Abstract
The hormonal form of vitamin D, 1,25-dihydroxyvitamin D3[1,25(OH)2D3], is a potent inhibitor of cellular proliferation as well as an inducer of differentiation of myeloid leukemic cells to macrophages. We have previously reported that a number of genes are upregulated by 1,25(OH)2D3 during myeloid differentiation, including the cyclin-dependent kinase (CDK) inhibitors p21, p27, 15, and p18, suggesting that cell cycle arrest and differentiation are tightly linked processes. We further explore here the relationship between growth inhibition and differentiation. We report that, upon 1,25(OH)2D3 treatment, U937 cells exhibited an early proliferative burst followed by growth inhibition and subsequent differentiation. Although CDK levels remain constant throughout, this transient increase in proliferation was accompanied by increases in cyclin A, D1, and E protein levels. p21 and p27 levels were also elevated during both the proliferative burst and subsequent inhibition of cell growth. Ectopic overexpression of p21 and/or p27 in U937 cells, in the absence of hormone, resulted in an induction of the expression of monocyte/macrophage-specific markers, whereas overexpression of p15 and p18 had no effect, suggesting that a subset of CDK inhibitors are important for both growth arrest and differentiation and that an early increase in proliferation is somehow a prerequisite for subsequent differentiation. However, no such biphasic behavior was detected in cells that are growth inhibited by 1,25(OH)2D3but do not differentiate, such as MCF-7 cells. Taken together, these results indicate that both growth stimulation and subsequent inhibition precede differentiation and involve induction of both cyclins and p21 and p27, whereas cell cycle arrest of differentiated cells can be achieved simply by elevations in CDK inhibitors.
IN MAMMALIAN CELLS, signals that induce or facilitate differentiation often do so through regulation of the cell cycle. However, how withdrawal from the cell cycle leads to differentiation is not yet completely understood. What is clear is that a mechanistic description of this process has broad implications spanning basic cell biology to clinical oncology, because aggressive tumors are often composed of de-differentiated cells.
To proceed from a proliferating cell to a differentiated state requires control of DNA replication. The decision of a cell to enter the DNA synthetic phase of the cell cycle (S-phase) and the actual onset of DNA replication are two events that are in part governed by G1 cyclins, which assemble as complexes with cyclin-dependent kinases (CDKs).1 A group of recently characterized small proteins that interact with and inhibit the activity of cyclin-CDK complexes, called CDK inhibitors (CKIs), act as negative regulators of growth by causing cells to arrest in G1 and withdraw from the cell cycle.2 Two families of CKIs have thus far been identified. The Cip/Kip family is composed of p21Cip1/Waf1,3-6p27Kip1,7,8 and p57Kip2.9 These proteins share homologies at their N-termini, defining a CDK-interacting domain. A second group, the Ink4 family, consists of p15Ink4b, p16Ink4a, p18Ink4c, and p19Ink4d.10-13 The Ink4 proteins contain four tandemly repeated ankyrin repeats and are structurally unrelated to the Cip/Kip family. Evidence is accumulating that CKIs are targets of extracellular and intracellular signals that regulate cell growth, differentiation, and apoptosis.14 For example, radiation-induced DNA damage elevates p53 levels, and this protein in turn transcriptionally induces p21 expression, resulting in cell cycle arrest.4 Transforming growth factor-β (TGF-β) acts as an antimitogenic factor primarily by inducing p15 and by translocating p27 from Cdk4 to Cdk2.15,16 MyoD upregulates p21 expression, which is correlated to muscle cell differentiation.17 Several other signals appear to converge on p21, including retinoic acid,18 steroid hormones,19,20 and epidermal growth factor (through STAT1).21
We have been studying how the active metabolite of vitamin D, 1,25(OH)2D3, acts as a general inhibitor of cellular proliferation and as an inducer of differentiation of myeloid leukemic cells. This seco-steroid signals through an intracellular receptor, the vitamin D3 receptor (VDR), that, as a member of the steroid/nuclear hormone receptor superfamily, controls gene expression by directly regulating the transcription of specific target genes. In its capacity as a regulator of cell growth and differentiation, some components of the cell cycle ought to be direct or indirect targets of 1,25(OH)2D3. For example, the expression of positive factors that stimulate cell growth, such as cyclins and CDKs, would be predicted to be inhibited by the ligand-receptor complex. Negative factors, such as CKIs, would be induced by VDR. Using a modified differential screen that enriched for genes that would be among the earliest regulated after the addition of 1,25(OH)2D3 in the myelomonoblastic leukemic cell line U937, we identified p21Waf1/Cip1 as such a direct VDR target gene.22 23 In addition to p21, 1,25(OH)2D3 induced the expression of p27Kip1. These results suggested that differentiation of U937 cells to monocyte/macrophages by 1,25(OH)2D3 was induced in part by a cell cycle arrest caused by increased levels of CKIs.
We wished to further explore the relationship between growth inhibition and differentiation using 1,25(OH)2D3 as a model system. We report here that, upon 1,25(OH)2D3 treatment, U937 cells surprisingly exhibited an early proliferative burst followed by growth inhibition and subsequent differentiation. Although CDK levels remained constant throughout, this transient increase in proliferation was accompanied by increases in cyclin A, D1, and E protein levels. p21 and p27 levels, but not p15 or p18, were also elevated during both the proliferative burst and subsequent inhibition of cell growth. Ectopic overexpression of p21 and/or p27 in U937 cells, in the absence of hormone, resulted in an induction of the expression of the monocyte/macrophage-specific membrane markers CD11b and CD14, whereas overexpression of the Ink 4 family members p15 and p18 had no effect. These results suggest that some, but not all, CKIs are important for both growth arrest and differentiation and that an early increase in proliferation in our system is a prerequisite for subsequent differentiation. No such biphasic behavior was detected in cells that retain the antiproliferative response to 1,25(OH)2D3 but that are not induced to differentiate, such as the breast carcinoma cell line MCF-7. Taken together, these results distinguish between growth arrest and differentiation, in that in the latter case, both growth stimulation and inhibition precede differentiation and involve induction of both cyclins and CKIs. In contrast, cell cycle arrest without induction of differentiation can be achieved simply by elevations in CKIs.
