP displayed both FXIIIa substrate and plasmin inhibitory activities comparable to those of native α2AP. To evaluate the effect of a mutation of the reactive-site Arg364 on plasmin inhibitory activity and on FXIIIa substrate activity, Arg364 was replaced with Ala. This mutant did not inhibit plasmin, but it did serve as an efficient FXIIIa substrate. When incorporated into human plasma fibrin clots or whole blood clots, the Arg364 → Ala α2AP mutant enhanced UK-induced fibrinolysis.
MATERIALS AND METHODS
Materials.
Human α2AP cDNA in the ZEM228R vector was a gift from Dr Donald Foster (ZymoGenetics, Seattle, WA). Native human α2AP was either purified from citrated plasma as previously described12 or purchased from Calbiochem (La Jolla, CA). Human FXIII was prepared from human plasma using a published procedure.13 Purified protein concentrations were determined using an extinction coefficient at 280 nm of 0.67014 or 1.3815 for 1 mg/mL solutions of α2AP or FXIII, respectively. Human thrombin was supplied by Dr D.L. Aronson (Division of Biological Standards, National Institutes of Health [NIH], Bethesda, MD). [14C]Methylamine (51.8 mCi/mmol) and [125I]human fibrinogen (5.77 mCi/mg) were purchased from New England Nuclear (Boston, MA), and ICN (Costa Mesa, CA), respectively. Other reagents were obtained from the following suppliers: pPIC3K yeast expression vector, Invitrogen (San Diego, CA); plasmin and the chromogenic plasmin substrate, S-2251(VLK-pNa), Chromogenix (Mölndal, Sweden); UK (Abbokinase), Abbott (Chicago, IL); 1-tosylamide-2-phenylethylchloromethyl ketone (TPCK)-treated trypsin, Sigma (St Louis, MO); and cyanogen bromide, Aldrich (Milwaukee, WI).
Construction of human α2AP expression vector and transformation of yeast.
Human α2AP cDNA with BamHI and NotI sites at the 5′ and 3′ ends, respectively, was amplified by the ligation-independent polymerase chain reaction (PCR) system16 using two oligonucleotide primers (5′-CUACUACUACUAGGATCCATGAACCAGGAGCAGGTGTCCCCAC for theBamHI site and 5′-CAUCAUCAUCAUGCGGCCGCTCACTTGGGGCTGCCAAAC for the NotI site) and a template α2AP cDNA provided by Dr Donald Foster. The amplified DNA was subcloned into the plasmid, pAMP2, using uracil DNA glycosylase.16The α2AP cDNA was cut out of pAMP2 with BamHI andNotI and cloned into the pPIC3K yeast expression vector that contained the bacterial kanamycin gene conferring G418-resistance. The resulting clone was termed human wild-type α2AP expression vector pPIC3K/AP.
The Arg364 → Ala mutant expression vector pPIC3K/APR364A was prepared using the PCR mutagenesis system16 and the synthetic primers (5′-AUGUCACUAUCCUCCTTCAGCGTGAAC and 5′-GGAUAGUGACAUGGCGGACATGGCAATG). The construct was authenticated by double-strand DNA sequencing using a Perkin-Elmer Model ABI-373A Sequencer and a fluorescent dye-labelled dideoxy terminator kit (Perkin-ElmerCorp, Foster City, CA). The wild-type and mutant vectors were digested with BglII and transformed into Pichia pastoris GS115 and proteinase-deficient SMD1168 host cells, respectively, by standard spheroplast procedures (Invitrogen instruction manual). His+ transformants were selected by plating on a defined minimal media (regeneration dextrose biotin [RDB]) lacking histidine, and then multicopy integrants were selected on yeast peptone dextrose (YPD) agar plates containing various concentrations of G418 (0 to 3 mg/mL).17
The G418 resistants were screened by determining the amount of recombinant α2AP in cell extracts from small cultures as follows. Ten-milliliter cultures were grown in BMGY medium (yeast extract, 10 g; peptone, 20 g; glycerol, 10 mL; biotin, 0.4 mg; yeast nitrogen base with ammonium sulfate, 13.4 g; and 100 mL of 1 mol/L potassium phosphate buffer, pH 6.0, per liter) at 29°C for 2 days and cells were harvested by centrifugation at 2,000g. Cell pellets were resuspended with 2 mL BMMY medium (same as BMGY with the exception that it contained 5 mL of methanol/L in place of glycerol), and then induction was continued for 2 days, with fresh methanol (5 mL/L) added every 24 hours. The cells were collected and resuspended in 0.2 mL of 10 mmol/L Tris·HCl, pH 7.5, and kept at 4°C. Acid-washed glass beads (diameter [d], 425 to 600 μm) were added and each suspension was vortexed for 30 seconds and returned to the ice bath for 30 seconds, with this sequence repeated seven times. The lysed cells were centrifuged in a microfuge for 10 minutes, and the clear supernatant was collected. To selectPichia strains, which produced the expected size of α2AP and the highest yield, cell extracts were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting (for detail, see “Polyacrylamide gel electrophoresis and immunoblotting”).
Expression and purification of recombinant α2AP.
