Abstract
We recently reported detection of a transient increase in circulating donor leukocytes (WBCs) in immunocompetent recipients 3 to 5 days posttransfusion (tx) (Blood 85:1207, 1995). We have now characterized survival kinetics of specific donor WBC subsets in additional tx populations. Eight female elective surgery patients (pts) were sampled pre-tx and on days 1, 3, 5, 7, and 14 post-tx. Ten female trauma pts transfused with a total of 4 to 18 U of relatively fresh red blood cells were sampled up to 1.5 years post-tx. WBC subsets from frozen whole blood were isolated using CD4, CD8 (T cell), CD15 (myeloid), and CD19 (B cell) antibody-coated magnetic beads. Donor WBCs were counted by quantitative polymerase chain reaction (PCR) of male-specific sex determining region (SRY) sequences. PCR HLA typing and mixed leukocyte reaction (MLR) between recipient and donor WBCs were performed on two of the trauma tx recipients who had long-term chimerism of donor cells post-tx. In 6 of 8 female surgery pts, circulating CD4+ male donor cells peaked at day 3 or 5 (0.01 to 1 cell/μL), followed by clearance by day 14. In 7 of 10 female trauma pts, we observed multilineage persistence of male donor WBCs (CD4, CD8, CD15, CD19) for 6 months to 1.5 years post-tx at concentrations of 10 to 100 cells/μL. In 2 trauma recipients studied, MLR showed no, or very low, response to WBC of the single donor implicated as the source of microchimerism by HLA typing. Establishment of long-term multilineage chimerism in trauma recipients is probably caused by engraftment of donor stem cells and mutual tolerance between recipient and donor leukocytes. A better understanding of factors determining clearance versus chimerism of transfused leukocytes is critical to prevention of alloimmunization and transfusion-induced graft-versus-host disease, and, potentially, to induction of tolerance for transplantation.
CELLULAR BLOOD components (whole blood, packed red blood cells [RBCs], and platelets) contain 108to 1010 donor leukocytes that are widely recognized as being responsible for a host of transfusion-related complications, ranging from chill-fever reactions to HLA alloimmunization with resulting platelet refractoriness, to fatal graft-versus-host disease (GVHD).1-10 Donor leukocytes also play an important role as vectors for transfusion-transmission of retrovirus and herpes virus infections,11 may act as allogeneic stimulators triggering reactivation of recipient viral infections,12 and may modulate the host’s immune system with both beneficial (eg, enhanced tolerance to renal transplantation)13 and deleterious (eg, increased risk of cancer recurrence14; susceptibility to post-operative infections15) effects.
Peripheral blood (PB) has been known for decades to contain primitive hematopoietic stem cells as well as intermediate types of committed progenitor cells.16 Collection of stem cells and committed progenitor cells from PB is now widely used to reconstitute the hematopoietic system of patients (pts) after intensive chemotherapy and total body irradiation. Manipulation of autologous or allogeneic PB stem cells is becoming increasingly sophisticated, both with respect to removal of tumor or alloreactive cells and introduction of genetic markers. Leukocytes have also been harvested from PB and transfused for treatment of posttransplant infections.17 Finally, transfusion of leukocytes for induction of tolerance before transplantation remains an active area of clinical and basic research.18-23
Despite the frequency and clinical importance of leukocytes in PB, relatively little is known about the kinetics of survival and clearance of donor leukocytes after standard blood transfusions (tx). We recently reported detection of a transient increase in circulating donor leukocytes 3 to 5 days post-tx in immunocompetent recipients.24 This phenomenon was reproduced in a canine transfusion model, where the transient donor leukocyte expansion phase was prevented by gamma irradiation of the canine blood before transfusion.24 These studies used quantitative allele-specific polymerase chain reaction (QAS-PCR) assays directed at a single-copy Y-chromosome gene or a series of HLA class II alleles to detect donor cells in total leukocyte preparations derived from fresh recipient blood samples.
To further characterize the survival kinetics of the donor cell populations in a recipient’s circulation post-tx, we developed a protocol to purify immunophenotypic subpopulations of leukocytes from frozen whole blood samples, followed by QAS-PCR to quantitate donor white blood cells (WBCs) in each subpopulation. Magnetic beads coated with different monoclonal antibodies (MoAbs), including anti-CD4 and anti-CD8 (T cells), anti-CD15 (myeloid), and anti-CD19 (B cells), were used in these studies. The protocol was then applied to serial pre- and post-tx samples collected from 8 female surgery pts who had been transfused with relatively fresh male blood components (nonleukodepleted, nonirradiated). We also applied this protocol to the pre- and post-tx samples collected from 10 female trauma pts who had received multiple transfusions during and subsequent to surgery. Together, these results show the spectrum of short- and long-term survival kinetics of donor leukocytes in additional recipient populations and identify the specific donor cells surviving in the recipients’ circulations.
MATERIALS AND METHODS
Subjects Studied and Transfusion Protocol
Elective surgery patients.
Twenty-five female pts who were followed in hospitals in Fukushima County, Japan, were scheduled for surgical procedure for which at least 1 U of unmanipulated (ie, not irradiated and not leukodepleted) packed red blood cells (RBCs) was likely to be transfused, and from whom autologous blood was not collected. Before selection for the study, the subjects’ charts were reviewed to determine indications for surgery and prior pregnancy and transfusion history, and to exclude medical conditions suggesting severe immunodeficiency such as leukemia or acquired immunodeficiency syndrome. The information for the 8 subjects transfused with at least 1 U of nonirradiated, nonleukodepleted packed RBCs from a male donor is summarized in Table1. EDTA-anticoagulated blood samples obtained from the pts pre-tx and at post-tx days 1, 3, 5, 7, and 14 were retrieved from the hematology laboratory daily. One-half–milliliter aliquots of well-mixed whole blood were frozen at −70°C immediately after collection. The serial samples from each individual were batched and tested together to minimize inter-run variation of the assay. All 8 pts had uneventful postoperative recoveries without reported transfusion reactions.
Severe trauma transfused patients.
Ten female automobile accident victims seen at the University of California Davis Medical Center in Sacramento were serially studied. All sustained serious multiple injuries, underwent surgery, and received 3 to 14 U of nonirradiated, nonleukodepleted packed RBCs from male donors (Table 2). All survived at least 6 months after the accident. EDTA-anticoagulated blood samples were serially collected up to 1.5 years after the accident. Aliquots of well-mixed whole blood were frozen at −70°C within 4 hours of collection. No signs or symptoms of GVHD were reported in these pts. Three additional pts who received blood only from female donors were similarly followed as “controls.”
Informed consent was obtained from trauma patients and their donors for follow-up specimen collection and HLA typing and mixed leukocyte reaction (MLR) studies. These studies were approved by the Sacramento Medical Foundation Institutional Review Board and the University of California, Davis, Human Subjects Review Committee; a similar protocol has been approved by the Committee on Human Research at the University of California, San Francisco, for the work done at Blood Centers of the Pacific. For the samples collected in Japan, Japanese researchers obtained a waiver of informed consent, because the samples were collected for routine testing and the residual blood was stored and used for this study.