MATERIALS AND METHODS
Cell culture and plasmids.
U937 human myelomonocytic cells (clone 4; provided by K. Nilsson, Uppsala University, Uppsala, Sweden24) were routinely maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum and 5 mmol/L L-glutamine. All CMV5 expression constructs were generous gifts of J. Massagué (Memorial Sloan Kettering Cancer Center, New York, NY).
Cell proliferation assay.
A colorimetric method was used to determine the number of viable cells in proliferation assays, by measuring the conversion of MTS(3-(4,5-dimethylthialzol-2-yl)-5-(3-carboxy-methoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) into the aqueous, soluble form called formazan (Promega, Madison, WI). Exponentially growing U937 cells were grown in the presence or absence of 1,25(OH)2D3 for 0 to 72 hours. The absorbance of formazan was measured at 490 nm 2 hours after addition of 20 μL of a combined MTS/PMS solution to each well in a 96-well plate. Data are expressed as the percentage of inhibition relative to untreated control cells. Each time point was assayed in triplicate.
Immunoprecipitation kinase assay and Western blot analysis.
Antibodies against the proteins p15Ink4b, p18Ink4c, p21Cip1, CDK4, CDK6, cyclin A, and cyclin E were purchased from Santa Cruz Biotechnology (Santa Cruz, CA); cyclin D1 was purchased from Neomarkers. Antibodies against the Cdk2 and p27 proteins were a generous gift from Drs J. Massague and A. Koff (Memorial Sloan Kettering Cancer Center, New York, NY), respectively. For immunoprecipitation experiments, cells were in 50 mmol/L HEPES, pH 7.5, 50 mmol/L NaCl, 1 mmol/L EDTA, 2.5 mmol/L EGTA, 10% glycerol, 0.1% Tween 20, 1 mmol/L dithiothreitol (DTT), 0.1 mmol/L Na-orthovanadate, 1 mmol/L NaF, 10 mmol/L β-glycerophosphate, and 1 mmol/L phenylmethyl sulfonyl fluoride (PMSF), 10 μg/mL leupeptin, and 10 μg/mL aprotinin on ice for 30 minutes with occasional vortexing. Lysates were clarified by centrifugation at 10,000g for 10 minutes at 4°C, after which they were incubated with a particular antibody and protein A-sepharose for 2 to 4 hours at 4°C. Immunocomplexes bound to protein A-sepharose were collected by centrifugation and washed 4 times with lysis buffer and twice with kinase buffer (50 mmol/L HEPES [pH 7.5], 10 mmol/L MgCl2, 1 mmol/L DTT). The kinase reaction was initiated by resuspending the beads in 30 μL kinase buffer containing 50 mmol/L HEPES (pH 7.5), 2.5 mmol/L EGTA, mmol/L DTT, 0.1 mmol/L Na-orthovanadate, 1 mmol/L Na-F, 10 mmol/L glycerophosphate, 50 μmol/L ATP, 10 μCi [γ-32P]ATP (3,000 Ci/mmol; NEN Dupont, Boston, MA), and 2 μg of glutathione S-transferase (GST)-tagged RB. After incubation at 30°C for 30 minutes with occasional mixing, the samples were boiled in sodium dodecyl sulfate (SDS) sample buffer and resolved by 12% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) followed by autoradiography. For Western blot analysis, 20 μg total cell extract was separated by SDS-PAGE (7.5% to 15%) and transferred to polyvinylidene fluoride (PVDF) membranes (Dupont, Wilmington, DE). Immunoblots were developed by use of ECL (Amersham, Arlington Heights, IL).
Transient transfections and fluorescence-activated cell sorting (FACS) analysis.
Early log-phase–growing U937 cells were transiently cotransfected with 10 μg pCMV5 vector with or without indicated insert and 8 μg pGreen Lantern-1, a plasmid containing the reporter gene Green Fluorescent Protein (GFP; GIBCO/BRL, Grand Island, NY). Cells were harvested, washed twice with phosphate-buffered saline (PBS), resuspended in 400 μL RPMI-1640, and electroporated (2,800 μF, R4, and 250 V) in 4-mm cuvettes and diluted in 20 mL RPMI-1640 complete medium containing 10% fetal calf serum (FCS). Forty-eight hours later, cells were harvested and analyzed by FACS analysis (Becton Dickinson, Mountain View, CA) using a phycoerythrin (PE)-conjugated CD11b antibody (Caltag, Burlingame, CA). For MCF-7 transfections, 1 × 107 60% to 80% confluent MCF7 cells were cotransfected with 2 μg cytomegalovirus (CMV)-GFP and 8 μg empty CMV5 vector or CMV5-driving specific CKIs (ie, p21, 27, p15, and p18) using electroporation (1,000 μF, R4, and 100 V). Forty-eight hours after electroporation, cells were harvested washed twice with PBS and resuspended in minimum essential medium (MEM) without additions. Nuclei were prepared from the GFP-positive cells, which were sorted using a cell sorter (Becton Dickinson). The DNA content of the nuclei was determined by flow cytometry.
Immunofluorescence.
Indirect immunofluorescence was performed on transiently transfected U937 cells using an unlabeled mouse antihuman CD11b primary antibody (Becton Dickinson) that was detected by a Texas red-conjugated donkey antimouse antibody (Jackson ImmunoResearch Labs, West Grove, PA) and the autofluorescent signal of the green fluorescent protein. U937 cells were transiently cotransfected with CMV-GFP and CMV-5 vector with or without insert as described above. Cells were harvested, washed with PBS + 1% bovine serum albumin (BSA) and incubated in the dark at 4°C with an unlabeled mouse antihuman CD11b primary antibody. After 30 minutes, the cells were washed again in PBS + 1% BSA and incubated with a Texas red-conjugated donkey antimouse antibody diluted 1:100. After 3 final washing steps with PBS + 1% BSA, the cells were concentrated and spun on a glass slide using a cytospin. Fluorescent signals were detected using a UV microscope.