The selected GS115 cells expressing wild-type α2AP or SMD1168 cells expressing mutant α2AP, were grown in 500 mL of BMGY medium in a 2-L baffled flask at 29°C to A600 of approximately 2.5. The cells were harvested and resuspended in 100 mL of BMMY medium and incubated at 29°C for 2 days. Cells were collected by centrifugation, washed once with cold 10 mmol/L Tris·HCl, pH 7.5, and resuspended in 10 mmol/L Tris·HCl, pH 7.5. Cells were lysed using a BeadBeater (Biospec, Bartlesville, OK), centrifuged at 3,000 rpm for 10 minutes in a HG-4 rotor (Sorvall, Norwalk, CT), and the supernatant stored at −80°C. After thawing, cell extracts were dialyzed against 10 mmol/L Tris·HCl, pH 7.5, and centrifuged to obtain a clear supernatant, which was applied at 1 mL/min to a 2.5 cm × 20 cm Q Sepharose Fast Flow column (Pharmacia, Piscataway, NJ) equilibrated with the same buffer. Subsequent to washing of nonbound components, a 200-mL salt gradient was developed (0 to 0.2 mol/L NaCl) at 2 mL/min while collecting 3-mL fractions. Fractions containing α2AP activity were pooled and applied to an affinity column (1.0 × 2.0 cm), which contained 15 mg of mouse monoclonal anti-human α2AP antibody (American Diagnostica, Inc, Greenwich, CT) conjugated to 1.5 mL of Affigel-10 (Bio-Rad, Richmond, CA). After washing with 10 mmol/L Tris·HCl, 0.5 mol/L NaCl and 0.05% Tween 20, pH 7.5, recombinant α2AP was eluted with 0.2 mol/L glycine (pH 2.5). Fractions were immediately neutralized by collection into tubes, which contained 1.0 mol/L Tris·HCl, pH 9.0. The neutralized samples were buffer-exchanged into 20 mmol/L Tris·HCl and 0.15 mol/L NaCl, pH 7.5, using a Centricon-30 (Amicon, Danvers, MA). Samples were stored at −80°C. Mutant α2AP displayed chromatographic behavior indistinguishable from that of wild-type.
Polyacrylamide gel electrophoresis and immunoblotting.
Purified wild-type and mutant α2AP proteins were analyzed by SDS-PAGE and immunoblotting. For SDS-PAGE, samples were brought to a final concentration of 0.5% (wt/vol) SDS, 1 mmol/L 2-mercaptoethanol, and then heated at 100°C for 2 minutes. Electrophoresis was performed on a Novex precast 10% to 20% acrylamide gradient gel using a Novex Xcell II apparatus (San Diego, CA). Gels were stained for protein with Coomassie blue (FastStain system, Zoion Research, Newton, MA). For Western immunoblotting, proteins from unstained gels were transferred to a nitrocellulose membrane using the Novex blot module, and then the membrane was treated with either an anti-α2AP polyclonal (Biodesign, Kennebunkport, ME) or monoclonal (American Diagnostica, Catalog No. 3612 or 3613) antibody18 and the horseradish peroxidase detection kit (Bio-Rad).
Trypsin digestion, mass spectrometry, cyanogen bromide cleavage, and peptide sequencing.
Recombinant α2AP was digested with trypsin, as Edman degradation of the intact protein yielded no sequence. A total of 1 μg of TPCK-treated trypsin was added to 51 μg (1 nmol/L) of mutant α2AP in 100 μL of 0.1 mol/L ammonium bicarbonate buffer (pH 8.2). Digestion was allowed to proceed at 37°C for 15 hours and then stopped by the addition of 2 μL of glacial acetic acid.
The tryptic digest mixture was purified by reverse-phase chromatography. A model UMA-600 high performance liquid chromatography (HPLC) system (Michrom BioResources, Inc, Auburn, CA) was equipped with a 1.0 mm × 150 mm Reliasil C18 column operated at a flow rate of 40 μL/min. Solvent A consisted of 0.1% trifluoroacetic acid in 2:98 (vol/vol) acetonitrile/water, while solvent B contained 0.085% trifluoroacetic acid in 95:5 (vol/vol) acetonitrile/water. For peptide isolation, 90% of the digest was injected onto the HPLC column equilibrated with 2% solvent B. Then the solvent composition was immediately changed to 20% solvent B, and a linear gradient from 20% to 60% solvent B was applied for 60 minutes. Peptide peaks were detected by absorbance at 215 nm wavelength. Each peak was manually collected. The remaining 10% of the tryptic digest was analyzed by HPLC-mass spectrometry. All HPLC conditions were equivalent to those used for peptide purification, except the column effluent was connected to an electrospray mass spectrometer (Sciex model API III; PE Biosystems, Foster City, CA). Peptide ions were detected in the mass range of 400 to 2,200 atomic mass units (amu) in the positive ion mode using an orifice voltage of 65 V. No peptides were observed that corresponded to the mass predicted for the unmodified amino-terminal tryptic peptide. However, one peptide mass was found that did not match any predicted tryptic peptide; its molecular weight of 1541.8 was consistent with the monoisotopic mass of the amino-terminal peptide with the addition of an acetylated Met residue.
The peptide of molecular weight 1541.8 was dried and then treated with 100 μL of 0.5 mol/L cyanogen bromide in 70% vol/vol formic acid/water. The reaction was continued for 15 hours at room temperature in the dark under nitrogen and then terminated by evaporation of the liquid with a gentle stream of nitrogen. The cyanogen bromide-treated peptide was dissolved in 20 μL of neat trifluoroacetic acid and applied to a polybrene-treated glass fiber filter for microsequencing. The protein sequencer, a Procise model 392 (PE Biosystems), was operated using the manufacturer’s standard procedure. Twelve cycles of Edman degradation were performed on the peptide.
Evaluation of recombinant α2AP as a plasmin inhibitor and FXIIIa substrate.