Cell/DNA Preparation and Processing
To characterize the post-tx survival kinetics of the donor leukocyte subpopulations in a recipient’s circulation, we developed a protocol to purify immunophenotypic leukocyte subsets from frozen EDTA-anticoagulated whole blood samples of recipients, followed by QAS-PCR to quantitate donor WBCs in each enriched subpopulation. Magnetic beads (Dynal, Inc, Lake Success, NY) coated with different MoAbs, including anti-CD4 and anti-CD8 (T cells), anti-CD15 (myeloid), and anti-CD19 (B cells), were used in this study. Immediately after thawing, 0.5-mL aliquots of frozen whole-blood samples collected from recipients were incubated separately with each MoAb-coated bead preparation for 20 minutes at 4°C on a rotator, following the manufacturer’s instructions to calculate the volume of beads being used. The beads were then washed twice, first with 1 mL phosphate-buffered saline (PBS) and then with 1 mL PCR solution A (100 mmol/L KCl; 10 mmol/L Tris, pH 8.3; 2.5 mmol/L MgCl2). After washing, the beads with bound specific WBC subpopulations were resuspended with 50 μL of solution A and 50 μL of PCR lysis solution B (10 mmol/L Tris, pH 8.3; 2.5 mmol/L MgCl2; 1% Tween-20; 1% NP40; and 0.4 mg/mL Proteinase K) and incubated at 60°C for 1.5 hours with vortexing every 20 minutes, followed by 95°C for 2 hours. The DNA lysates from each serial bleed were then tested together in duplicate, in a single hybridization/polyacrylamide gel electrophoresis (PAGE)/autoradiography run, along with analysis of duplicate standard dilution series. By testing the serial samples from each pt in a single run, we controlled for possible patient-specific factors (eg, variable background recipient leukocyte concentrations) and eliminated potential inter-run variables, such as slight differences in PCR reaction conditions or 32P-specific activity. If the PCR signal in the recipient sample was stronger than the highest standard, the DNA lysate of that sample was further diluted (eg, 1:10, 1:100, or 1:1,000) and reamplified.
Sensitivity, Specificity, and Reproducibility of QAS-PCR
To document the sensitivity and reproducibility of the assay over the quantitative range investigated, a series of replicate analyses of female whole-blood samples spiked with varying amounts of blood from male donors were performed following the protocol described above. To test assay specificity we tested samples from 30 female blood donors and from 3 females who underwent surgery subsequent to trauma and who received blood only from female donors.
QAS-PCR for Y-Chromosome
A 148-bp region of the sex-determining region of the human Y-chromosome (SRY)25 was amplified using allele-specific primer pair SA (5′ CGCATTCATCGTGTGGTCTCGCG 3′) and SD (5′ CTGTGCCTCCTGGAAGAATGGCC 3′). The PCR reaction mixture consisted of 100 mmol KCl; 20 mmol Tris HCl, pH 8.3; 2.5 mm MgCl2; 0.02% gelatin; 1 pmol/μL of each primer; and 0.04 U/μL Thermalase (IBI, New Haven, CT). DNA lysate (25 μL) was added to 50 μL of PCR reaction mixture, followed by amplification for 30 cycles consisting of 30 seconds at 95°C and 2 minutes at 70°C. Specific amplified product was detected by oligomer liquid hybridization using a32P-labeled probe, SB (5′ GAGGCGCAAGATGGCTCTAGAG 3′).25,26 The probe was end-labeled at 37°C for 1 hour in a 40-μL solution consisting of 7 mmol Tris HCL, pH 7.6; 10 mmol MgCl2; 15 mmol dithiothreitol; 10 U T4 polynucleotide kinase (New England Biologicals, Cambridge, MA); 40 μCi32P-adenosine triphosphate (32P-ATP; Dupont, Wilmington, DE); and 30 pmol probe. After incubation, the volume was adjusted to 400 μL by addition of 0.75 mmol/L NaCl and 10 mmol/L EDTA solution. For hybridization, 10 μL of this probe mix was added to 30 μL of postamplification specimen and incubated for 5 minutes at 59°C after a 5-minute denaturation at 95°C. To each hybridized sample, 10 μL of loading buffer (0.25% bromphenol blue, 0.25% xylene cyanol, and 30% glycerol) were added, and this mixture was subjected to 6% PAGE at 12.5 V/cm. After running, the gel was exposed to XAR-5 autoradiographic film (Kodak, Rochester, NY) with enhancing screen for 30 minutes, 2 hours, and overnight at room temperature. Duplicate standard curves composed of 10-fold serial dilutions of male donor cells in pre-tx female recipient cells (103, 102, 101, 100) were analyzed in parallel with clinical samples and used to interpolate donor leukocyte concentration in test samples. Autoradiographs were analyzed using the Millipore BioImage Electrophoresis Analyzer (Millipore, Ann Arbor, MI) with Whole Band Analyzer application software (Millipore).24 Image analysis was performed on the dilution (neat, 1:10, 1:100, or 1:1,000) and film exposure (30 minutes, 2 hours, or overnight) that was within the linear range of the corresponding standard curve on that film. Values from duplicate determinations were averaged. If two dilutions (eg, neat and 1:10) were within the dynamic range, both were analyzed and the results were averaged (after adjusting for the dilution factor). The reproducibility of the image analysis system was assessed by replicate (5×) scanning of 23 sample data points spanning the assay’s dynamic range (2 to 500 cells): the mean percent coefficient of variation was 8.3%, with a range of 3.7% to 17.8%.
QAS-PCR for HLA Typing and Quantitation of Specific Donor Cells Surviving in the Circulation
To optimize the sensitivity and specificity of the assay for specific HLA types, a specific positive DNA lysate was spiked into a negative DNA lysate to create a series of spiked samples containing different concentration of specific positive DNA. For each specific primer pair, different concentrations of Mg2+, different amplification programs, and different reaction buffers were used to amplify the series of spiked samples to identify the optimal DNA assay conditions. All sequences of primers and probes, as well as amplification conditions, are listed in the Appendix. Assay sensitivities ranged from 10 to 100 cells/mL.
Mixed Leukocyte Reactions (MLR)
Fresh Ficoll-Hypaque (Robbins Scientific, Sunnyvale, CA) purified peripheral blood mononuclear cells from two transfusion recipients (1 × 105 cells) were mixed with an equal number of inactivated stimulator cells from their male blood donors. (Fresh cells from applicable blood donors were inactivated by incubation with 0.25 mg/mL of mitomycin C for 20 minutes at 37°C in a 5% CO2-95% air-humidified incubator.) Cultures were set up in triplicate in 96-well round-bottom trays and were incubated in 0.2 mL of RPMI 1640 supplemented with 2 mmol/L glutamine, an antibiotic/antimycotic, and 10% newborn calf serum. Plates were incubated at 37°C in 5% CO2-95% air for 5 days before pulsing for 6 hours with 1.0 μCi/well of tritiated thymidine (in 25 μL). After incubation with tritiated thymidine, cells were recovered by filtration on glass fiber filters and thoroughly air dried. Individual circles from the filter were removed, placed in scintillation vials, and counted (within 6 to 8 hours after adding cocktail) on a beta scintillation counter with windows set for3H.