RESULTS
Effect of 1,25(OH)2D3 on cell growth and differentiation of U937 cells.
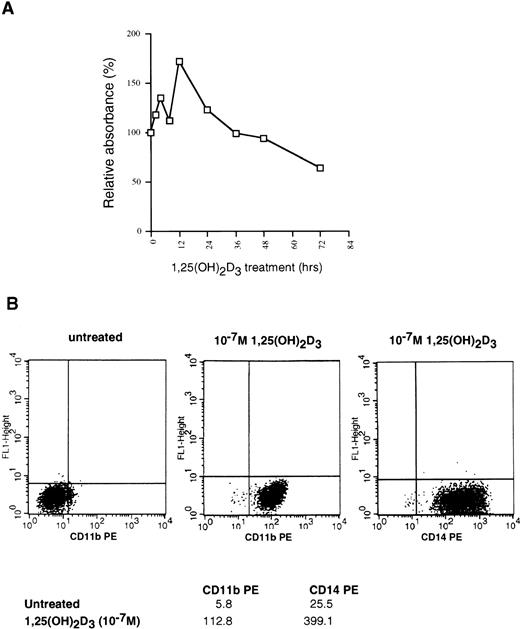
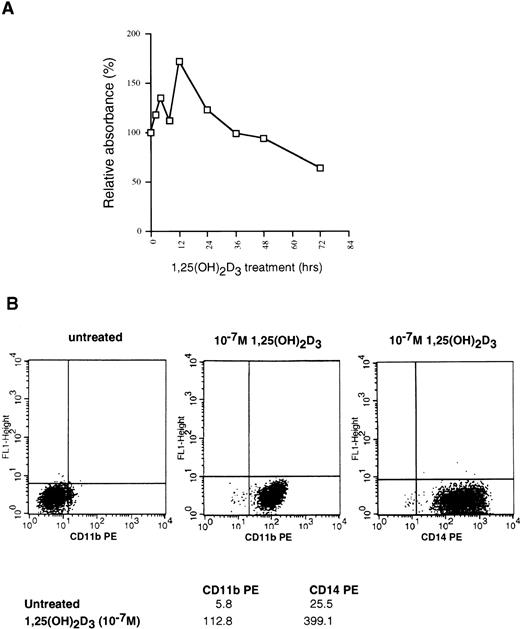
U937 cells are myelomonocytic cells that can be induced to differentiate into monocyte/macrophages by several agents, including dimethyl sulfoxide, 12-O-tetradecanoylphorbol-13-acetate, retinoic acid, and 1,25(OH)2D3. To begin an investigation of the relationship between cell cycle arrest and differentiation in these cells as conferred by 1,25(OH)2D3, we initially sought to confirm that our U937 cells responded to treatment with this ligand. To examine growth inhibition by 1,25(OH)2D3, we used a colorimetric assay that quantifies the number of viable cells in proliferation by measuring the conversion of a substrate, MTS, into formazan by dehydrogenase enzymes found in metabolically active cells. The quantity of formazan, measured by the absorbance at 490 nm, is directly proportional to the number of living cells in culture. The percentage of growth inhibition caused by 1,25(OH)2D3 addition to U937 cells over a 72-hour period, relative to untreated controls, is shown in Fig 1A for each time point. Surprisingly, U937 cells exhibited two closely linked proliferative bursts in the first 12 hours after hormone treatment, after which growth was inhibited up to 50%. This transient increase in proliferation followed by inhibition is highly characteristic of these cells, because it was consistently observed in six such growth curves (data not shown). Growth arrest, beginning 36 hours after ligand addition, occurs from a block at G1, with a substantial accumulation of cells in G1 not evident until 72 hours after 1,25(OH)2D3treatment.22 As shown in Fig 1B, continuous exposure to the ligand for 72 hours elicited a pronounced increase in the expression of the monocyte/macrophage-specific markers CD11b and CD14, consistent with the notion that growth arrest is closely linked to differentiation in this cell type.
Effects of 1,25(OH)2D3 on U937 cells. (A) Proliferation assay. Early log-phase growing U937 cells were treated with 1 × 10−7 mol/L 1,25(OH)2D3 or ethanol (no ligand control) for 0 to 72 hours. At the indicated timepoints, cells were harvested and incubated with MTS/PMS and absorbance at 490 nm determined, as described in Materials and Methods. All time points were assayed in triplicate and expressed as the percentage of inhibition relative to untreated controls. (B) Differentiation assay. 1,25(OH)2D3- or ethanol-treated cells were harvested after 72 hours and incubated with PE-conjugated CD11b or CD14 antibodies and analyzed for positive staining by FACS. Mean fluorescent intensities for each antibody are shown at the bottom of the graphs.
Effects of 1,25(OH)2D3 on U937 cells. (A) Proliferation assay. Early log-phase growing U937 cells were treated with 1 × 10−7 mol/L 1,25(OH)2D3 or ethanol (no ligand control) for 0 to 72 hours. At the indicated timepoints, cells were harvested and incubated with MTS/PMS and absorbance at 490 nm determined, as described in Materials and Methods. All time points were assayed in triplicate and expressed as the percentage of inhibition relative to untreated controls. (B) Differentiation assay. 1,25(OH)2D3- or ethanol-treated cells were harvested after 72 hours and incubated with PE-conjugated CD11b or CD14 antibodies and analyzed for positive staining by FACS. Mean fluorescent intensities for each antibody are shown at the bottom of the graphs.
Induction of the CKIs and cyclins by 1,25(OH)2D3.