Plasmin inhibitory activity of recombinant α2AP was analyzed by modification of a published method.10 In brief, assays were performed in 96-well plates at 22°C using S-2251 as the plasmin chromogenic substrate. α2AP (14.3 nmol/L) was mixed with plasmin (14.3 nmol/L) in 40 mmol/L Tris·HCl and 150 mmol/L NaCl, pH 7.5. In the control, α2AP was omitted. After preincubation for 0, 0.5, 1, 3, 5, or 15 minutes, 0.4 mmol/L S-2251 and 3.3 mmol/L ε-aminocaproic acid10 were added. Then after a 15-minute incubation, hydrolysis of S-2251 by residual plasmin was measured at A405 using a Vmax Kinetic Spectrophotometer (Molecular Devices, Menlo Park, CA).
Kinetic efficiencies of FXIIIa for native and recombinant α2AP proteins were determined using a modification of a published method.19 Assays were performed at 37°C in 40 mmol/L Tris·HCl and 150 mmol/L NaCl, pH 7.5, containing selected amounts of native or recombinant α2AP, 2 mmol/L [14C]methylamine, 31 nmol/L FXIII, 0.1 U/mL thrombin, and 5 mmol/L CaCl2. The reaction mixture was coprecipitated after addition of 1/4 vol of 10 mg/mL bovine serum albumin followed by 10 vol of 7.5% trichloroacetic acid (TCA). The precipitate was collected on filter discs and washed exhaustively with 5% TCA, and then the incorporation of [14C]methylamine was determined by measuring protein-bound radioactivity. All kinetic studies were performed under conditions where no more than 10% of the [14C]methylamine substrate was consumed during the reaction period. Kinetic data were analyzed using the computer program Hyperbolic Regression Analysis of Enzyme Kinetic Data, Version 1.02a (Dr J.S. Easterby, Department of Biochemistry, The University, Liverpool, UK).
Measurement of plasma and whole-blood clot lysis.
Blood was drawn from five normal donors into plastic tubes containing a 10% vol of 3.8% sodium citrate and then pooled. Platelet-poor plasma was prepared by centrifugation at 2,500g for 10 minutes. After preincubation of mutant α2AP with plasma, instant fibrin clot formation and initiation of fibrinolysis was achieved by adding a mixture of thrombin, CaCl2, and UK to give final concentrations of 1 U/mL, 20 mmol/L, and 200 U/mL, respectively. The rate of plasma clot lysis was determined by a turbidimetric microtiter plate method.20 21 Turbidity change was monitored as absorbance at 405 nm on a Vmax microtiter plate-reader (Molecular Devices, Menlo Park, CA) operating under SOFTmax version 2.31 (Molecular Devices) software control.
For measurement of blood clot lysis, pooled citrated whole blood was supplemented with 5 μL (2.1 μCi) of [I125]fibrinogen/mL, transferred to microfuge tubes in 200-μL aliquots, mixed with selected concentrations of UK and mutant α2AP, clotted with 10 μL of a mixture of 40 U/mL thrombin to 0.4 mol/L CaCl2, and then incubated at 37°C and observed for clot lysis with time over a 4-hour period. At each selected time point, tubes were centrifuged in a microfuge at 16,000g for 3 minutes and the radioactivity in 10 μL of supernatant was quantitated using a Beckman Gamma 4000 Counter (Beckman, Palo Alto, CA). The percentage of whole blood clot lysis was expressed as (Supernatant Radioactivity − Blank)/(Total Radioactivity − Blank) × 100, where the blank value equaled the radioactivity in the supernatant from clots to which no UK had been added.
Radioiodination of α2AP and measurement of incorporation into fibrin.
Native α2AP and recombinant α2AP proteins were radioiodinated using Lactoperoxidase 125I Labeling Kit (ICN) to specific activities of 1.3 × 106 cpm/μg and 1.7 × 106 cpm/μg, respectively. Incorporation of native α2AP or mutant α2AP into fibrin was measured by a published method.8 Selected concentration of mutant α2AP were each mixed with a trace amount (1.0 × 105 cpm) of 125I-native α2AP or 125I-modα2AP, and added to platelet-poor plasma. Each mixture was clotted by adding a thrombin-CaCl2 mixture. For a control, EDTA and iodoacetamide were added to inhibit FXIIIa-mediated cross-linking. After a 30-minute incubation, each clot was washed and counted for radioactivity. The amount of native α2AP or mutant α2AP cross-linked into fibrin was calculated using the radioactivity of the washed clot and the specific activity of native α2AP or mutant α2AP in the clotting mixture.
RESULTS AND DISCUSSION
Expression of recombinant α2AP.