RESULTS
Sensitivity, Specificity, and Reproducibility of QAS PCR Assays
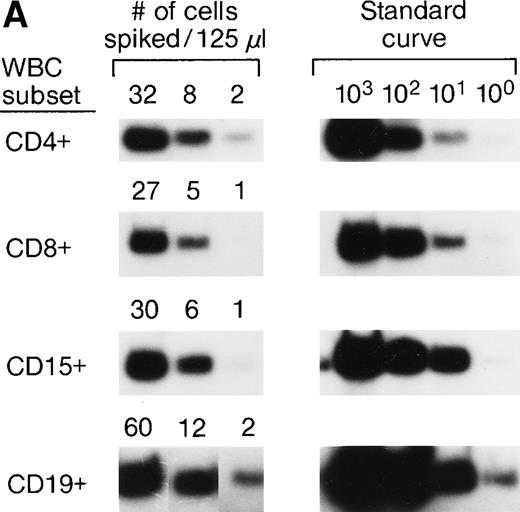
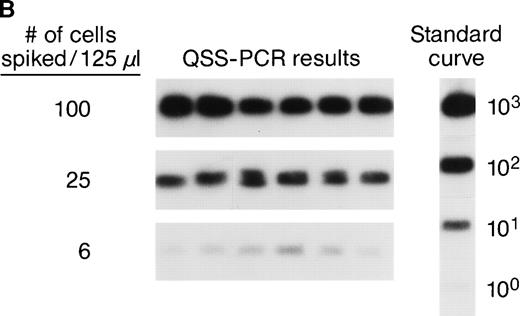
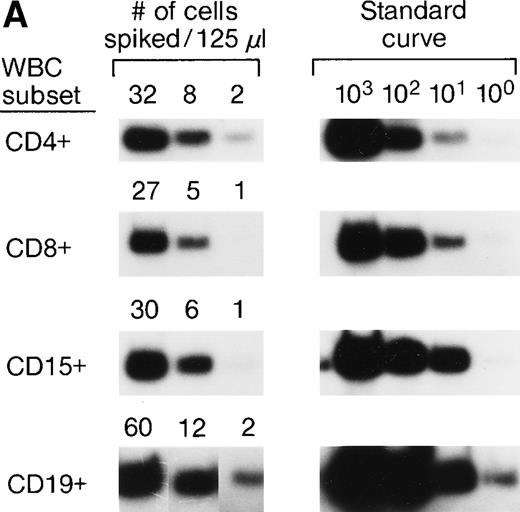
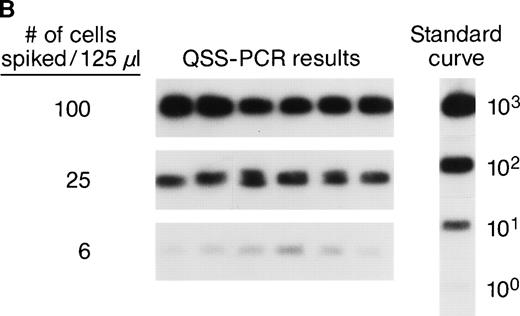
The accuracy and precision of QAS-PCR have been previously described,27 as has adaptation of the assay to analysis of frozen whole-blood samples.28 To document the sensitivity and reproducibility of the protocol we developed to quantitate donor leukocytes in enriched subpopulations derived from frozen whole-blood samples from female recipients, four different male blood donor samples were characterized for various concentrations of their major leukocyte subsets by flow cytometry; serial fourfold dilutions were prepared, and selected dilutions were spiked into four different female (recipient) whole blood samples. This resulted in the theoretical levels of male cells indicated in Fig 1A. Each dilution was then processed through the assay protocol in replicates of six. Figure 1A shows representative results of assay sensitivity for all four WBC subsets. The assay protocol was able to detect as few as 1 to 5 spiked male donor CD4+, CD8+, CD15+, and CD19+ cells in the input volume (125 μL) of female frozen whole blood. Figure 1B shows representative results of assay reproducibility for male donor CD4+ cells spiked into female whole blood. The coefficients of variation (CVs) of the CD4+ cell assay ranged from 26% to 68% at spiking levels of 100, 25, and 6 male CD4+cells. The CVs of the CD8+ cell assay were 32%, 43%, and 98%, respectively, for spiking levels of 50, 13, and 5 male CD8+ cells. The CVs for the three different spiking levels for CD15+ and CD19+ cell assays were 20%, 25%, or 26% and 28%, 39%, or 87%, respectively. These results indicate that the assays have CVs of 20% to 98% over this dynamic range and can, therefore, discriminate a fourfold difference in male donor cell concentration. To evaluate assay specificity, we tested samples from 30 female blood donors with no history of transfusion. In addition, samples were sent under code for 3 female pts with traumatic injuries who received blood only from female donors, along with the samples from the 10 females who received blood from male donors as part of their treatment for serious trauma. All samples from females who received no male blood were negative for Y-chromosome.
(A) Representative results of sensitivity of QAS-PCR detection of male donor WBCs in enriched subpopulations from spiked female blood. Four different male donor blood samples with known leukocyte concentrations were diluted fourfold, and serial dilutions were spiked into four different female recipient whole blood samples. For the assay of CD4+ cells, three different levels of male CD4+ cells (32, 8, and 2) were spiked into female blood, and for CD8+ cells, 27, 5, or 1 male CD8+ cells were spiked. For the CD15+ cell assay, 30, 6, or 1 male CD15+ cells were spiked, and for the CD19+ cell assay, samples containing 60, 12, or 2 male CD19+ cells were created. Each dilution was then processed through the assay protocol. (B) Representative results of QAS-PCR reproducibility (6×) for male donor CD4+ cells spiked into female whole blood.
(A) Representative results of sensitivity of QAS-PCR detection of male donor WBCs in enriched subpopulations from spiked female blood. Four different male donor blood samples with known leukocyte concentrations were diluted fourfold, and serial dilutions were spiked into four different female recipient whole blood samples. For the assay of CD4+ cells, three different levels of male CD4+ cells (32, 8, and 2) were spiked into female blood, and for CD8+ cells, 27, 5, or 1 male CD8+ cells were spiked. For the CD15+ cell assay, 30, 6, or 1 male CD15+ cells were spiked, and for the CD19+ cell assay, samples containing 60, 12, or 2 male CD19+ cells were created. Each dilution was then processed through the assay protocol. (B) Representative results of QAS-PCR reproducibility (6×) for male donor CD4+ cells spiked into female whole blood.
Post-tx Short-Term Survival Kinetics of Nonirradiated Donor Leukocytes in Female Elective Surgery Patients
Pre-tx and serial post-tx samples were collected from 8 female pts who received at least 1 U of nonirradiated male blood (cellular) components. QAS-PCR results of Y-chromosome-positive WBCs in total leukocyte preparations of serial blood samples collected from these 8 transfusion recipients showed similar clearance kinetics of donor leukocytes to those we previously reported.20 At 24 hours post-tx, more than 99.9% of male donor leukocytes had been cleared out from the circulation of all 8 female transfusion recipients. However, in 6 of 8 recipients, we observed a substantial increase of Y-chromosome–positive donor leukocytes in the recipients’ circulations at 3 or 4 days post-tx. No Y-chromosome–positive donor leukocytes were detected in the recipients’ circulations at 7 to 14 days post-tx in any of these 8 recipients.