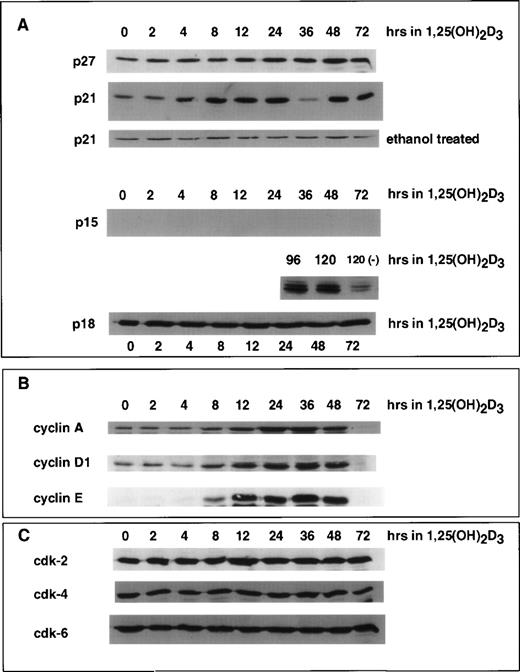
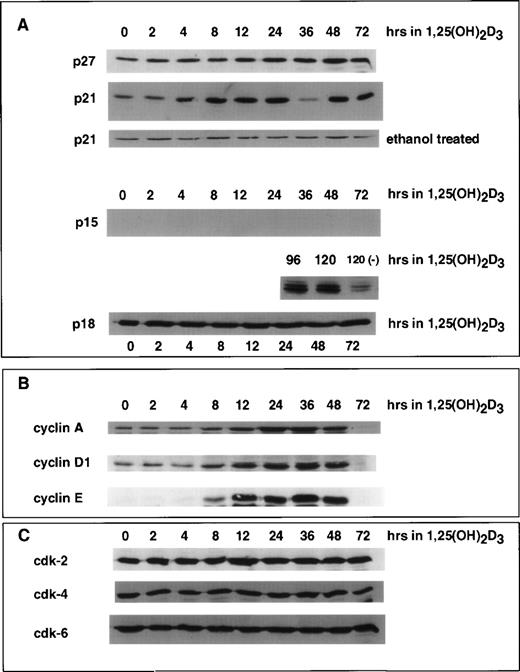
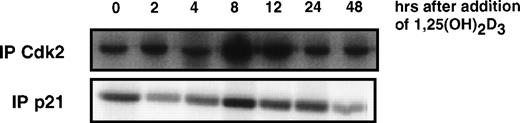
We previously showed that differentiation of U937 cells by both 1,25(OH)2D3 and retinoic acid is accompanied by an increase in the levels of p21 and p27 mRNA and protein.18 22 In the case of p21, this increase is due to a direct activation of transcription by the cognate nuclear receptors for these ligands, VDR and RAR, respectively, activating at specific DNA elements within the p21 promoter. As determined by immunoblotting shown in Fig 2A, the time course of 1,25(OH)2D3 induction of p21 protein was rapid and detectable within 4 hours after the addition of hormone and remained elevated through the 72 hours of treatment. Whole cell extracts isolated from control cells treated with ethanol showed constant basal expression levels of p21 through 72 hours of growth. p27 protein did not increase in response to 1,25(OH)2D3 until 12 to 24 hours after the addition of ligand. Interestingly, an increase in p15 protein was undetectable through 72 hours of treatment. Only after 96 hours could we detect an induction of p15, and this increase was quite strong. In contrast, p18 protein levels remained unchanged over the entire period of 1,25(OH)2D3 treatment.
Effect of 1,25(OH)2D3 on the levels of CKIs (A), cyclins (B), and CDKs (C) in U937 cells. Whole cell extracts isolated from cells grown in the absence or presence of 1,25(OH)2D3 (1 × 10−7 mol/L) for 0 to 72 hours were analyzed by Western blot. Twenty micrograms of whole cell extract was used in each lane and probed with antibodies to the indicated proteins. As a control, cells were grown for 72 hours in the absence of ligand (ethanol alone), and a representative Western blot probed with p21 antibody is shown in (A). Note that for p15 protein, samples were assayed up to 120 hours of treatment with ligand.
Effect of 1,25(OH)2D3 on the levels of CKIs (A), cyclins (B), and CDKs (C) in U937 cells. Whole cell extracts isolated from cells grown in the absence or presence of 1,25(OH)2D3 (1 × 10−7 mol/L) for 0 to 72 hours were analyzed by Western blot. Twenty micrograms of whole cell extract was used in each lane and probed with antibodies to the indicated proteins. As a control, cells were grown for 72 hours in the absence of ligand (ethanol alone), and a representative Western blot probed with p21 antibody is shown in (A). Note that for p15 protein, samples were assayed up to 120 hours of treatment with ligand.