The methylotropic yeast, Pichia pastoris, is reported to be an excellent host for the production of recombinant proteins.11 In comparison to mammalian cells, Pichia pastoris cultures are reported to provide: (1) fast and inexpensive protein production, (2) high yields of intracellular proteins, (3) high levels of protein secretion into an almost protein-free medium, and (4) ease of fermentation to high cell density. Initially we attempted to express α2AP using a yeast secretory expression vector (pPIC9) to ensure that posttranslational modification of recombinant α2AP occurred, as native human α2AP is glycosylated and contains disulfide bonds.2,22 Recombinant α2AP from culture media of yeast transformed with the pPIC9 expression vector showed a broad band between 51 and 65 kD on SDS-PAGE and immunoblot analysis (data not shown). The amino-terminal sequence analysis of this purified recombinant α2AP showed that at least two different forms of α2AP existed in the sample. One was the expected mature α2AP having Asn at the amino-terminal end, while the other was proteolyzed at the Lys12-Leu13 bond with loss of 12 amino acid residues from its amino-terminus. Besides proteolysis, another probable reason why recombinant α2AP showed such a broad band is that the protein may have been posttranslationally modified by heterogeneous glycosylation because it has four potential N-linked glycosylation sites.2
To circumvent these impediments, an intracellular expression system17 was used to produce nonglycosylated α2AP. A pPIC3K vector with the alcohol oxidase 1 promoter was selected as the expression vector. This vector contained theTn903kanr gene, which confers G418-resistance, and when integrated in a high copy number, marked G418-resistance and a high-level of expression has been reported in most cases.17After initial selection of the expression vector-transformed cells for His+, the transformants were plated on YPD agar containing G418. Forty-eight colonies that grew on 2 mg/mL G418 were identified for further assessment of protein expression level. Initially, the cell extract from a 10-mL culture of each colony was examined by SDS-PAGE to determine the molecular size and production level of recombinant α2AP, but because of relatively low levels of expression and the presence of yeast cellular proteins, it was difficult to detect recombinant α2AP protein on Coomassie blue–stained SDS-PAGE gels. However, immunoblot analysis of the cell extracts (each 4 μg protein) showed a single 51-kD protein band, which corresponded to the size of the protein predicted from the cDNA inserted into the expression vector; also, each transformant had a different band-intensity (data not shown). Plasmin inhibitory activity was measured in the extracts (8 μg protein) from each yeast culture transformed with either wild-type or mutant α2AP expression vector. Plasmin inhibitory activity in wild-type extracts correlated directly with Western blot band intensities; however, mutant extracts and cell extracts from control yeast without the expression vector, were indistinguishable in their lack of inhibitory activity. These results showed that both recombinant wild-type and mutant α2AP had the same molecular size and that wild-type α2AP functioned as a plasmin inhibitor, whereas the mutant protein did not. Wild-type and mutant α2AP transformants that showed the highest band-intensity on immunoblot analysis were selected for large-scale culture.
Structural characterization of recombinant α2AP.
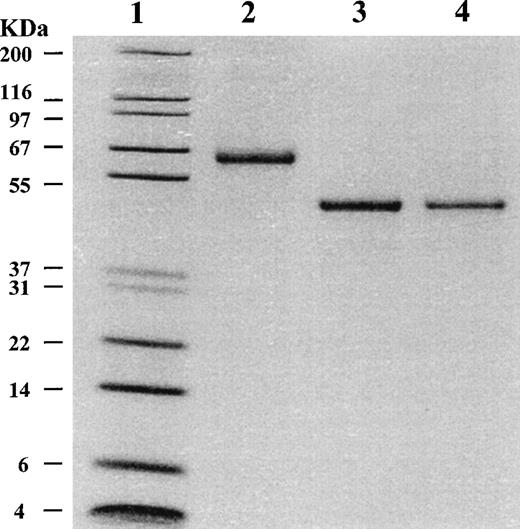
Both purified wild-type and mutant α2AP proteins were essentially equal in size by SDS-PAGE analysis (Fig 1, lanes 3 and 4, respectively), and both migrated faster than native glycosylated α2AP protein (Fig 1, lane 2). Native α2AP (66 kD), which is secreted by liver cells into plasma, contains 452 amino acids and has a calculated molecular mass of 51 kD; it contains carbohydrate bound at Asn87, Asn256, Asn270, and Asn277.2 The recombinant α2AP bands migrated on SDS-PAGE to a position consistent with a molecular mass of 51 kD, the expected size for nonglycosylated α2AP. On Western blot, both recombinant forms of α2AP were recognized by polyclonal and monoclonal antibodies against native α2AP.
SDS-PAGE analysis of purified 2AP. Purified 2AP proteins were analyzed on a 10% to 20% polyacrylamide gradient gel under reducing conditions. Lane 1, molecular-weight standards with sizes shown on the left; lane 2, 1.0 μg of native human plasma 2AP; lane 3, 1.0 μg of recombinant wild-type 2AP; lane 4, 0.6 μg of recombinant mutant 2AP.
SDS-PAGE analysis of purified 2AP. Purified 2AP proteins were analyzed on a 10% to 20% polyacrylamide gradient gel under reducing conditions. Lane 1, molecular-weight standards with sizes shown on the left; lane 2, 1.0 μg of native human plasma 2AP; lane 3, 1.0 μg of recombinant wild-type 2AP; lane 4, 0.6 μg of recombinant mutant 2AP.
Ten cycles of Edman degradation performed on either wild-type or mutant α2AP yielded no amino acid sequence, suggesting that the amino-terminus was blocked. To identify the blocking group, mutant α2AP was digested by trypsin and subjected to HPLC and mass spectrometry. Only one significant peptide did not match the mass of any predicted tryptic peptide from mutant α2AP. It had a mass of 1541.8 daltons, which was consistent with that calculated for the 12-amino acid amino-terminal peptide of α2AP (Asn-Gln-Glu-Gln-Val-Ser-Pro-Leu-Thr-Leu-Leu-Lys-) with the addition of an acetylated Met. It has been reported that in yeast the initiator Met residue can remain and become acetylated if the protein or peptide has Asn, Asp, or a Glu as the residue penultimate to the initiator Met.23 To confirm that the peptide with an observed mass of 1541.8 was Ac-Met-Asn-Gln-Glu-Gln-Val-Ser-Pro-Leu-Thr-Leu-Leu-Lys-, approximately 100 pmol of the purified peptide was treated with cyanogen bromide to cleave the Ac-Met and then subjected to amino acid sequence determination. The amino acid sequence of the cyanogen bromide-treated peptide was found to be identical to the predicted 12 amino acid sequence of the amino-terminus of α2AP. Because trypsin is inhibited by wild-type α2AP, the amino-terminal fragment of wild-type recombinant α2AP was not determined. To confirm the structural authenticity of the reactive-site region of wild-type α2AP by internal amino acid sequence, it was incubated with thrombin at 37°C for 1 hour, as it has been reported that native α2AP inhibits thrombin by first forming a stable 1:1 molar complex before cleavage of α2AP to produce a carboxyl-terminal 10-kD fragment.24 On SDS-PAGE analysis of the thrombin-treated sample, it was observed that the intensity of the 51-kD wild-type α2AP band decreased, and new 41-kD and 10-kD bands appeared (data not shown). After electroblotting to a polyvinylidene difluoride membrane, Edman sequence analysis of the 10-kD band (Met365-Ser-Leu-Ser-Ser-Phe-Ser-Val-Asn-Arg374-) showed that thrombin cleavage of wild-type α2AP occurred between the reactive site Arg364 and Met365.