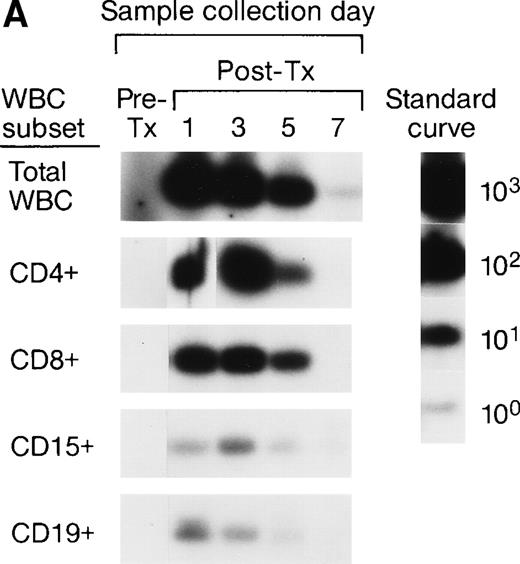
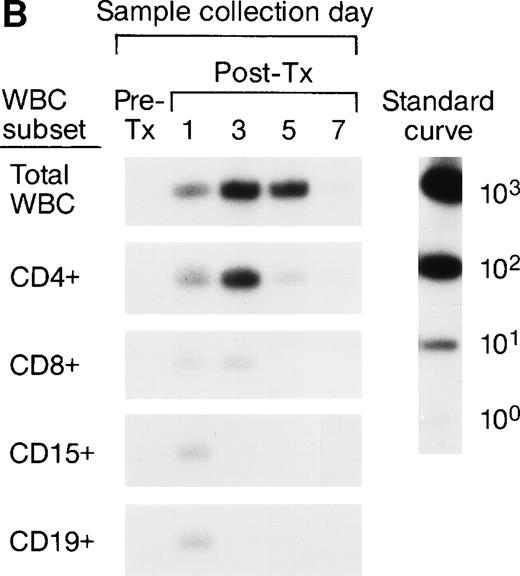
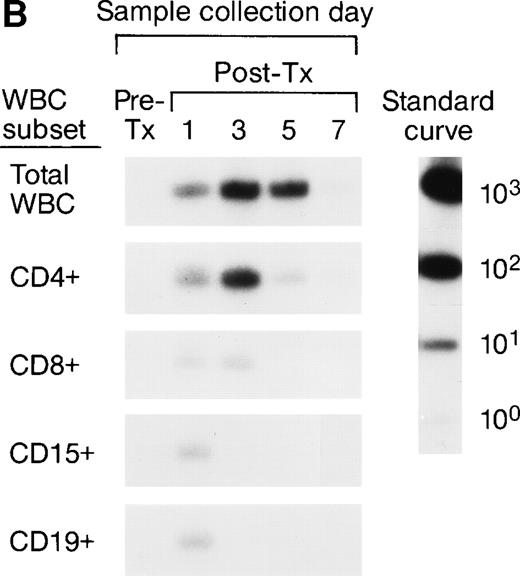
The serial pre- and post-tx frozen whole-blood samples collected from the female elective surgery pts were also subjected to enrichment of leukocyte subpopulations including CD4, CD8, CD15, and CD19 cells, followed by amplification and quantitation using Y-chromosome–specific primers. The serial samples collected from 2 recipients were completely negative, suggesting the concentrations of male donor leukocyte subsets were below the detection level of our assay system. For the remaining 6 recipients, we were able to analyze the short-term survival kinetics of donor leukocyte subsets in the recipients’ circulations. The QAS-PCR results of serial samples collected from 2 representative female recipients are shown in Fig 2A and B. In Fig 2A, the QAS-PCR signals of donor CD4+ and CD15+ cells peaked at day 3 post-tx and disappeared by day 7 post-tx. We also observed the persistence of CD8+ donor cells in this recipient’s circulation up to 5 days post-tx, but there was no increase of CD8+ levels detected in these serial post-tx samples. In Fig 2B, the QAS-PCR signals of donor total leukocytes and CD4+ cells also peaked at day 3 post-tx and disappeared by day 7 post-tx in this pt. There was no post-tx increase of donor CD8+, CD15+, or CD19+cells observed in this tx recipient.
Representative QAS-PCR results of short-term survival kinetics of male donor leukocyte subsets in female transfusion recipients 1 and 2 (A and B). The pre- (pre-tx) and post-tx (post-tx) serial frozen whole-blood samples collected from female recipients were subjected to the enrichment of leukocyte subpopulations including CD4, CD8, CD15, and CD19 cells, followed by amplification and quantitation using Y-chromosome–specific primers.
Representative QAS-PCR results of short-term survival kinetics of male donor leukocyte subsets in female transfusion recipients 1 and 2 (A and B). The pre- (pre-tx) and post-tx (post-tx) serial frozen whole-blood samples collected from female recipients were subjected to the enrichment of leukocyte subpopulations including CD4, CD8, CD15, and CD19 cells, followed by amplification and quantitation using Y-chromosome–specific primers.
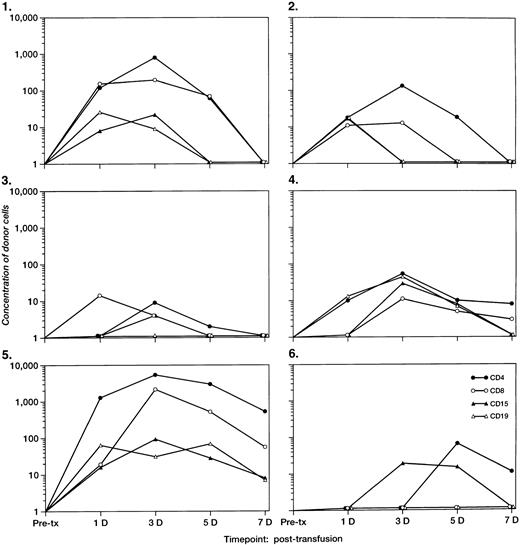
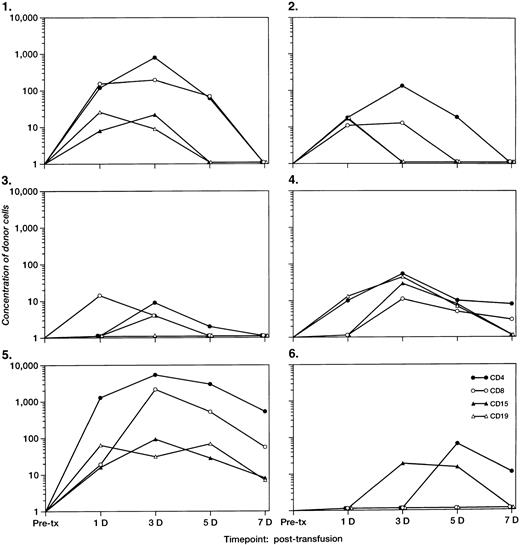
The concentrations of donor leukocyte subpopulations derived from QAS PCR for the 6 recipients with detectable microchimerism are plotted in Fig 3. An increase in circulating Y-chromosome–positive donor cells in the enriched CD4+cells was observed in all 6 recipients at day 3 or day 5 post-tx. Although the post-tx increase in donor CD8+, CD15+, and CD19+ cells was not as significant as that of donor CD4+ cells, an increase of QAS-PCR signal of donor cells in the enriched subpopulations of CD8+ T cells and CD19+ B cells was observed among 3 recipients at 3 to 5 days post-tx. We also found that, for 5 recipients, the Y-chromosome–positive donor cells in the enriched CD15+WBC subpopulation peaked at 3 or 4 days post-tx but disappeared after 7 days post-tx. QAS-PCR signal of Y-chromosome–positive donor cells in total and all enriched leukocyte subsets derived from recipient blood samples had disappeared by day 14 post-tx in all surgery pts.
The concentrations per milliliter of short-term survival of donor leukocyte subpopulations (including CD4, CD8, CD15, and CD19) derived from QAS-PCR results for the six female tx recipients who had detectable male cells 3 to 4 days post-tx. The pre- and post-tx serial frozen whole-blood samples collected from six female recipients were subjected to enrichment of leukocyte subpopulations including CD4, CD8, CD15, and CD19 cells, followed by amplification and quantitation using Y-chromosome–specific primers. The y-axis represents the concentration of donor cells per milliliter of recipient’s blood.