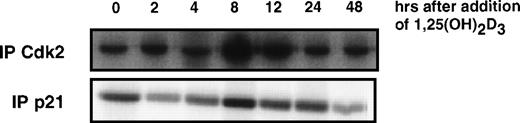
Because cell cycle arrest can be accompanied by increases in CKIs and/or decreases in cyclins or CDKs, we also analyzed the protein levels of various CDKs and cyclins after the addition of 1,25(OH)2D3 to U937 cells, as shown in Fig 2B and C. Analysis of the relative levels of these proteins by immunoblotting showed that, whereas the levels of CDK2, CDK4, and CDK6 were unaffected by 1,25(OH)2D3, there was an initial increase in cyclin A, D1, and E beginning 8 to 24 hours after the addition of the ligand, followed by a decrease beginning at 48 hours that lead to essentially undetectable levels at 72 hours (Fig2B). Because the early increase in cyclin levels was not expected, functional activity of the cyclin/CDK complexes was assayed at various times after the addition of 1,25(OH)2D3. As shown in Fig 3, Cdk2-associated kinase activity, which is rate-limiting for progression into S-phase,1 in fact exhibited a dramatic increase in activity 8 hours after the addition of 1,25(OH)2D3, which was followed by a loss of activity 12 hours after treatment. Notably, the increase and subsequent loss of Cdk2 kinase activity closely followed the kinetics of cell growth as determined in Fig 1A. However, this pattern was not due to changes in Cdk2 protein levels, because CDK levels were unaffected by 1,25(OH)2D3 (Fig 2C). Rather, the pattern in the kinase activity correlates with both the early increase and subsequent loss of cyclins, especially cyclins A, D1, and E (Fig 2B), as well as the more gradual increase in p21 and p27 beginning 4 to 12 hours posttreatment, respectively (Fig 2A). The initial increase in p21 levels may in fact relate more to the early proliferative burst observed in these cells, because p21 protein levels increase much earlier than p27 after 1,25(OH)2D3 addition. In addition, an initial increase in p21-associated kinase activity was detected 8 hours after the addition of 1,25(OH)2D3 (Fig 3). These data support the notion that p21 can act both as a positive and negative factor in cell growth, where at low levels, an initial role for p21 is as an assembly factor for kinase complexes, and at higher levels it acts to inhibit the kinase activity of cyclin/CDK complexes.25 26
1,25(OH)2D3 induces an initial increase followed by a rapid decrease in Cdk2- and p21-associated kinase activities in U937 cells. (A) Cdk2- or (B) p21-containing complexes were immunoprecipitated from U937 lysates at the indicated times after 1,25(OH)2D3 exposure using anti-Cdk2 or p21 antibodies, and subsequent kinase reactions were performed using GST-RB as a substrate.
1,25(OH)2D3 induces an initial increase followed by a rapid decrease in Cdk2- and p21-associated kinase activities in U937 cells. (A) Cdk2- or (B) p21-containing complexes were immunoprecipitated from U937 lysates at the indicated times after 1,25(OH)2D3 exposure using anti-Cdk2 or p21 antibodies, and subsequent kinase reactions were performed using GST-RB as a substrate.
Role of CKIs in the induction of U937 differentiation.
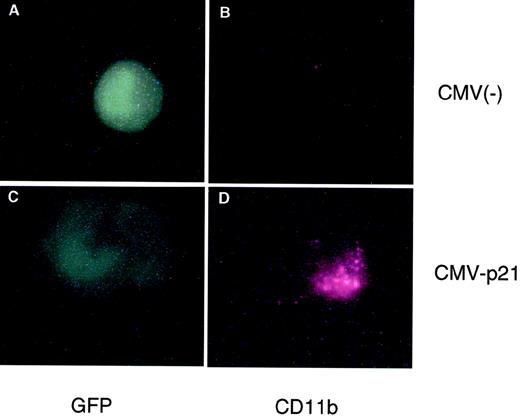
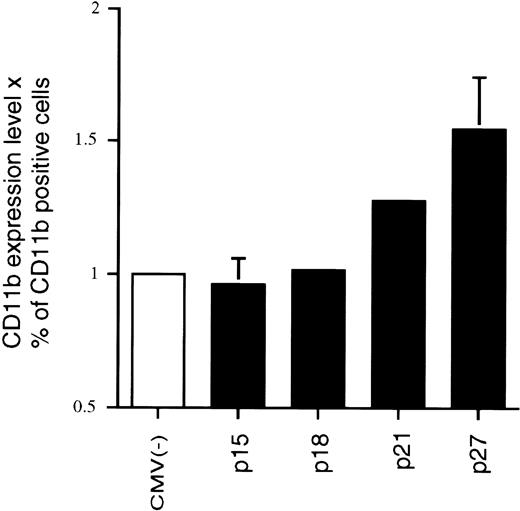
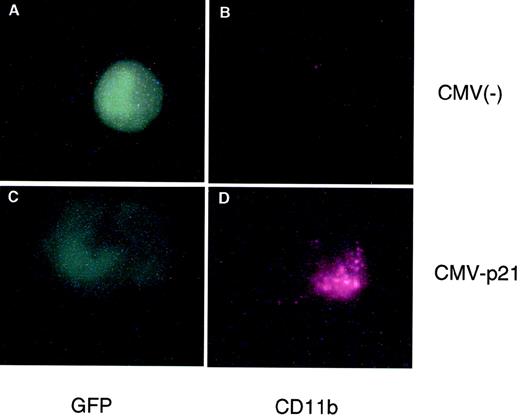
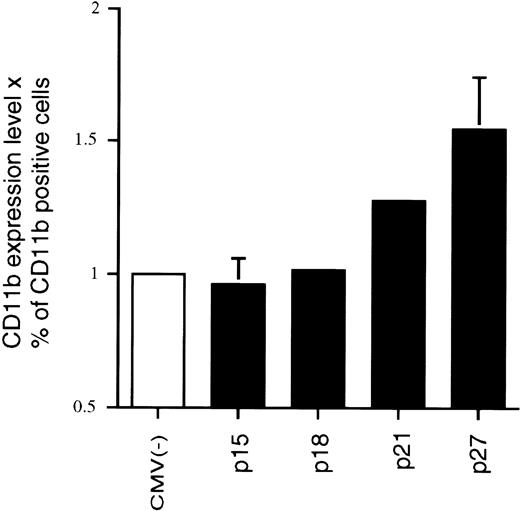
We originally reported that the correlation between 1,25(OH)2D3-induced differentiation of U937 cells and the accompanying increase in p21 and p27 levels appeared to be causal; that is, overexpression of these CKIs can in fact lead to a certain level of macrophage differentiation, as measured by increases in macrophage-specific markers CD11b or CD14.22 23 Because p15 levels also increase in response to 1,25(OH)2D3, although with a delayed kinetics, we wondered if Ink4 CKIs could also facilitate differentiation of U937 cells and if there were additive or cooperative effects between Cip/Kip and Ink4 CKIs. To address this question, exponentially growing U937 cells were cotransfected by electroporation with a CMV-driven expression plasmid containing p15, p18, p21, or p27, together with a CMV-driven GFP-expressing plasmid. The latter plasmid was used to select specifically for transfected cells by only analyzing GFP and CD11b doubly transfected cells, as measured by flow cytometry analysis. Figure 4 shows a cell cotransfected with CMV-GFP and CMV-empty vector [CMV(−); Fig 4A and B] that was GFP-positive but did not express CD11b, indicating that CMV(−) was not able to induce the expression of CD11b in U937 cells. The lower panels (Fig 4C and D) show a cell that was cotransfected with CMV-p21 and CMV-GFP. This cell was both GFP- and CD11b-positive and, moreover, showed morphological characteristics of a differentiated cell (Fig 4D). Qualitatively similar results were obtained when CD14 expression was determined (data not shown). This result confirms that ectopic overexpression of p21 alone, independent of 1,25(OH)2D3, is able to induce expression of macrophage-specific markers in U937 cells. Quantitatation of these data showed that both the percentage of CD11b-positive cells as well as the mean level of CD11b expression per cell (mean fluorescent intensity) was affected by the increased expression of p21 (Fig 5) relative to the CMV empty vector. We therefore quantitated the consequence of overexpression of the various CKIs on CD11b levels as the product of the mean CD11b expression level per cell and the percentage of CD11b-positive cells, as shown in Fig 5. As is apparent from the quantitations, ectopic overexpression of the Ink4 family members p15 and p18 had no effect on CD11b levels as compared with the CMV control vector. However, p21 and p27 were able to induce an increase in the expression of the differentiation marker (Fig 5). Combinations of the two Ink4, or Cip/Kip inhibitors, had no or little additive or cooperative effect, respectively (data not shown). These results suggest that, in terms of their abilities to facilitate differentiation, Ink4 CKIs play roles that are quite distinct from Cip/Kip CKIs.