Characterization of recombinant α2AP as a plasmin inhibitor and FXIIIa substrate.
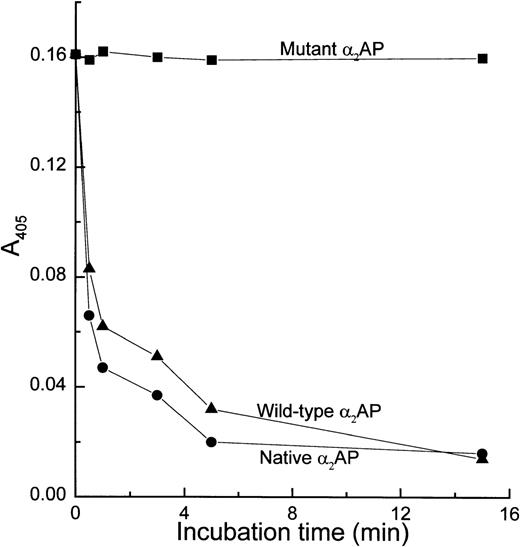
The plasmin-inhibitory activity of both wild-type and mutant recombinant α2AP proteins was measured and compared with that of native α2AP (Fig 2). Both the wild-type and native α2AP proteins rapidly inhibited plasmin activity, while mutant α2AP showed no plasmin-inhibitory activity. This result provided convincing evidence that nonglycosylated recombinant wild-type α2AP isolated from the cytosol of yeast possessed plasmin inhibitory activity comparable to glycosylated native α2AP, which is in accord with reports that nonglycosylated recombinant serine proteinase inhibitors, such as α1-antitrypsin and α1-chymotrypsin, have inhibitory activities essentially the same as their glycosylated native counterparts.25-27Hence, just as glycosylation in these serpins appears to be unimportant for expression of their proteinase inhibitory activities, the same appears to be the case for α2AP.
Plasmin-inhibitory activity of native, wild-type, and mutant 2AP. Each 2AP was incubated with an equimolar amount of plasmin for selected incubation periods, and then residual plasmin activity was assayed using a plasmin chromogenic substrate (S-2251) as described in Materials and Methods. Each data point is the average of two experiments.
Plasmin-inhibitory activity of native, wild-type, and mutant 2AP. Each 2AP was incubated with an equimolar amount of plasmin for selected incubation periods, and then residual plasmin activity was assayed using a plasmin chromogenic substrate (S-2251) as described in Materials and Methods. Each data point is the average of two experiments.
Results from previous studies2 showed that native α2AP has two disulfide bonds Cys31-Cys113 and Cys64-Cys104, but recently this has been disputed by others22 who found only a single disulfide bond (Cys31-Cys104) in native α2AP and reported that its reduced and alkylated form had essentially the same association rate constants (kass) for inhibition as native α2AP. Because this study showed that disulfide bond formation was not essential for inhibitory function of α2AP, disulfide-bond assignment in our recombinant forms of α2AP was not performed.
To assess the role of Arg364 in plasmin inhibition, mutant α2AP was prepared by replacing Arg364 with Ala. This mutant form could not inhibit plasmin (Fig 2). Previously, we reported that treatment of native α2AP with the Arg-specific reagent, phenyglyoxal,8 caused complete loss of plasmin inhibitory function. Our data from chemically modified, and now from genetically engineered α2AP, support previous reports7 9 that indicated the reactive-site Arg364 is critical to the function of α2AP as a plasmin inhibitor.
We also assessed both recombinant α2AP proteins as FXIIIa substrates by measuring FXIIIa-catalyzed [14C]methylamine incorporation into the antiplasmin molecule.19 Human plasma FXIIIa was determined to have apparent Km values of 5.52 μmol/L, 5.48 μmol/L, and 5.35 μmol/L, for wild-type, mutant, and native α2AP proteins, respectively, thereby demonstrating that each of the recombinant forms of α2AP retained full affinity for FXIIIa. Also, the overall kinetic efficiencies (kcat/Km) of wild-type (719 min−1/mmol/L) and mutant (725 min−1/mmol/L) α2AP proteins were shown to be comparable to that for native α2AP (716 min−1/mmol/L). Both recombinant α2AP proteins functioned as well as native α2AP when used as FXIIIa substrates, despite each having an additional residue, acetylated Met, as the amino-terminus. Considering the added amino-terminal acetylated Met, our findings contrast to those previously reported for two recombinant forms of α2AP: one having three9 extra amino-terminal amino acids and no longer a FXIIIa substrate; and the other recombinant form having 1210 additional amino-terminal residues, and markedly inefficient as a FXIIIa substrate. The differences between our findings and these two previous reports9 10 suggest that the length or type of residues in the amino-terminal extension are critical for the interaction of FXIIIa with the α2AP molecule.
Acceleration of lysis of plasma and whole blood clots by mutant α2AP.