The concentrations per milliliter of short-term survival of donor leukocyte subpopulations (including CD4, CD8, CD15, and CD19) derived from QAS-PCR results for the six female tx recipients who had detectable male cells 3 to 4 days post-tx. The pre- and post-tx serial frozen whole-blood samples collected from six female recipients were subjected to enrichment of leukocyte subpopulations including CD4, CD8, CD15, and CD19 cells, followed by amplification and quantitation using Y-chromosome–specific primers. The y-axis represents the concentration of donor cells per milliliter of recipient’s blood.
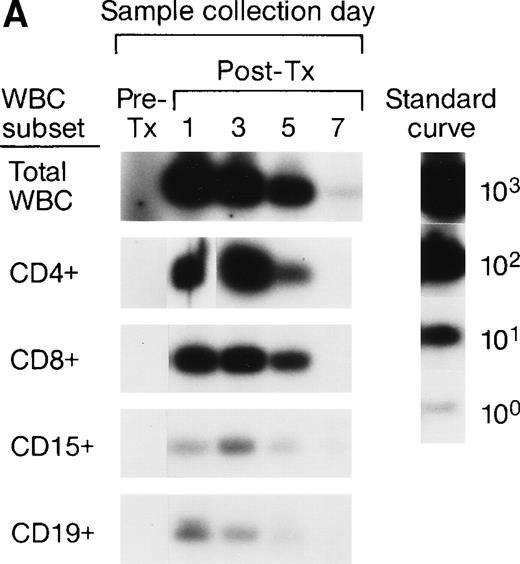
Long-Term Survival of Donor Leukocyte Subpopulations in Transfused Trauma Patients
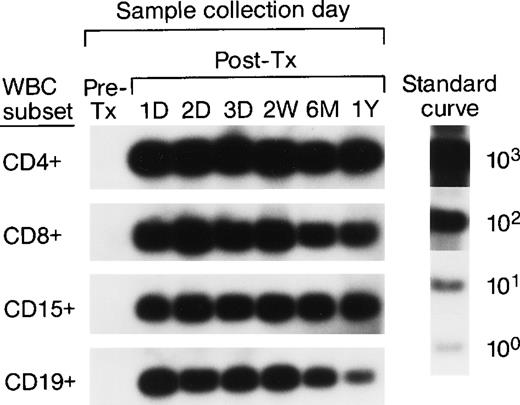
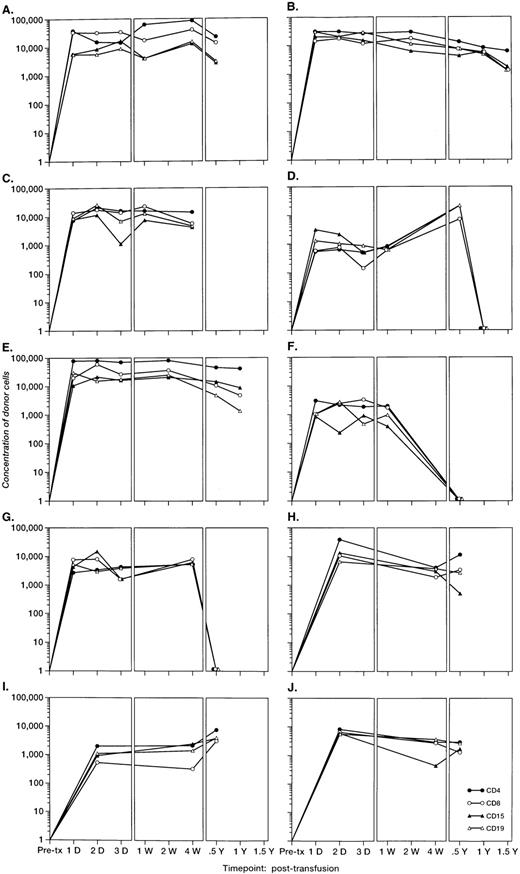
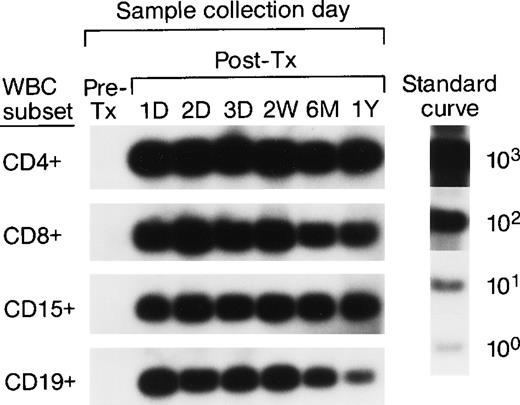
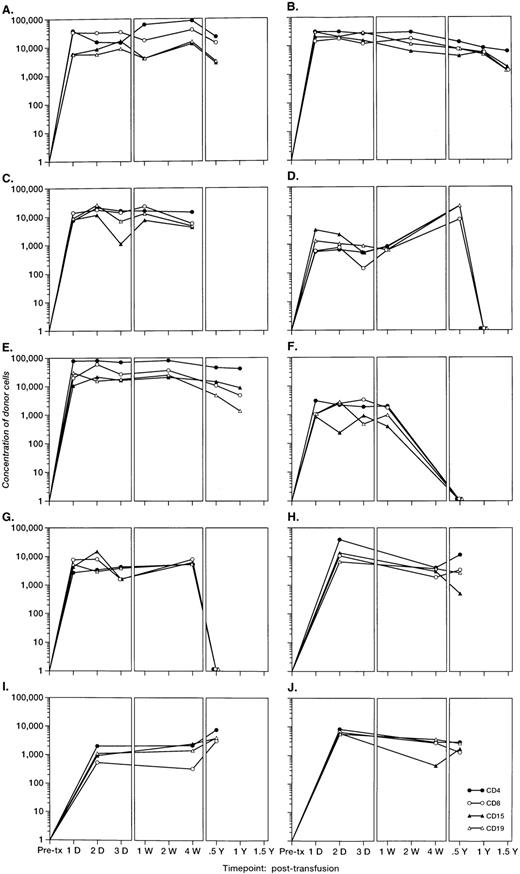
To investigate the survival of donor leukocyte subpopulations in transfused trauma pts, pre-tx and serial post-tx blood samples were collected from 10 females who had undergone severe trauma and were transfused with at least 1 U of nonirradiated, nonleukoreduced packed RBCs from a male donor. All samples were tested under code along with 30 nontransfused female blood donor and serial pre- and post-tx samples collected from 3 “control” female recipients transfused only with female blood (see above). Two pts (B and E) had samples collected up to 1.5 years post-tx; 1 pt had samples collected up to 1 year post-tx, and in 6 pts samples were collected up to 6 months post-tx. Representative QAS-PCR results (including CD4+, CD8+, CD15+, and CD19+, in pre- and post-tx samples) for female pt E are shown in Fig 4. QAS-PCR signal for Y-chromosome–positive cells persisted from 1 day post-tx through 1.5 years post-tx for all four leukocyte subsets, and the donor CD4 counts were between 42 and 80 cells/μL. The concentrations of donor cells derived from QAS-PCR results of all samples collected from the 10 female trauma pts who received male donor blood are graphically summarized in Fig 5. The durations, concentrations, and phenotypes of donor cells circulating in trauma recipients’ blood were remarkable compared with the low-level, transient persistence observed in elective surgery patients. Between 0.5% and 10% of circulating WBCs in these recipients were donor derived, and the chimerisms involved multiple lineages (CD4, CD8, CD15, and CD19) of donor leukocytes. Pts B and E had the longest follow-up (1.5 years post-tx) and showed long-term donor leukocyte survival in their blood, including all four leukocyte subsets: CD4+, CD8+, CD15+, and CD19+. Five additional pts (A, D, H, I, J) showed donor cell survival up to 6 months post-tx; 1 of these (subject D) became negative for male donor cells by 1 year of post-tx follow-up while the other 4 were positive at the last sampling time point. The samples collected from the 2 remaining trauma recipients (subjects F and G) became negative for donor cells between 4 months and 6 months post-tx.