Transient overexpression of p21 induces macrophage differentiation of U937 cells. Shown is an immunofluorescent double staining of a transfected cell. Exponentially growing U937 cells were cotransfected with an empty CMV expression vector (top panel) or a CMV vector containing p21 cDNA (bottom panel) together with a CMV plasmid expressing Green Lattern Protein, a modified version of GFP. Green fluorescence was used as a marker for transfected cells (A and C). CD11b expression was detected using a Texas red-conjugated antimouse secondary antibody (B and D). Note the morphological change evident in the CMV-p21-transfected cell (C) compared with the CMV(−) cell (A).
Transient overexpression of p21 induces macrophage differentiation of U937 cells. Shown is an immunofluorescent double staining of a transfected cell. Exponentially growing U937 cells were cotransfected with an empty CMV expression vector (top panel) or a CMV vector containing p21 cDNA (bottom panel) together with a CMV plasmid expressing Green Lattern Protein, a modified version of GFP. Green fluorescence was used as a marker for transfected cells (A and C). CD11b expression was detected using a Texas red-conjugated antimouse secondary antibody (B and D). Note the morphological change evident in the CMV-p21-transfected cell (C) compared with the CMV(−) cell (A).
Effect of overexpression of various CKIs on the expression of the monocyte/macrophage-specific membrane marker CD11b, independent of 1,25(OH)2D3. Transfections and FACS analyses were performed as described in Fig 4 and quantitated by determining the product of the mean CD11b expression levels and the percentage of CD11b-positive cells. Each value is the mean of a minimum of four independent experiments. The effect of the empty CMV control vector was set at 1 for each individual experiment to enable comparison of different experiments.
Effect of overexpression of various CKIs on the expression of the monocyte/macrophage-specific membrane marker CD11b, independent of 1,25(OH)2D3. Transfections and FACS analyses were performed as described in Fig 4 and quantitated by determining the product of the mean CD11b expression levels and the percentage of CD11b-positive cells. Each value is the mean of a minimum of four independent experiments. The effect of the empty CMV control vector was set at 1 for each individual experiment to enable comparison of different experiments.
Effect of 1,25(OH)2D3 on the breast cancer cell line MCF-7.
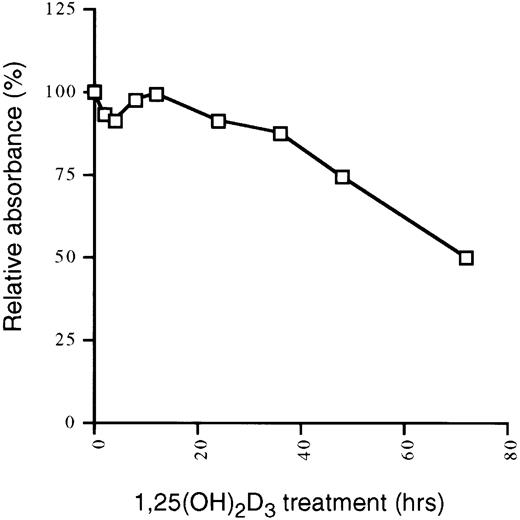
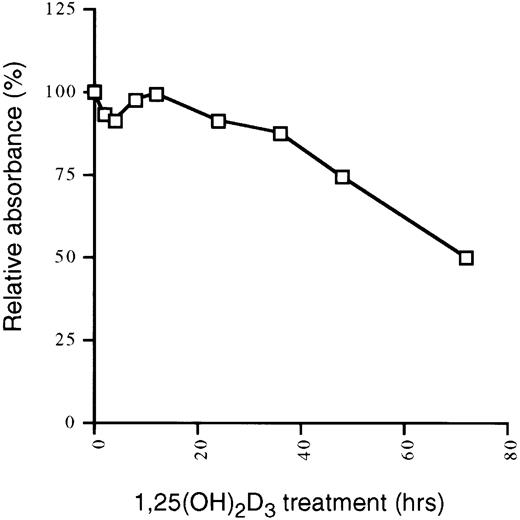
The preceding results demonstrated that a transient proliferative burst precedes growth arrest and differentiation in U937 cells in response to 1,25(OH)2D3. The increase in proliferation is accompanied by an increase in cyclins, and the subsequent growth arrest and differentiation coincides with an increase in CKI levels. We next asked if this pattern is also observed in cells that are only growth inhibited, but not differentiated, by 1,25(OH)2D3. MCF-7 cells are a breast tumor cell line that are growth inhibited by physiological concentrations of 1,25(OH)2D3 (Fig6). To compare the response of this cell line to the de-differentiated U937 line, we again examined expression levels of different components of the cell cycle by immunoblotting whole cell extracts from MCF7 cells grown in the presence or absence of 1,25(OH)2D3for 0 to 48 hours. As shown in Fig 7A, levels of both p21 and p27, but not p15, were elevated 8 to 24 hours after the addition of the hormone. As was the case for U937 cells, Cdk2, Cdk4, and Cdk6 were not affected by the addition of 1,25(OH)2D3, but in contrast to the U937 pattern, cyclin A, cyclin D1, and cyclin E protein levels were also not affected by the treatment (Fig 7B and C). Thus, growth inhibition of these cells is accompanied only by an increase in p21 and p27, which is synchronous with the accumulation of cells in G1 (data not shown). The lack of any kind of proliferative burst, as seen in differentiating U937 cells, is reflected in the constancy in the cyclin levels over the 48 hours of treatment. These results suggest that cells that differentiate require an initial proliferative burst, accompanied by transient increases in cyclin levels. Cells that only experience a cell cycle withdrawal do so in part through late increases in CKIs such as p21 and p27, without accompanying alterations in cyclin levels.
1,25(OH)2D3 inhibits the growth of MCF-7 cells. MCF-7 cells seeded at 1 × 104 cells/mL in 96-well plates were treated with 1,25(OH)2D3 (1 × 10−7 mol/L) for 0 to 72 hours. The absorbance at 490 nm was measured 2 hours after the addition of 20 μL MTS/PMS. Cells were assayed in triplicate and data were expressed as the percentage of inhibition relative to untreated controls for each individual timepoint. The percentage of dead cells was less than 5% at each time point measured.
1,25(OH)2D3 inhibits the growth of MCF-7 cells. MCF-7 cells seeded at 1 × 104 cells/mL in 96-well plates were treated with 1,25(OH)2D3 (1 × 10−7 mol/L) for 0 to 72 hours. The absorbance at 490 nm was measured 2 hours after the addition of 20 μL MTS/PMS. Cells were assayed in triplicate and data were expressed as the percentage of inhibition relative to untreated controls for each individual timepoint. The percentage of dead cells was less than 5% at each time point measured.
Effect of 1,25(OH)2D3 on the levels of CKIs (A), cyclins (B), and CDKs (C) in MCF-7 cells. Whole cell extracts isolated from cells grown in the absence or presence of 1,25(OH)2D3 (1 × 10−7 mol/L) for 0 to 48 hours were analyzed by Western blot. Twenty micrograms of whole cell extract was used in each lane and probed with antibodies to the indicated proteins. As a control, cells were grown for up tp 48 hours in the absence of ligand (ethanol alone), and p21 levels were determined (shown in [A]).
Effect of 1,25(OH)2D3 on the levels of CKIs (A), cyclins (B), and CDKs (C) in MCF-7 cells. Whole cell extracts isolated from cells grown in the absence or presence of 1,25(OH)2D3 (1 × 10−7 mol/L) for 0 to 48 hours were analyzed by Western blot. Twenty micrograms of whole cell extract was used in each lane and probed with antibodies to the indicated proteins. As a control, cells were grown for up tp 48 hours in the absence of ligand (ethanol alone), and p21 levels were determined (shown in [A]).
Role of CKIs in cell cycle arrest of MCF-7 cells.
Cell cycle distribution analysis showed that addition of 1,25(OH)2D3 to exponentially growing MCF7 cells induced cell cycle arrest primarily in G1 (Table 1). Are the CKIs each sufficient to induce this cell cycle arrest independent of 1,25(OH)2D3, as we observed in U937 cells? Exponentially growing MCF7 cells were cotransfected, using electroporation, with a CMV-driven vector driving p15, p21 or p27 expression, together with CMV-GFP. The sorted GFP-positive cells were then subjected to cell cycle distribution analysis by flow cytometry. Table 1 demonstrates that, relative to the CMV(−) control, ectopically overexpressed p15, p21, or p27 were able to induce a strong cell cycle arrest exclusively in G1 and independent of the presence of 1,25(OH)2D3 in MCF7 cells. These results indicate that both Ink4a and Cip/Kip CKIs can facilitate cell cycle arrest in MCF7 cells. In contrast, it appears that the link between growth arrest and induction of differentiation may be a feature of only the Cip/Kip class of CKIs, because neither p15 nor p18 could affect differentiation of U937 cells (Fig 5).
DISCUSSION
In this study, we have presented evidence demonstrating that cell cycle arrest accompanies differentiation in myeloid leukemic U937 cells treated with 1,25(OH)2D3. This effect is facilitated in part by elevations in the levels of CDK inhibitors p21 and p27, which in the case of p21, we previously demonstrated to be a direct transcriptionally regulated target of 1,25(OH)2D3 through its cognate nuclear receptor VDR. Paradoxically, growth arrest and differentiation of this cell line was found to be preceded by two transient proliferative bursts that were accompanied by increases in the levels cyclin A, D1, and E, as well as p21-associated kinase activity, shortly after the addition 1,25(OH)2D3. At the same time, p21 protein levels were also observed to increase. These seemingly contradictory effects in cells poised to differentiate, as opposed to cells that can only cell cycle arrest, such as MCF-7, suggest that differentiation in response to agents such as 1,25(OH)2D3 require a rapid, transient increase in proliferation before arresting in G1 and subsequently differentiating. Remarkably, we first detected the upregulation of both the p21 and cyclin A genes in a differential screen we performed in U937 cells treated for 4 hours with 1,25(OH)2D3.22,23 Recently, Meyyappan et al27 reported that cyclin D2 mRNA levels increased in both primary and established murine fibroblasts upon contact inhibition and serum starvation. They speculated that cyclin D2 sequesters CDK2 into an inactive complex, preventing it from being activated by other cyclins.
Alterations in cyclin levels were not observed in a breast cancer cell line, MCF-7, and two other cell lines that are strongly growth inhibited by 1,25(OH)2D3, LNCaP (prostate tumor) and HeCAT (keratinocyte) (data not shown). In these cells, only increases in p21 and p27 were detected. An additional difference we found between growth arrest and the subsequent induction of differentiation is that both classes of CKIs we tested (ie, Cip/Kipv Ink4a) were able to confer growth arrest to differentiated MCF-7 cells when transiently overexpressed (Table 1). In contrast, only p21 or p27, but not p15 or p18, could facilitate growth arrest and differentiation when overexpressed in U937 cells.
It is tempting to speculate that this difference in functionality relates to the early increase in p21 (and perhaps p27) levels after the addition of 1,25(OH)2D3 to U937 cells, which in turn correlates to both the proliferative burst detected in these cells within 12 hours of hormone addition and the proposed role of these cell cycle inhibitors as assembly factors of cyclin-CDK complexes at low stoichiometries. It has been reported that p21 and p27 can be found in proliferating cells as part of active kinase complexes.25,28 At low concentrations, p21 may promote the assembly of active kinase complexes, whereas at higher concentrations it inhibits its activity.26 Perhaps a transient increase in proliferation is a requisite event that sets up a cellular milieu leading to differentiation. In fact, in primary mouse keratinocytes treated with calcium, p21 levels increase in a proliferative, predifferentiation phase, and then decrease or disappear as cells arrest and begin to differentiate.29 This is similar to the p21-associated kinase activity pattern we observed in U937 cells after 1,25(OH)2D3 treatment (Fig 3). Thus, p21 appears to be important in the early stages of differentiation, including, perhaps, enhancing cell proliferation, and then must step aside to permit cells to proceed with their differentiating program. In cells that are only rendered senescent, such as MCF-7, the scenario may be less complex, where upregulation of CDK inhibitors leading to arrest in G1 appears to suffice.
ACKNOWLEDGMENT
The authors thank T. Delohery and A. Koff for important suggestions and discussions. We are indebted to J. Massagué, K. Nilsson, and M. Uskokovic for various reagents used here.
Supported by National Institutes of Health Grants No. DK-45460 and DK-52621 to L.F. and by MSKCC Support Grant No. CA-08748. N.Y.R. was supported in part by a fellowship from the Netherlands Organization for Scientific Research (NWO). L.F. is a Scholar of the Leukemia Society of America.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Leonard P. Freedman, PhD, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, New York, NY 10021; e-mail: l-freedman@ski.mskcc.org.

![Fig. 7. Effect of 1,25(OH)2D3 on the levels of CKIs (A), cyclins (B), and CDKs (C) in MCF-7 cells. Whole cell extracts isolated from cells grown in the absence or presence of 1,25(OH)2D3 (1 × 10−7 mol/L) for 0 to 48 hours were analyzed by Western blot. Twenty micrograms of whole cell extract was used in each lane and probed with antibodies to the indicated proteins. As a control, cells were grown for up tp 48 hours in the absence of ligand (ethanol alone), and p21 levels were determined (shown in [A]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/8/10.1182_blood.v93.8.2721/5/m_blod40828007w.jpeg?Expires=1768091291&Signature=B5NESiNPi19OQholcaAQjKZ8D5Oj0GZ-Y7N9Xi0VJHjE2~r7mHiSvP1Y8IsPQsfkw0sj2u5V9yHYqX4Avb6A1GUtvrtmC2n1xD-O9mCOj6HkdrvO9a9yLaE4rNP7goK5cuEpyi4DrdGfnZXaFIFx6Kd4CHAuqsgFYcONZPMwXAx2LkhjBNwYgi1bwmOJ8pBfheXBiWC3yXasD~6IcANnBIKNdhOjU4-7NUsbwtSZkZ~yvrDfkiXc61Xb1WEBZdIlkgurn~8jG6GvbLdmgI3dgBPjKffmaWoq3iD4SshCbhaXEnm~Z~DH4wXSVnPj2h-odFRF-O0mru0C40YdbHCeng__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 7. Effect of 1,25(OH)2D3 on the levels of CKIs (A), cyclins (B), and CDKs (C) in MCF-7 cells. Whole cell extracts isolated from cells grown in the absence or presence of 1,25(OH)2D3 (1 × 10−7 mol/L) for 0 to 48 hours were analyzed by Western blot. Twenty micrograms of whole cell extract was used in each lane and probed with antibodies to the indicated proteins. As a control, cells were grown for up tp 48 hours in the absence of ligand (ethanol alone), and p21 levels were determined (shown in [A]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/8/10.1182_blood.v93.8.2721/5/m_blod40828007w.jpeg?Expires=1768091292&Signature=nUcFSV7wPuKTrzXjlAUkvInQyUWJCXtcJOEDZPk-3tCi1aSO0Eqn76DXTPzhdKtgyM6OGXvG8NWoUYhQoHVwc1cN8Xxmr192dcxiwZpXqJcF3agI39EUh~p6rVPtJLYM2S18~7ycnuxSHXKZiDiud4TZVRPFMHb6Lh4gZ0FIwzxc7VuZ8A~lcyqWB5byyCwiDwmoI2udgJR~jr9A0OyIL-1Nb2XoF6BUP8Uzes4VIYuNuMZkNBYbuMWmA9OOrjuA~nz55ZCV-d3xo1~FpARxastLfkMeT4GGo8tmZoPYxBFd8KnwPvgM3-cfpQtmZCPsBJe3Ej8Z0frENDOvYtQx3A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)