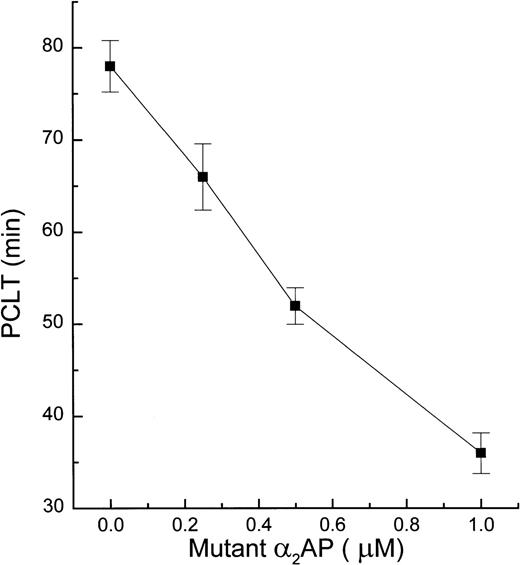
Previously we reported that α2AP modified by the Arg-specific reagent, phenylglyoxal, acted to enhance UK-induced blood clot lysis by competitive inhibition of factor XIIIa–mediated incorporation of native unmodified α2AP into fibrin.8 Less fibrinogenolysis was also observed when chemically modified α2AP was present in whole blood during fibrin clot formation, presumably because less UK was required to produce equipotent fibrinolysis when compared with control samples, which lacked chemically modified α2AP. In the present study, Arg364-mutated α2AP did not inhibit plasmin, but it remained a good FXIIIa substrate. Therefore, it was tested to determine if its incorporation into fibrin would allow UK-induced fibrinolysis to proceed more rapidly in human plasma and whole blood clots. A simple turbidimetric microtiter plate method20 21for plasma clot lysis was used to determine UK-induced plasma clot lysis times (PCLT). In the presence of 0.25 μmol/L mutant α2AP, PCLT was reduced by 15% (Fig 3). Further reductions in PCLT of 33% and 54% occurred with mutant α2AP concentrations of 0.50 and 1.0 μmol/L, respectively.
Effect of mutant 2AP on PCLT. PCLT experiments were performed in a reaction mixture containing citrated plasma, various concentrations of mutant 2AP, 1 U/mL thrombin, 20 mmol/L CaCl2, and 200 U/mL UK. Plasma samples were preincubated with mutant 2AP for 10 minutes at 22°C before adding a fresh mixture of UK, thrombin, and CaCl2. Turbidity was monitored as absorbance at 405 nm. PCLT was determined as the midpoint between the highest absorbance and constant lower absorbance.31
Effect of mutant 2AP on PCLT. PCLT experiments were performed in a reaction mixture containing citrated plasma, various concentrations of mutant 2AP, 1 U/mL thrombin, 20 mmol/L CaCl2, and 200 U/mL UK. Plasma samples were preincubated with mutant 2AP for 10 minutes at 22°C before adding a fresh mixture of UK, thrombin, and CaCl2. Turbidity was monitored as absorbance at 405 nm. PCLT was determined as the midpoint between the highest absorbance and constant lower absorbance.31
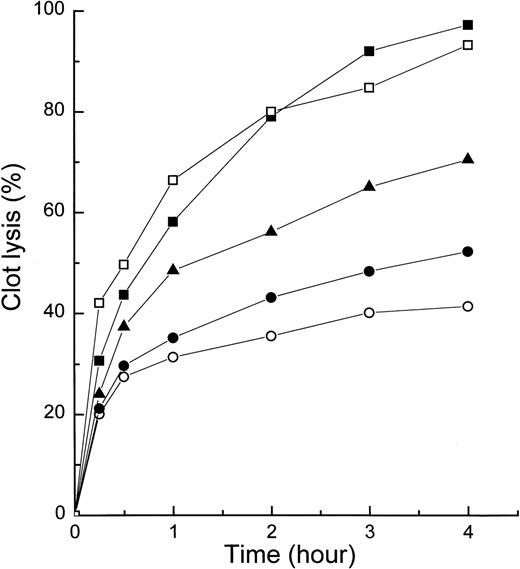
When mutant α2AP was tested for its ability to enhance clot lysis in whole blood (Fig 4), whole-blood samples supplemented with [I125]fibrinogen and defined concentrations of mutant α2AP (0.25, 0.5, and 1.0 μmol/L) were clotted by adding a freshly prepared mixture of thrombin, CaCl2, and UK. At specified times, each clot was centrifuged and radioactivity released over time into the supernatant taken as a measure of fibrin clot lysis rate. As shown in Fig 4, mutant α2AP clearly accelerated whole blood clot lysis in a dose-dependent manner. The rate of clot lysis induced by 40 U/mL UK with 1.0 μmol/L mutant α2AP was similar to that induced by 80 U/mL UK without mutant α2AP.
Effect of mutant 2AP on whole-blood clot lysis. Whole blood containing 125I-labeled fibrinogen and selected concentrations of mutant 2AP and UK was clotted by the addition of thrombin and CaCl2. Fibrinolysis was measured by counting radioactivity of the supernatant at each time point. It was expressed as the percentage of total radioactivity as described in Materials and Methods. Samples contained 40 U/mL UK alone (○), 40 U/mL UK and 0.25 μmol/L mutant 2AP (•), 40 U/mL UK and 0.5 μmol/L mutant 2AP (▴), 40 U/mL UK and 1.0 μmol/L mutant 2AP (▪), and 80 U/mL UK alone (□). Each data point is the average of two experiments.
Effect of mutant 2AP on whole-blood clot lysis. Whole blood containing 125I-labeled fibrinogen and selected concentrations of mutant 2AP and UK was clotted by the addition of thrombin and CaCl2. Fibrinolysis was measured by counting radioactivity of the supernatant at each time point. It was expressed as the percentage of total radioactivity as described in Materials and Methods. Samples contained 40 U/mL UK alone (○), 40 U/mL UK and 0.25 μmol/L mutant 2AP (•), 40 U/mL UK and 0.5 μmol/L mutant 2AP (▴), 40 U/mL UK and 1.0 μmol/L mutant 2AP (▪), and 80 U/mL UK alone (□). Each data point is the average of two experiments.