QAS-PCR results (including CD4+, CD8+, CD15+, and CD19+) for female trauma tx recipient E. All samples were subjected to the enrichment of CD4+, CD8+, CD15+, and CD19+ leukocyte subpopulations, followed by amplification and hybridization using human Y-chromosome–specific primers and probe.
QAS-PCR results (including CD4+, CD8+, CD15+, and CD19+) for female trauma tx recipient E. All samples were subjected to the enrichment of CD4+, CD8+, CD15+, and CD19+ leukocyte subpopulations, followed by amplification and hybridization using human Y-chromosome–specific primers and probe.
The concentrations per milliliter of Y-chromosome–positive donor cells derived from QAS PCR results of samples collected from the 10 female trauma patients. All frozen whole-blood samples were subjected to the enrichment of CD4+, CD8+, CD15+, and CD19+ leukocyte subpopulations, followed by amplification, hybridization, and quantitation using human Y-chromosome–specific primers and probe. Y-axis represents the concentration of donor cells per milliliter of recipient’s blood.
The concentrations per milliliter of Y-chromosome–positive donor cells derived from QAS PCR results of samples collected from the 10 female trauma patients. All frozen whole-blood samples were subjected to the enrichment of CD4+, CD8+, CD15+, and CD19+ leukocyte subpopulations, followed by amplification, hybridization, and quantitation using human Y-chromosome–specific primers and probe. Y-axis represents the concentration of donor cells per milliliter of recipient’s blood.
HLA Analysis of MLR of Cells From Female Trauma Patients
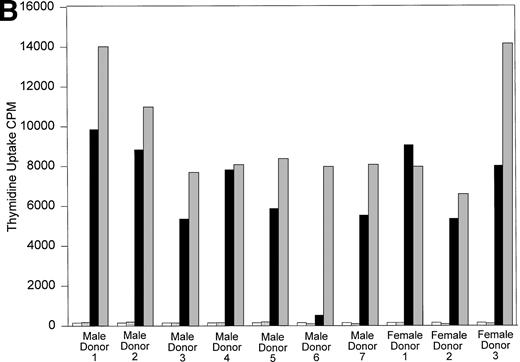
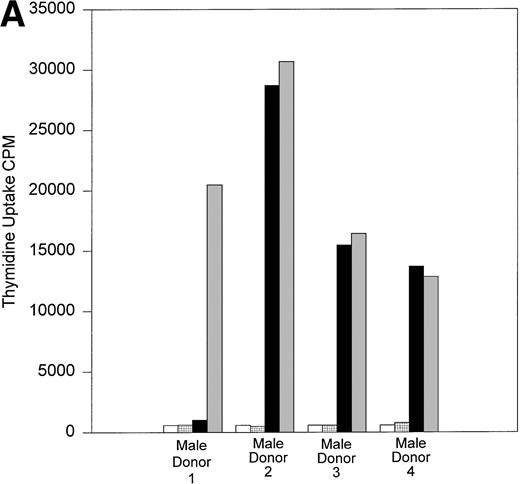
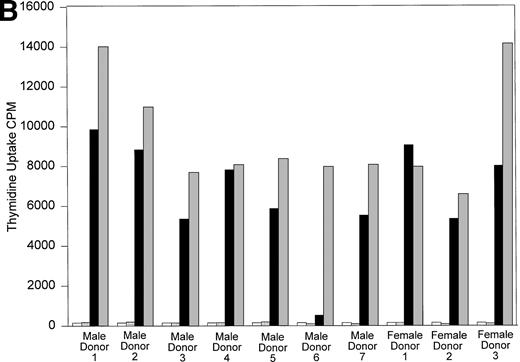
All 10 trauma pts received multiple transfusions in a short time period (<1 week). To determine if HLA similarity between donor and recipient played a role in the long-term post-tx chimerism, we performed serological HLA typing of pts B and D, and all their donors. The results of serological HLA typing are shown in Tables 3 and4. The MLR results are shown in Fig 6A and B. Control MLRs of recipient cells versus autologous mitomycin C–treated recipient cells and donor cells versus autologous mitomycin c–treated donor cells all gave results of less than 516 cpm (results not shown). In vitro, cells from recipient B had a very low response when incubated with the cells of male donor 1, compared with the response to the cells of male donors 2, 3, or 4. This result suggests that recipient B did not recognize the cells of male donor 1 as foreign, and that male donor 1 is the likely source of donor cells surviving in recipient B’s circulation. The HLA types between recipient B and her male blood donors showed no unusual HLA compatibility to explain why only cells from donor 1 persisted in this recipient’s circulation. Indeed, the cells from donor 1 responded in MLR against recipient B at a level comparable to that seen with the other donors whose cells did not engraft. Similarly, the cells of recipient D had minimum response to the cells of a single male donor (donor 6; see Fig 6B). Again, there was no unusual HLA compatibility, and the cells from male donor 6 showed strong response in MLR to the cells of recipient D, suggesting the cells of male donor 6 were capable of recognizing recipient D’s cells as “foreign” before tx.
(A) One-way MLR: Recipient B and donors. Results of one-way MLR between recipient B and her four male donors. White bars represent the tritiated thymidine uptake of cells from recipient B mixed with mitomycin C–treated autologous cells (control). Patterned bars represent tritiated thymidine uptake of the indicated donor cells mixed with their own mitomycin C-treated cells (control). Black bars represent the response of cells from recipient B to mitomycin C–treated stimulator cells from the indicated donor. Gray bars represent the indicated donor cell response to mitomycin C–treated stimulator cells from recipient B. Results shown are the mean cpm of triplicate cultures. (B) One-way MLR: Recipient D and donors. Results of one-way MLR between recipient D and her seven male and three female donors. White bars represent the tritiated thymidine uptake of cells from recipient D mixed with mitomycin C–treated autologous cells (control). Patterned bars represent tritiated thymidine uptake of the indicated donor cells mixed with their own mitomycin C–treated cells (control). Black bars represent the response of cells from recipient D to mitomycin C–treated stimulator cells from the indicated donor. Gray bars represent the indicated donor cell response to mitomycin C–treated stimulator cells from recipient D. Results shown are the mean cpm of triplicate cultures.
(A) One-way MLR: Recipient B and donors. Results of one-way MLR between recipient B and her four male donors. White bars represent the tritiated thymidine uptake of cells from recipient B mixed with mitomycin C–treated autologous cells (control). Patterned bars represent tritiated thymidine uptake of the indicated donor cells mixed with their own mitomycin C-treated cells (control). Black bars represent the response of cells from recipient B to mitomycin C–treated stimulator cells from the indicated donor. Gray bars represent the indicated donor cell response to mitomycin C–treated stimulator cells from recipient B. Results shown are the mean cpm of triplicate cultures. (B) One-way MLR: Recipient D and donors. Results of one-way MLR between recipient D and her seven male and three female donors. White bars represent the tritiated thymidine uptake of cells from recipient D mixed with mitomycin C–treated autologous cells (control). Patterned bars represent tritiated thymidine uptake of the indicated donor cells mixed with their own mitomycin C–treated cells (control). Black bars represent the response of cells from recipient D to mitomycin C–treated stimulator cells from the indicated donor. Gray bars represent the indicated donor cell response to mitomycin C–treated stimulator cells from recipient D. Results shown are the mean cpm of triplicate cultures.
Identification of Specific Donor Cells Surviving in Recipients’ Circulations
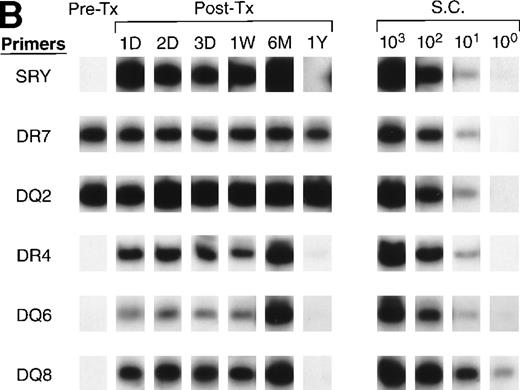
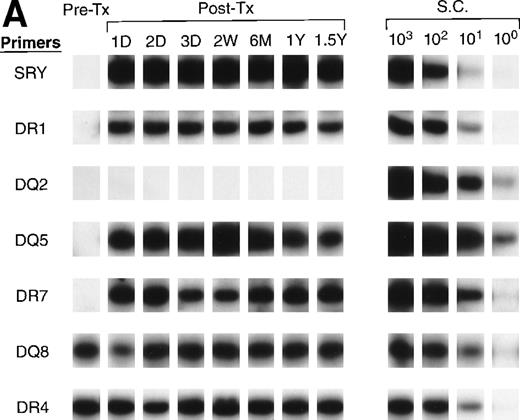
To further identify the specific donor cells surviving in a recipient’s circulation from among the tx from many donors, we optimized a panel of primer pairs specific to HLA DR (alleles 1, 2, 3, 4, 7, 8, 11, 12, 13, 15, 16) and DQ (alleles 2, 4, 5, 6, 7, 8) alleles (see Appendix). Pre- and post-tx serial samples collected from recipients B and D and samples collected from all donors were amplified with specific primer pairs to HLA DR and HLA DQ to identify the specific donor cells that survived in each recipient’s circulation. The HLA typings of recipients and donors were used to identify alleles that could distinguish the specific surviving donor cells among the many transfusion donors. For recipient B (Fig7A), both pre- and post-tx samples were negative for DQ2. Therefore, surviving donor leukocytes from male donors 2 and 4 were excluded. The pre-tx sample was negative for DR7 and DQ5, but the post-tx samples were positive for those alleles that were present in male donor 1. In recipient B the surviving donor cells are therefore most likely from male donor 1.
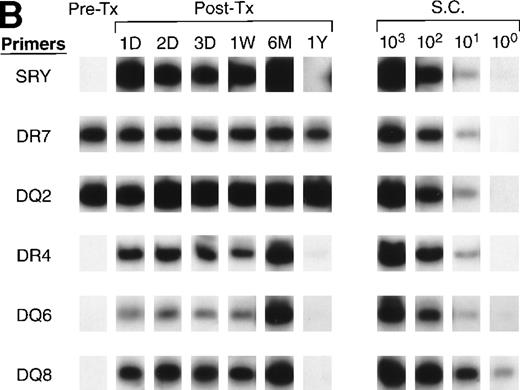
QAS-PCR results of HLA DR and DQ amplification for samples collected from recipients B (A) and D (B). Pre-tx and serial post-tx samples collected from female trauma tx recipients B and D were amplified with a panel of primer pairs specific to HLA DR (1, 2, 4, 7) and DQ (2, 4, 5, 6, 7, 8) and hybridized with specific probes. The results of DR2 and DQ6 were positive and DQ4 and DQ7 negative at all time points, and are not shown for recipient B; the results of DR1, DR2, DQ4, DQ5, and DQ7 were negative at all time points and are not shown for recipient D.
QAS-PCR results of HLA DR and DQ amplification for samples collected from recipients B (A) and D (B). Pre-tx and serial post-tx samples collected from female trauma tx recipients B and D were amplified with a panel of primer pairs specific to HLA DR (1, 2, 4, 7) and DQ (2, 4, 5, 6, 7, 8) and hybridized with specific probes. The results of DR2 and DQ6 were positive and DQ4 and DQ7 negative at all time points, and are not shown for recipient B; the results of DR1, DR2, DQ4, DQ5, and DQ7 were negative at all time points and are not shown for recipient D.
All of the pre- and post-tx samples collected from recipient D were negative for DR 1, 2, 11, and DQ 4, 5 (data not shown), which indicated that all male and female donors except male donor 6 could be excluded as the source of surviving donor cells. Figure 7B shows that, in the amplification with DR4, DQ6, and DQ8 primers for recipient D samples, the pre-tx sample was negative; however, all the post-tx samples were positive for all three alleles, showing that the donor cells surviving in recipient D’s circulation after transfusion were probably derived from male donor 6, who is positive for DR4, DQ6, and DQ8.
DISCUSSION
In a previous publication we reported a transient increase of donor leukocytes in the circulation of immunocompetent elective surgery pts at day 4 post-tx, which we hypothesized might represent one arm of an in vivo MLR, with activated donor T lymphocytes proliferating in an abortive GVHD reaction to HLA-incompatible recipient cells.24 In the present study, we observed a similar transient increase of donor total leukocytes in recipient’s circulation at 3 to 5 days post-tx in 6 of 8 additional pts undergoing elective surgery for various conditions, including orthopedic procedures and cancer resections (Table 1). To further understand the mechanism of post-tx proliferation of donor leukocytes, we developed a protocol to purify WBC subpopulations from recipient’s frozen whole-blood samples. By combining magnetic beads and QAS-PCR, we optimized an assay protocol to characterize the post-tx survival kinetics of donor leukocyte subpopulations in a recipient’s circulation. We were able to detect as few as 1 to 5 male donor CD4+, CD8+, CD15+, and CD19+ cells in 125 μL of female recipient blood containing approximately 1 × 105, 5 × 104, 4.5 × 105, and 3 × 104 of these cells, respectively. Using this protocol, we observed a marked increase of male donor CD4+ T cells at day 3 to 5 post-tx in all 6 of 8 female recipients whose post-tx samples contained detectable male donor leukocytes. We also observed an increase of donor CD8+ T cells, CD15+ myeloid cells, and CD19+ B cells in some of these recipients between day 3 and day 5 post-tx. The proliferation of donor CD4+ and CD8+ T cells supports the concept that this increase might represent one arm of an in vivo MLR in post-tx recipients’ circulation. The presence of CD19+ and CD15+ indicates broader expansion of committed progenitor cells, perhaps driven by MLR-cytokine stimulation. The subsequent clearance of proliferating donor leukocytes in these pts undergoing elective surgery with significant enough blood loss to require blood transfusion presumably results from allograft rejection by immunocompetent recipient cells.
Although a post-tx, two-way, MLR was proposed over 25 years ago by Schechter et al,29 no one has previously documented which leukocyte subpopulations are involved and proliferate in the reaction. Indeed, the MLR is an in vitro model of T-cell recognition of foreign, major histocompatibility complex (MHC) gene products, and is used as a predictive test of cell-mediated graft rejection.30 Two populations of alloreactive T cells are stimulated and proliferate during an in vitro allogeneic MLR, and each responding T-cell subset recognizes a different MHC gene product. One type of T cell expresses the CD8 but not CD4 molecule, usually functioning as a cytolytic T lymphocyte; the second type is the cytokine-producing CD4+ helper T-cell specific for foreign protein antigens. Therefore, based on the in vitro model, the MLR alone cannot explain all the results presented in our study, especially the post-tx increase of donor CD15+ myeloid cells, which usually don’t proliferate in the MLR.