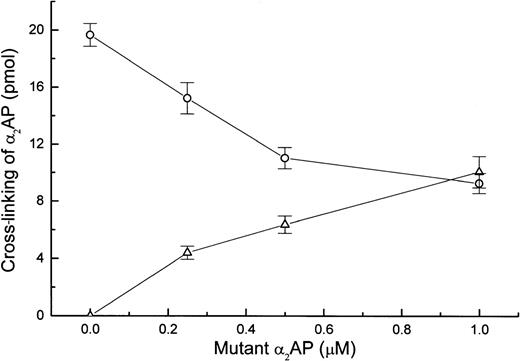
Inhibition of native α2AP cross-linking to fibrin by mutant α2AP.
To determine if the enhancement of fibrin clot lysis by mutant α2AP was due to a reduced content of cross-linked native α2AP, plasma was clotted in the presence of selected concentrations of mutant α2AP, and the amount of native α2AP or mutant α2AP that cross-linked to fibrin was determined. FXIIIa-catalyzed cross-linking of native α2AP to fibrin decreased as the concentration of mutant α2AP increased (Fig 5). Fifty percent inhibition of native α2AP cross-linking was obtained with approximately 1 μmol/L mutant α2AP. Also, in a separate experiment using purified fibrinogen, thrombin, and FXIII, the efficiencies with which native and mutant α2AP could be cross-linked into fibrin were compared using a published method,8 and in each case, cross-linking reached a maximum level within 10 minutes and then plateaued. These results show that (1) mutant α2AP has equivalent kinetics of cross-linking into fibrin as native α2AP, and (2) it competes effectively with native α2AP for becoming cross-linked to fibrin.
Effect of mutant 2AP on cross-linking of native 2AP to fibrin in plasma. Plasma samples containing a trace amount of 125I-native 2AP or 125I-mutant 2AP were clotted in the presence of specified concentrations of mutant 2AP. After a 30-minute incubation, the amount of cross-linked native 2AP (○) or mutant 2AP (▵) was determined in triplicate as described in Materials and Methods.
Effect of mutant 2AP on cross-linking of native 2AP to fibrin in plasma. Plasma samples containing a trace amount of 125I-native 2AP or 125I-mutant 2AP were clotted in the presence of specified concentrations of mutant 2AP. After a 30-minute incubation, the amount of cross-linked native 2AP (○) or mutant 2AP (▵) was determined in triplicate as described in Materials and Methods.
Cleavage of mutant α2AP by plasmin and thrombin.
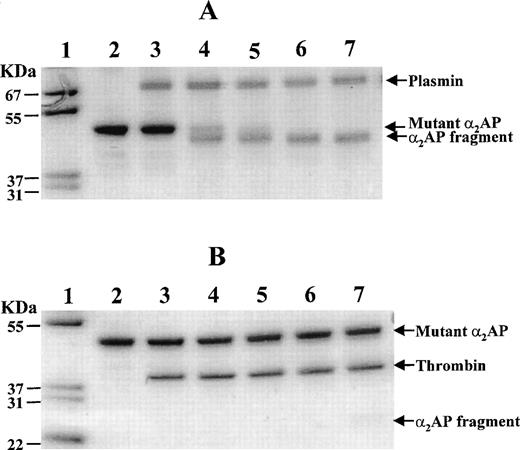
The ability of mutant α2AP to withstand digestion by either plasmin or thrombin was evaluated in a purified system (Fig 6). Such information could be important to the consideration of mutant α2AP as a fibrinolysis enhancer, or as part of engineered bifunctional therapeutic agent for localization on fibrin. In preliminary experiments, SDS-PAGE analysis indicated that plasmin digestion (40 minutes) generated a fragment (49 kD) missing a peptide mass of 2 kD when compared with the mobility of nondigested mutant α2AP (51 kD). As shown in Fig 6A, the 49-kD polypeptide appeared within 0.5 minutes and was maximally detected at 4 minutes of digestion, by which time the 51-kD mutant α2AP had disappeared completely. The 49-kD fragment remained stable up to 10 minutes. To determine the plasmin cleavage site in mutant α2AP, the 49-kD peptide band was transferred onto a polyvinylidene difluoride membrane, stained, excised, and sequenced. Its amino-terminal sequence was Leu-Gly-Asn-Gln-Glu-Pro-Gly-Gly-Gln-Thr-, demonstrating that the Lys12-Leu13 bond of the 51-kD mutant α2AP had been cleaved by plasmin. These data indicated that the amino-terminal region of our recombinant mutant α2AP not only contained an efficient FXIIIa substrate site (Gln2), but also an efficient plasmin substrate site (Lys12-Leu13), suggesting that this region of the protein is exposed and accessible to solvent. Unfortunately, no three-dimensional structural studies of any α2AP molecule, native or recombinant, have been performed that might allow further insights about conformational relationships of the cleaved peptide region.
Time course of plasmin and thrombin cleavage of mutant 2AP. Mutant 2AP was assessed for its sensitivity to cleavage by plasmin (A) and thrombin (B) using SDS-PAGE under nonreducing conditions. Mutant 2AP (3.5 μg) was incubated with 1.2 μg plasmin or 1.2 μg thrombin at 37°C in 15 μL of 40 mmol/L Tris·HCl, pH 7.5 containing 0.15 mol/L NaCl. An aliquot (3 μL) of the incubation mixture was taken at each time point and analyzed on 10% to 20% polyacrylamide gradient gels. Lanes 1 and 2 show molecular weight markers and untreated mutant 2AP, respectively. Lanes 3 through 7 show samples incubated for 0, 0.5, 1, 4, and 10 minutes, respectively.