In 1962, Goodman and Hodgson first introduced the term “blood stem cell” into the literature; in 1964, Cavins and colleagues successfully transplanted cryopreserved blood stem cells into irradiated dogs.31 Peripheral WBC counts of dogs exposed to gamma irradiation and given an infusion of autologous WBCs began to increase 5 to 10 days post-tx. In 1967, Storb and colleagues performed similar studies to show the existence of hematopoietic stem cells in the PB of dogs in the allogeneic transfusion situation.31The timing (3 to 5 days post-tx) we observed for the increase of donor CD15+ and CD19+ T cells in our recipients’ circulations is consistent with the observations described by Cavins and Storb, assuming a lag-time of 2 to 5 days between the earliest detection of increasing donor cells by our PCR assay and their counting method. Therefore, the transient post-tx increase of donor cells we observed in recipients probably represents a combination of an allogeneic lymphocyte reaction of donor cells to recipient cells and the expansion of committed donor progenitor cells, which are eventually eradicated by most hosts’ immune systems.
Long-term chimerism up to many years has been documented after solid-organ transplantation32-34 in which immunosuppressive drugs were used to prevent the rejection of graft by host. Male fetal progenitor cells have been reported to persist in maternal blood for as long as 27 years postpartum.35 For transfusion recipients, very few studies address the question of long-term survival of donor cells post-tx. In the second part of the present study, we report that long-term survival of blood donor cells is relatively common for severe trauma pts who received multiple transfusions of blood components acutely. In contrast to the results we report in this study, Wenk and Chiafari36 recently reported that in a limited number of transfused adult patients, donor alleles were not detected by PCR. The discrepancy between Wenk and Chiafari’s study and ours is likely attributable to the differences in sensitivity of PCR techniques used in the studies. Our assays have sensitivities of approximately 10 cells per mL of blood containing 106 to 107 recipient leukocytes (1 donor cell to 105to 106 recipient cells). In contrast, the methods used by Wenk and Chiafari cannot detect donor cells present at less than 104 to 105 per mL recipient’s blood (1 donor cell to 102 recipient cells) (unpublished data, June 1998).
The durations, concentrations, and phenotypes of donor cells circulating in these multiply transfused trauma recipients’ blood were remarkable. Up to 5% of some recipients’ WBCs were donor derived, and the chimerisms included multiple lineages (CD4, CD8, CD15, and CD19) of donor leukocytes. The establishment of post-tx long-term chimerism seems to indicate the occurrence of mutual tolerance in the recipient’s circulation between the host’s immune system and certain donor cells, because there were no symptoms or signs of GVHD observed in these female trauma pts, nor was there rejection of the donors’ cells for, in some cases, over a year. In the experiments involving in vitro MLR between donor cells and cells of trauma pts B and D, we observed that the lymphocytes of the latter did not react to the cells of the specific male donors implicated as chimeric; in contrast, the cells from these specific male donors did react against the cells of the trauma pts. It is unclear why the specific alloreactive donor cells did not attack recipients’ tissues, even though donor cells could proliferate in response to recipient cells in vitro in a one-way MLR. It is possible that potentially alloreactive (naive) donor T cells were educated in the recipient’s thymus (or other sites) not to respond to the recipient’s tissue (ie, tolerated). Alternatively, the alloreactive (educated) donor lymphocytes could have been selectively eliminated by the recipient’s immune system.
The mechanism of long-term donor cell survival in transfused trauma pts is unclear. All trauma pts were transfused with multiple units in a short period of time, and all underwent surgery. Both tx and surgery are known to be immunosuppressive.37-45 Hemorrhage alone reportedly induces a marked suppression of many cellular and humoral immune functions including cytokine release,46-49 antigen presentation by macrophages,50 and humoral immunity.51,52 Trauma has also been associated with immunosuppression,53 including dysfunction in macrophage function,54 stimulation of inflammatory mediators,55 and cytokine release.56,57Trauma reportedly also increases the frequency of hematopoietic progenitor cells.58 The combination of trauma and hemorrhage might cause a prolonged depression in cellular immunity.59,60 T cells collected from tissue such as lymph vessels are affected by trauma and hemorrhage to a greater degree than PB T cells measured soon after injury.61
We found no clear correlation between the severity of the initial injury, duration of subsequent surgery, number or order of units transfused, previous transfusion history, splenectomy, or HLA compatibility and the subsequent survival of donor cells in the recipients’ circulations. Transfused blood was relatively fresh, with at least 1 male unit of blood stored for less than 15 days transfused into each trauma pt. Leukocytes in whole blood do not lose their ability to respond to mitogen in vitro until after approximately 2 weeks of storage at 4°C.62 63 Temporary immunosuppression combined with exposure to a large number of antigens from multiple donors could explain why some donor’s leukocytes are not recognized by the recipient, eg, why there is apparent tolerance to only one donor but not others encountered in the same time frame.
The traditional “self ” and “non-self ” discrimination theory of the immune system does have some inconsistencies. Why doesn’t a mother reject her partly foreign fetus? Why aren’t tumors seen as foreign and attacked? Matzinger et al64-66 recently proposed a “danger” model that may apply in our study. The idea is that the immune system responds not to “non-self ” but to dangerous entities. To be dangerous, damage must be caused. As long as no damage is being caused to the recipient cells by the donor cells, the recipient would not necessarily attack the donor cells. As soon as any damage is caused by the donor cells, they would be eliminated by the recipient. Although our one-way MLR studies showed that donor cells proliferated in vitro in response to recipient cells, they may not have been cytotoxic in vivo. Donor cells may have been tolerized when exposed to a large number of antigens in a multiply transfused pt. This temporary tolerance may be broken by some mechanism that activates the donor cells, such as viral infection. Once the donor cells become activated and recognized as cytotoxic to the recipient, they would be eliminated by the recipient’s immune system. This might explain why in some of the transfused trauma pts in the study the circulating (proliferating) donor cells were eventually cleared.
In conclusion, the present studies document transient persistence of transfused donor cells in immunocompetent surgery pts for several days after blood tx, while multiply transfused trauma victims had evidence of donor cell chimerism for up to 1.5 years after transfusion without evidence of GVHD. Further studies that may prove useful in understanding mechanisms leading to chimerism include (1) comparison of the immunological reactivity of fresh donor cells with chimeric donor cells isolated from the recipient’s blood, and (2) immunological and T-cell receptor repertoire studies to compare the difference of surface antigen expression and T-cell receptor utilization between recipient blood-derived and donor blood-derived donor cell populations. Additional clinical studies are also warranted to determine if these observations are generalizable to male transfusion recipients and to develop a better understanding of factors determining rapid clearance versus persistent chimerism of transfused leukocytes. These studies should yield critical insights relevant to prevention of alloimmunization and transfusion-induced GVHD, as well as approaches toward induction of tolerance for tissue and organ transplantation and active immunotherapy.
Supported in part by National Heart, Lung and Blood Institute Award No. PO1-HL-54476.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Michael P. Busch, MD, PhD, Vice President, Research and Scientific Services, Blood Centers of the Pacific, 270 Masonic Ave, San Francisco, CA 94118; e-mail: mpbusch@itsa.ucsf.edu.