Time course of plasmin and thrombin cleavage of mutant 2AP. Mutant 2AP was assessed for its sensitivity to cleavage by plasmin (A) and thrombin (B) using SDS-PAGE under nonreducing conditions. Mutant 2AP (3.5 μg) was incubated with 1.2 μg plasmin or 1.2 μg thrombin at 37°C in 15 μL of 40 mmol/L Tris·HCl, pH 7.5 containing 0.15 mol/L NaCl. An aliquot (3 μL) of the incubation mixture was taken at each time point and analyzed on 10% to 20% polyacrylamide gradient gels. Lanes 1 and 2 show molecular weight markers and untreated mutant 2AP, respectively. Lanes 3 through 7 show samples incubated for 0, 0.5, 1, 4, and 10 minutes, respectively.
Thrombin cleavage of mutant α2AP was also examined using SDS-PAGE (Fig 6B). No cleavage products were observed in the first 4 minutes of digestion, but at 10 minutes, a weakly stained broad band (≈25 kD) appeared (Fig 6B, lane 7). In a separate experiment, 10.2 μg of mutant α2AP was incubated with an equimolar amount of thrombin for 40 minutes, and the incubation mixture was then examined by SDS-PAGE. Approximately 10% of the 51-kD mutant α2AP disappeared as a 25-kD broad band appeared. The latter peptide band was excised and sequenced. A single amino-terminal sequence of Thr-Tyr-Pro-Leu-Arg-Trp-Phe-Leu-Leu-Glu- was found, confirming that the Arg232-Thr233 bond had been cleaved by thrombin. It appeared that the broad 25-kD band might contain both the Ac-Met-Asn1-Arg232 peptide fragment and a Thr233-Lys452 peptide fragment. Three pieces of evidence were consistent with this hypothesis: (1) the calculated masses of the amino-terminal and carboxyl-terminal fragments, 27.1 and 24.8 kD, respectively, were similar; (2) the amino-terminal fragment had potential disulfide bonding site(s) that could make the peptide move faster on nonreducing SDS-PAGE; and (3) no other fragments were detected on the SDS gel. Hence, in contrast to cleavage by thrombin between the reactive site Arg364 and Met365 of wild-type α2AP, it appeared that the Arg232-Thr233 bond of mutant α2AP is uniquely cleaved by thrombin, but much more slowly than the cleavage of the Lys12-Leu13 bond by plasmin.
Although mutant α2AP was cleaved by plasmin and thrombin, it enhanced plasminogen activator-induced fibrinolysis in plasma and whole blood clot assays. Most likely this can be explained by rapid cross-linkage of mutant α2AP to fibrin28before mutant α2AP could be cleaved by either plasmin or thrombin. Once cross-linked to fibrin, not only did the mutant α2AP block potential cross-link sites for native α2AP, but it also rendered fibrin more susceptible to proteolysis, as it lacked the ability to inhibit plasmin. It should be emphasized that even if the cross-linked mutant α2AP molecule were to be cleaved by plasmin or thrombin, the cross-link site would still be occupied and therefore blocked.
CONCLUDING REMARKS
Unlike previous reports by two other groups,9,10 the recombinant α2AP we expressed could be cross-linked into fibrin as well as native plasma α2AP by FXIIIa catalysis. To our knowledge, this represents the first report of recombinant wild-type or mutant α2AP, which retains full affinity for FXIIIa and kinetic efficiencies (kcat/Km) comparable to that for native plasma α2AP. Neither the lack of glycosylation or the addition of an acetylated Met on its amino-terminus affected its function as a FXIIIa substrate or its plasmin inhibitory capacity, but the Arg364 mutation did abolish the ability to inhibit plasmin. When cross-linked into fibrin by FXIIIa catalysis, mutant α2AP enhanced plasminogen activator-induced fibrinolysis primarily by reducing the amount of native α2AP that ordinarily becomes incorporated into fibrin during blood clot formation. Results presented here are consistent with a previous report that a synthetic 12-residue peptide derived from the amino-terminal sequence of α2AP competitively inhibited the cross-linking of native α2AP to fibrin, and as a consequence, enhanced fibrinolysis.29,30 However, the concentration of the amino-terminal peptide needed for the fibrinolysis-accelerating effect30 was approximately 1,000-fold higher than in the case of the Arg364-mutant α2AP described here. Presumably the high FXIIIa-affinity and high fibrin-specificity of α2AP require not only the FXIIIa-substrate site in the amino-terminal region, but also other regions of the α2AP molecule as well. At this time, however, it is not known how much of the structure of α2AP is essential to achieve maximal affinity and efficiency as a FXIIIa substrate.
Because the mutant α2AP in this report became cross-linked to fibrin as effectively as native α2AP, it may have potential as a fibrinolytic enhancer, or as the amino-terminal portion of potential therapeutic proteins engineered for specific targeting into fibrin. Albeit entirely speculative, if given in instances such as after certain intravascular procedures or during a thrombosis-in-progress (eg, a stroke), developing thrombi might then contain less native α2AP and as a consequence, be more susceptible to dissolution at lower doses of plasminogen activators with diminished risk of hemorrhage.
Supported by the William K. Warren Medical Research Institute and in part by the Oklahoma Center for the Advancement of Science and Technology (to K.N.L.) and Presbyterian Health Foundation (to K.N.L.).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Kyung N. Lee, PhD, W.K. Warren Medical Research Institute, PO Box 26901, BSEB-306, Oklahoma City, OK 73190; e-mail: kyung-lee@ouhsc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal