Abstract
When active bone marrow release is induced by inflammatory stimuli, it is associated with an increase in L-selectin expression on circulating polymorphonuclear leukocyte (PMN). This contrasts sharply with glucocorticoid-induced granulocytosis that is associated with decreased L-selectin expression on PMN. The present study was designed to determine if the reduced L-selectin expression observed after glucocorticoid treatment is the result of suppression of L-selectin synthesis in the bone marrow. New Zealand white rabbits treated with dexamethasone (2.0 mg/kg, a single dose intravenously) were shown to have decreased L-selectin expression on circulating PMN 12 to 24 hours after treatment (P < .01) with a return to baseline levels by 48 hours. When dexamethasone was administered 48 hours after the bone marrow PMN were pulse labeled with the thymidine analogue, 5′-bromo-2′-deoxyuridine (BrdU), L-selectin expression on BrdU-labeled PMN released from the bone marrow was decreased (P< .01). Dexamethasone decreased L-selectin expression on segmented PMN in the bone marrow (P < .05) but not on PMN already in the circulation. We conclude that glucocorticoids decrease L-selectin expression on circulating PMN by downregulating L-selectin expression in the maturation pool of bone marrow and speculate that this is an important glucocorticoid effect that influences the recruitment of PMN into inflammatory sites.
L-SELECTIN HAS A crucial role in the initial attachment of circulating polymorphonuclear leukocyte (PMN) to vascular endothelium during the initiation of PMN recruitment into a systemic inflammatory site.1-3 L-selectin expression is low in the mitotic pool, increases as they mature in the postmitotic pool of bone marrow,4,5 and is constitutively expressed on circulating PMN.6 PMN released from the bone marrow by inflammatory stimuli such as complement fragments, pneumonia, and endotoxin express higher levels of L-selectin than their circulating counterpart,7-9 and they progressively lose this L-selectin as they age in the circulation.10
Glucocorticoids also induce the release of PMN from the bone marrow,11-13 but recent studies have established that, in contrast to inflammatory stimuli, this release is associated with a decrease in L-selectin expression on bovine, rat, and human PMN.14-17 The L-selectin expression on leukocytes is regulated by proteolytic cleavage from the cell surface3that may occur in the presence18,19 or absence of cell activation in vitro.20-22 Rapid shedding of L-selectin occurs in vivo after stimuli such as endotoxin and C5a.9,23Glucocorticoid treatment, on the other hand, results in a slow decrease in the L-selectin expression on circulating PMN that only becomes detectable at 8 hours after administration in intact animals or human14,16 and cannot be demonstrated in vitro.20,24 25
The present study is based on the hypothesis that glucocorticoids decrease the L-selectin expression on circulating PMN by releasing PMN that express low levels of L-selectin from the bone marrow. To test this hypothesis, we studied the effects of dexamethasone on the L-selectin expression on PMN in the bone marrow, on PMN newly released from the bone marrow into the circulation by glucocorticoids, and on PMN already in the circulation.
MATERIALS AND METHODS
Animals
Female New Zealand White rabbits (n = 38; weight, 2.3 to 3.2 kg) were used in this study. They were anesthetized by a subcutaneous injection of fentanyl (0.02 mg/kg) and droperidol (1.0 mg/kg) at each time point of blood collection or intravenous (IV) injection, and 2% to 2.5% of halothane and a local injection of 0.75% of bupivacaine were added at each time point of bone marrow sampling. All these studies were approved by the Animal Experimentation Committee of the University of British Columbia.
Study Design
Effect of dexamethasone on L-selectin expression on all PMN in the circulation.
Rabbits received 2.0 mg/kg of dexamethasone in 3 mL of saline (n = 7) or 3 mL of saline (n = 6) IV. Blood samples were obtained from the central ear artery at 24 hours and just before dexamethasone or saline injection and at 2, 4, 6, 12, 24, 48, 72, 96, 120, 144, and 168 hours after dexamethasone or saline injection.
Effect of dexamethasone on L-selectin expression on PMN already in the circulation.
To determine if dexamethasone had any effect on the L-selectin expression on PMN already in the circulation, the PMN of donor rabbits (n = 5) were labeled with the thymidine analogue, 5′-bromo-2′-deoxyuridine (BrdU; Sigma Chemical, St Louis, MO), at 25 mg/kg/d for 7 days and the BrdU-labeled PMN harvested on day 8 were transferred to recipient rabbits as 15 mL of whole blood. This method of labeling results in approximately 80% BrdU-labeling of PMN and was previously described in more detail.26 The recipients received 2.0 mg/kg of dexamethasone (Boucherville, Quebec, Canada) in 3 mL of saline (n = 5) or 3 mL of saline (n = 4) IV 1 hour after the blood transfusion. This time point was selected to administer dexamethasone because previous studies from our laboratory using a similar method showed that labeled PMN infused into rabbits reached stable counts in the circulation 1 hour after a blood transfusion.26 Blood samples were obtained from the central ear artery just before dexamethasone or saline injection and at 2, 4, 6, 12, and 24 hours after dexamethasone or saline injection. We have selected these time points because the effects of dexamethasone on demargination of PMN were shown to reach a peak at 6 hours after treatment and to disappear 12 hours after treatment in Nakagawa et al.27
Effect of dexamethasone on L-selectin expression on PMN released from the bone marrow.
The PMN in the bone marrow of 12 rabbits were pulse labeled with IV administration of 100 mg/kg of BrdU. The BrdU was infused at a concentration of 10 mg/mL in normal saline over 15 minutes. Forty-eight hours later, either 2.0 mg/kg of dexamethasone in 3 mL of saline (n = 6) or 3 mL of saline (n = 6) was administered intravenously. This time point was selected because previous studies have shown a rapid release of BrdU-labeled PMN from the bone marrow27 28 48 hours after labeling. Blood samples were obtained from the central ear artery just before dexamethasone or saline injection and at 12 and 24 hours after dexamethasone or saline injection.
Effect of dexamethasone on L-selectin expression on PMN in the bone marrow.
A 0.2-mL sample of bone marrow was aspirated from the left iliac bone just before the intravenous injection of dexamethasone (2.0 mg/kg, n = 4) and from the right iliac bone 6 hours after dexamethasone injection using an 18-G spinal needle. This time point was selected because L-selectin on circulating PMN was still normal with little release of BrdU-labeled PMN from the marrow by dexamethasone.27 Smears were made immediately and allowed to air dry.
Flow Cytometry
Blood collected in EDTA tubes was used to immunolabel circulating PMN for the presence of surface L-selectin and CD18 with a commercially available kit (Coulter Clone; Coulter Immuno, Hialeah, FL). Briefly, 100 μL of whole blood was incubated for 10 minutes with either anti–L-selectin monoclonal antibody (MoAb) DREG-200 (kind gift of Dr E.C. Butcher, Stanford University School of Medicine, Palo Alto, CA) or the anti-CD18 MoAb 60.3 (kind gift of Dr J. Harlan, University of Washington, Seattle, WA). Nonimmune mouse IgG was used as negative control. After labeling with fluorescein isothiocyanate (FITC)-conjugated goat antimouse secondary antibodies, red blood cells were lysed, and the remaining leukocytes were washed twice and fixed with 1% paraformaldehyde. Flow cytometry was performed using analysis gates for PMN from typical forward and side angle light scattering (model, Profile Epics 2; Coulter Electronics, Hialeah, FL). We have used this whole blood method rather than isolated PMN to avoid possible cell activation associated with PMN purification.29 A total of 3,000 cells/specimen were evaluated, and the results are expressed as the mean fluorescence intensity.
Determination of BrdU-Labeled PMN in Circulation
Sample preparation.
One milliliter of blood collected in tubes containing acid-citrate-dextrose (ACD) was used to obtain leukocyte-rich plasma. Erythrocytes in the ACD blood sample were allowed to sediment for 25 to 30 minutes after the addition of an equal volume of 4% dextran (average molecular weight, 162,000; Sigma) in PMN buffer (138 mmol/L NaCl, 27 mmol/L KCl, 8.1 mmol/L Na2HPO47H20, 1.5 mmol/L KH2PO4, and 5.5 mmol/L glucose, pH 7.4). The resulting leukocyte-rich plasma was cytospun onto 3-aminopropryl-tri-ethoxysilane-coated slides by cytocentrifugation at 180g with a Cytospin 2 (Shandon Lab Products, Chestire, UK) for 4 minutes. The cytospin specimens were air-dried and stained using the alkaline phosphatase and anti-alkaline phosphatase (APAAP) method30 to determine the fraction of the BrdU-labeled PMN in each specimen.
Immunoenzymatic staining of PMN for BrdU.
Cytospin prepared from peripheral blood were stained for the presence of nuclear BrdU by the APAAP technique as previously descried.26 Briefly, the slides were fixed in methanol for 10 minutes and then digested at 37°C for 15 minutes in a 0.04% pepsin solution acidified to pH 2.5. Incubating slides in 2 N HCl at 37°C for 60 minutes denatured DNA in the samples. The 2 N HCl was neutralized by washing the slides three times with 0.1 mol/L borate buffer, pH 8.5, each for 10 minutes. The slides were incubated with 5% rabbit serum for 15 minutes before application of 2 μg/mL mouse MoAb against BrdU (DAKO Laboratories, Copenhagen, Denmark) for 60 minutes in a humidity chamber at room temperature. Nonimmune mouse IgG (5 μg/mL) and omission of the primary antibody were used as negative controls. A 1:20 dilution of rabbit antimouse IgG (DAKO) was applied for 30 minutes, followed by the antimouse IgG alkaline phosphatase-conjugated complex (DAKO) in a 1:50 dilution for 30 minutes. All antibodies were prepared in 50 mmol/L Tris hydrochloride and 150 mmol/L NaCl, pH 7.6 (TBS), with 1% bovine serum albumin, and slides were washed in 0.1% Tween 20 (Fisher Scientific, Fair Lawn, NJ) in TBS twice for 10 minutes between each antibody application. The alkaline phosphatase was developed for 20 minutes in 50 mL TBS at pH 8.7 after the addition of mixture of 0.5 mL of 4% sodium nitrite, 0.2 mL of 5% fuschin (Merck, Rahway, NJ) in 2 mol/L HCl, and 50 mg naphtol AS-BI phosphate (Sigma) dissolved in 0.3 mL N,N-dimethylformamide. Endogenous alkaline phosphatase was blocked by the addition of 17.5 mg levamisole (Sigma) to the color reaction. The preparations were counterstained with Mayer’s hematoxylin for 5 seconds, dehydrated through graded alcohol from 70% to 100% and xylene, and then mounted and evaluated on a Zeiss Universal Research light microscope (Model 2R; Zeiss, Oberkochen, Germany) at 400× magnification.
Number of BrdU-labeled PMN in circulation.
The total white blood cell (WBC) counts were determined on a model SS80 Coulter Counter (Coulter Electronics). Differential counts of PMN were obtained by counting 100 leukocytes in a randomly selected field of view on Wright’s-stained blood smears. Using these values, the number of BrdU-labeled PMN was calculated. The number of BrdU-labeled PMN in the circulation of each recipient was expressed as a percentage of the total number of labeled PMN originally infused and corrected for the calculated blood volume31 of the recipient in the following manner: %PMNBrdUcirc = (PMNcirc × BV × fractionPMNBrdUrecipient) × 100/PMNBrdUinfused (1), where %PMNBrdUcircrepresents the number of BrdU-labeled PMN in the circulation as a fraction of the total number of BrdU-labeled PMN infused; PMNcirc, the calculated number of PMN (per milliliter) in the circulation (WBC count × fraction of leukocyte that are PMN); BV, calculated blood volume (in milliliters); fractionPMNBrdUrecipient, the fraction of BrdU-labeled PMN on a cytospin of peripheral blood in the recipient; and PMNBrdUinfused, the number (per milliliter) of BrdU-labeled PMN infused (PMN count/mL × mL of blood infused × fractionPMNBrdU).
Determination of L-Selectin Expression on BrdU-Labeled PMN in Circulation
Double immunoenzymatic staining of PMN for L-selectin and BrdU.
Cells on cytospins were stained for the presence of both cell surface L-selectin (red) and nuclear BrdU (blue) using the APAAP and a modification of a previously described method.9 Briefly, surface L-selectin was stained for in the first part of the double staining procedure followed by nuclear BrdU staining. Cells were fixed in acetone for 10 minutes and incubated with 5% rabbit serum for 15 minutes before application of 5 μg/mL of DREG-200 for 60 minutes in a humidity chamber at room temperature. Rabbit antimouse IgG, followed by the antimouse IgG alkaline phosphatase-conjugated complex, was applied as described earlier. Slides were washed in TBS twice for 10 minutes between each antibody application. The alkaline phosphatase was developed and then fixed with 1% paraformaldehyde for 20 minutes. After a 10-minute wash, slides were incubated in methanol for 10 minutes at room temperature and then at 37°C for 15 minutes in a 0.08% pepsin solution acidified to pH 2.5. Denaturing in HCl and neutralizing of HCl was performed as described earlier. These steps were followed by the second APAAP procedure, in which mouse MoAb against BrdU was used as the primary antibody and 0.1% Tween 20 in TBS, pH 7.6, was used to wash slides. The alkaline phosphatase was developed with a commercially available kit, HistoMark Blue (Kirkegaard and Perry, Gaitherburg, MD), for 10 minutes in the dark. Slides were washed with tap water, mounted in an aqueous medium (Gelvatol), and analyzed on a light microscope.
Evaluation of L-selectin expression on BrdU-labeled PMN.
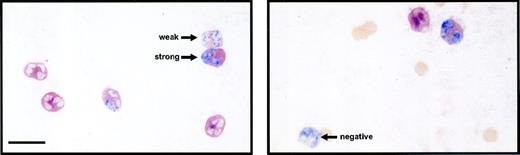
BrdU-labeled PMN was divided into three groups according to the intensity of surface staining of L-selectin using an arbitrarily designated grading system. Strong represents cells with more than 75% of their surface stained deep red, weak represents cells with positive and/or less than 75% of their surface stained deep red, and negative represents cells with no (background) red stain. The slides were coded and examined without knowledge of the group or the sampled time. Fields were selected in a systematic randomized fashion and 100 cells were evaluated per specimen. All cells of interest in a selected field were evaluated, except if the cell was broken or overlapping with other cells. This grading system was evaluated for both interobserver and intraobserver variability.
Determination of L-Selectin Expression on PMN in Bone Marrow
Immunoenzymatic staining of PMN for L-selectin.
Bone marrow smears were fixed in acetone for 10 minutes before immunocytochemical staining using the APAAP technique and DREG-200 to label L-selectin as described earlier. The preparations were counterstained with Mayer’s hematoxylin for 20 seconds, dehydrated through graded alcohol from 70% to 100% and xylene, mounted, and evaluated on a light microscope. One hundred cells in randomly selected fields were evaluated and graded according to the intensity of staining for L-selectin as strong, weak, or negative. Just segmented and band PMN were evaluated.
Statistical Analysis
All values are expressed as means ± SEM. Temporal changes in mean fluorescence intensity and BrdU-labeled PMN counts in the circulation were analyzed using an analysis of variance for repeated measurements. The interobserver and intraobserver variation of the grading the intensity of staining for L-selectin were evaluated by calculating the Pearson coefficient of mean-square contingency (R2) for each grade and expressing R2 as a fraction of the maximum possible value, R2max, which represent the value of the Pearson χ2 coefficient if the there is 100% agreement in the grading score between or within observers; thus, R2max = ±0.82.32 Results in immunocytochemical staining were analyzed using paired or two-sample t-test, and Bonferroni corrections were made for multiple comparisons. Statistical significance was defined as a P value of less than .05.
RESULTS
Effect of Dexamethasone on L-Selectin Expression on all PMN in the Circulation
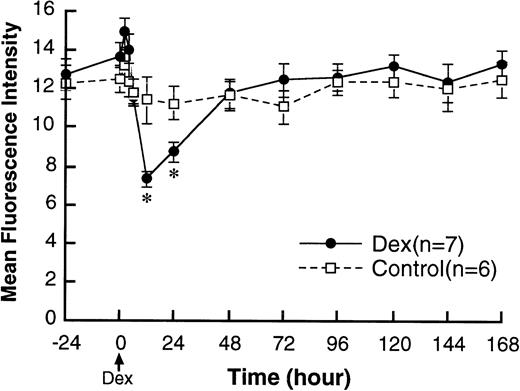
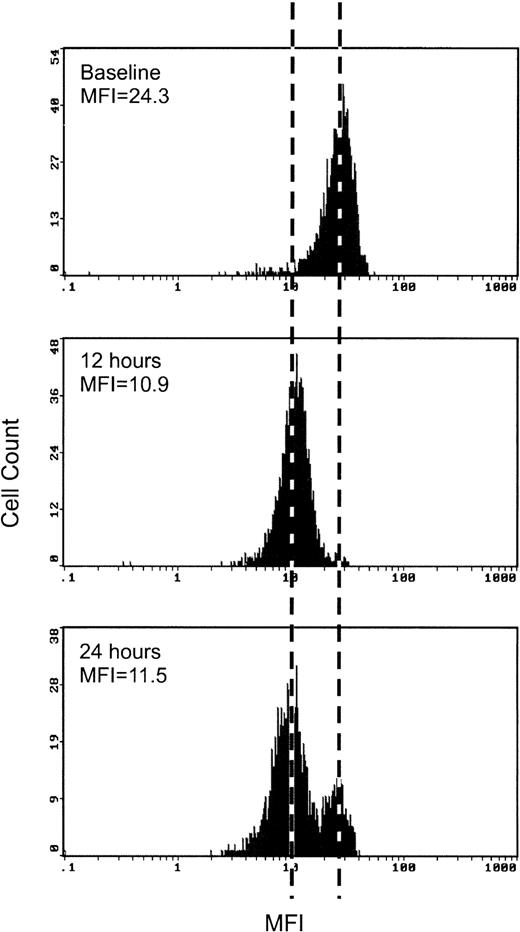
Dexamethasone decreased the mean fluorescence intensity of L-selectin on circulating PMN. The maximum effect was seen at 12 hours after dexamethasone treatment and L-selectin levels were back to pretreatment levels at 48 hours (Fig 1). Figure 2 shows a typical flow cytometry result of L-selectin measurement from one of the dexamethasone-treated rabbits. The curve was shifted to the left by dexamethasone at 12 hours after treatment. Although the mean fluorescence intensity was still low at 24 hours after treatment, there was a distinct double peak in the curve at 24 hours. A new population on PMN with normal baseline levels of L-selectin (right) appears, with still a significant population expressing low levels of L-selectin (left). CD18 was also measured as a marker of cell activation, but it did not change with dexamethasone treatment (data not shown).
Changes in L-selectin expression on PMN in the circulation after IV injection of either 2.0 mg/kg dexamethasone (n = 7) or saline (n = 6). Values are expressed as the means ± SEM of mean fluorescence intensity. Dexamethasone decreased L-selectin on circulating PMN with no change in control rabbits. The effect of dexamethasone to decrease L-selectin became detectable only at 12 hours after treatment. *P < .01 compared with control; Dex, dexamethasone.
Changes in L-selectin expression on PMN in the circulation after IV injection of either 2.0 mg/kg dexamethasone (n = 7) or saline (n = 6). Values are expressed as the means ± SEM of mean fluorescence intensity. Dexamethasone decreased L-selectin on circulating PMN with no change in control rabbits. The effect of dexamethasone to decrease L-selectin became detectable only at 12 hours after treatment. *P < .01 compared with control; Dex, dexamethasone.
A representative flow cytometric measurement of L-selectin in a dexamethasone-treated rabbit. The curve was shifted to left by dexamethasone at 12 hours after treatment. At 24 hours after treatment, two distinct populations of cells are seen. One population shows lower levels of L-selectin expression similar to the expression at 12 hours after treatment (left) and the another shows higher levels of L-selectin expression similar to the expression of baseline (right).
A representative flow cytometric measurement of L-selectin in a dexamethasone-treated rabbit. The curve was shifted to left by dexamethasone at 12 hours after treatment. At 24 hours after treatment, two distinct populations of cells are seen. One population shows lower levels of L-selectin expression similar to the expression at 12 hours after treatment (left) and the another shows higher levels of L-selectin expression similar to the expression of baseline (right).
Effect of Dexamethasone on L-Selectin Expression on PMN Already in the Circulation
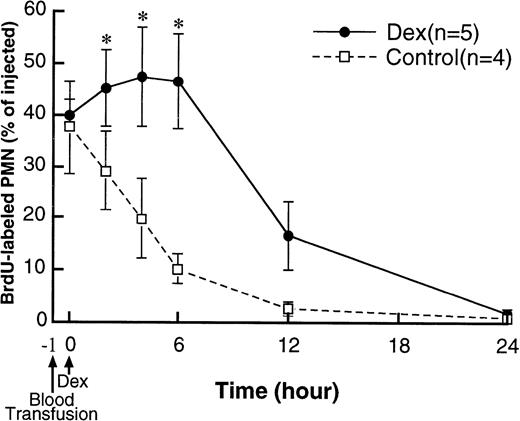
Figure 3 shows the clearance of BrdU-labeled PMN from the circulation of recipients. After transfusion of the BrdU-labeled PMN, 1 hour was allowed to achieve a stable count of BrdU-labeled PMN in the circulation.26 The fractions of the transfused BrdU-labeled PMN at baseline (1 hour after blood transfusion) were similar in both the dexamethasone-treated rabbits (40% ± 2.8%) and control rabbits (38% ± 9.1%). In control rabbits, BrdU-labeled PMN present in the circulation gradually decreased and almost disappeared by 24 hours after treatment. BrdU-labeled PMN increased after dexamethasone treatment (P < .05 at 2 to 6 hours compared with control), then decreased and almost disappeared by 24 hours after treatment. Figure 4 shows the PMN stained for both L-selectin (red) and BrdU (blue). Interobserver and intraobserver variation in the immunocytochemical evaluation of L-selectin expression was small, with the R2/R2max values greater than 0.75.7 The cells graded as either strong or negative gave the most reproducible results and were used to compare differences. The influence of the double labeling procedure on the presence of surface L-selectin and nuclear BrdU expression was evaluated by comparing the number of positive PMN for each antigen with paired slides stained for a single antigen using APAAP method as described above in 10 randomly selected slides. No significant difference was found (data not shown).
The clearance of transferred BrdU-labeled PMN from the circulation of recipients. The fractions of the transfused BrdU-labeled PMN 1 hour after blood transfusion were similar in both dexamethasone-treated and control rabbits. In controls, BrdU-labeled PMN in the circulation gradually decreased and almost disappeared by 24 hours after treatment. In dexamethasone-treated rabbits, BrdU-labeled PMN first increased after treatment and then decreased and disappeared by 24 hours after treatment. *P < .05 compared with control; Dex, dexamethasone.
The clearance of transferred BrdU-labeled PMN from the circulation of recipients. The fractions of the transfused BrdU-labeled PMN 1 hour after blood transfusion were similar in both dexamethasone-treated and control rabbits. In controls, BrdU-labeled PMN in the circulation gradually decreased and almost disappeared by 24 hours after treatment. In dexamethasone-treated rabbits, BrdU-labeled PMN first increased after treatment and then decreased and disappeared by 24 hours after treatment. *P < .05 compared with control; Dex, dexamethasone.
Double immunolabeling of circulating PMN for surface L-selectin (red) and nuclear BrdU (blue). BrdU-labeled PMN were classified according to the intensities of L-selectin expression as strong, weak, and negative. The bar represents 10 μm.
Double immunolabeling of circulating PMN for surface L-selectin (red) and nuclear BrdU (blue). BrdU-labeled PMN were classified according to the intensities of L-selectin expression as strong, weak, and negative. The bar represents 10 μm.
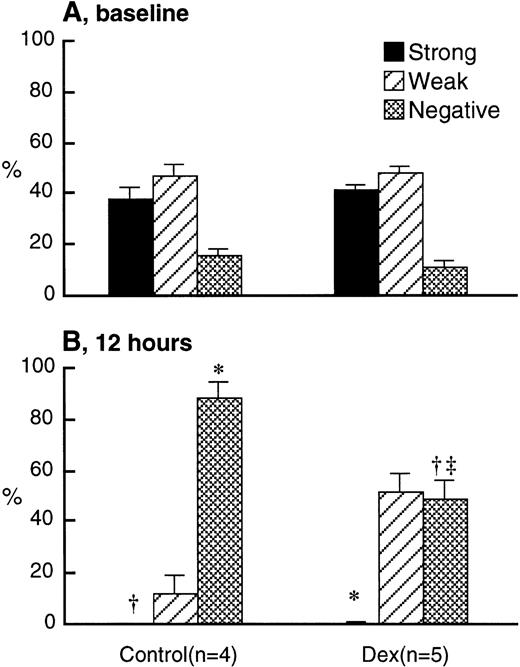
L-selectin expression on BrdU-labeled PMN at baseline was similar in dexamethasone-treated and control rabbits (Fig 5A). Although L-selectin–negative BrdU-labeled PMN were increased by 12 hours in both dexamethasone-treated and control rabbits, the decrease in L-selectin was larger in the control than in the dexamethasone-treated rabbits (Fig 5B). Too few BrdU-labeled PMNs were in the circulation at 24 hours to make a reproducible measurement.
L-selectin expression on BrdU-labeled PMN transferred from the donors into the circulation of recipients after IV injection of either 2.0 mg/kg dexamethasone (n = 5) or saline (n = 4) at baseline (A) and 12 hours after treatment (B) using the immunocytochemical grading system (see Materials and Methods). Values are expressed as the means ± SEM. At baseline, L-selectin expression was similar in both groups. Twelve hours after treatment, there was a significant decrease in the L-selectin expression in both groups. However, this decrease was larger in the controls than the dexamethasone-treated rabbits. *P < .01 compared with baseline; †P < .05 compared with baseline; ‡P < .01 compared with control; Dex, dexamethasone.
L-selectin expression on BrdU-labeled PMN transferred from the donors into the circulation of recipients after IV injection of either 2.0 mg/kg dexamethasone (n = 5) or saline (n = 4) at baseline (A) and 12 hours after treatment (B) using the immunocytochemical grading system (see Materials and Methods). Values are expressed as the means ± SEM. At baseline, L-selectin expression was similar in both groups. Twelve hours after treatment, there was a significant decrease in the L-selectin expression in both groups. However, this decrease was larger in the controls than the dexamethasone-treated rabbits. *P < .01 compared with baseline; †P < .05 compared with baseline; ‡P < .01 compared with control; Dex, dexamethasone.
Effect of Dexamethasone on L-Selectin Expression on Younger PMN Recently Released From the Bone Marrow
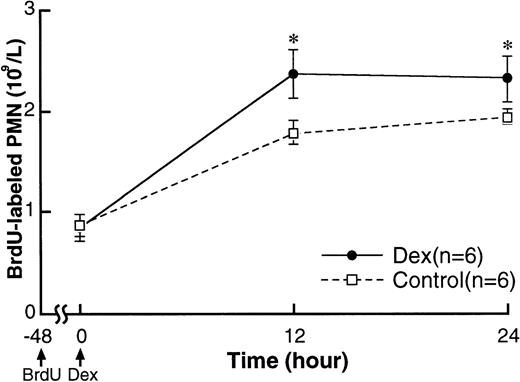
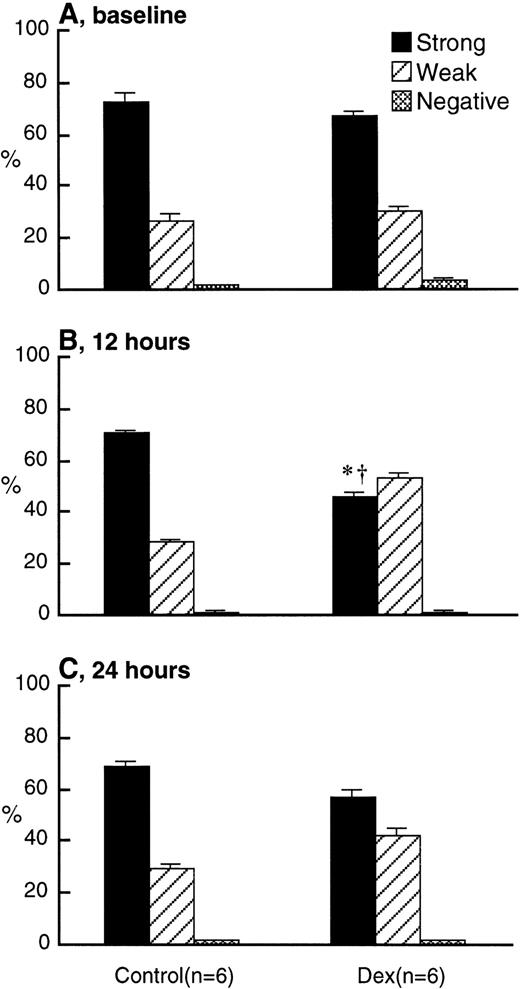
Figure 6 shows the appearance of BrdU-labeled PMN from the bone marrow into the circulation. BrdU-labeled PMN counts at 48 hours after labeling (baseline) were approximately 0.9 × 109/L in both experimental and control rabbits. BrdU-labeled PMN increased by 12 hours and remained high until 24 hours in both groups but were higher in the dexamethasone-treated compared with the control rabbits. L-selectin expression on BrdU-labeled PMN at baseline were similar in dexamethasone-treated and control rabbits (Fig 7A) and were higher than in older PMN already in the circulation (Fig 5A). L-selectin expression on BrdU-labeled PMN did not change throughout the study period in controls, but decreased by 12 hours after treatment in dexamethasone-treated rabbits and remained low until 24 hours (Fig 7B and C). This was evident by the decrease of BrdU-labeled PMN with strong expression of L-selectin in the dexamethasone-treated rabbits.
Changes in BrdU-labeled PMN counts in the circulation after IV injection of either 2.0 mg/kg dexamethasone (n = 6) or saline (n = 6). BrdU (100 mg/kg) was administered intravenously 48 hours before dexamethasone treatment. Values are expressed as the means ± SEM of the number of BrdU-labeled PMN in circulation. BrdU-labeled PMN counts increased by 12 hours and remained high until 24 hours in both groups. The counts were higher in dexamethasone-treated rabbits than in controls. *P < .05 compared with control; Dex, dexamethasone.
Changes in BrdU-labeled PMN counts in the circulation after IV injection of either 2.0 mg/kg dexamethasone (n = 6) or saline (n = 6). BrdU (100 mg/kg) was administered intravenously 48 hours before dexamethasone treatment. Values are expressed as the means ± SEM of the number of BrdU-labeled PMN in circulation. BrdU-labeled PMN counts increased by 12 hours and remained high until 24 hours in both groups. The counts were higher in dexamethasone-treated rabbits than in controls. *P < .05 compared with control; Dex, dexamethasone.
L-selectin expression on BrdU-labeled PMN in the circulation at baseline (A), at 12 hours (B), and at 24 hours (C) after IV injection of either 2.0 mg/kg dexamethasone (n = 6) or saline (n = 6) using the immunocytochemical grading system (see Materials and Methods). Values are expressed as the means ± SEM. The percentage of BrdU-labeled PMN with strong expression of L-selectin was low at 12 and 24 hours after dexamethasone treatment, with no change in the controls. *P < .01 compared with baseline; †P < .01 compared with control; Dex, dexamethasone.
L-selectin expression on BrdU-labeled PMN in the circulation at baseline (A), at 12 hours (B), and at 24 hours (C) after IV injection of either 2.0 mg/kg dexamethasone (n = 6) or saline (n = 6) using the immunocytochemical grading system (see Materials and Methods). Values are expressed as the means ± SEM. The percentage of BrdU-labeled PMN with strong expression of L-selectin was low at 12 and 24 hours after dexamethasone treatment, with no change in the controls. *P < .01 compared with baseline; †P < .01 compared with control; Dex, dexamethasone.
Effect of Dexamethasone on L-Selectin Expression on PMN in the Bone Marrow
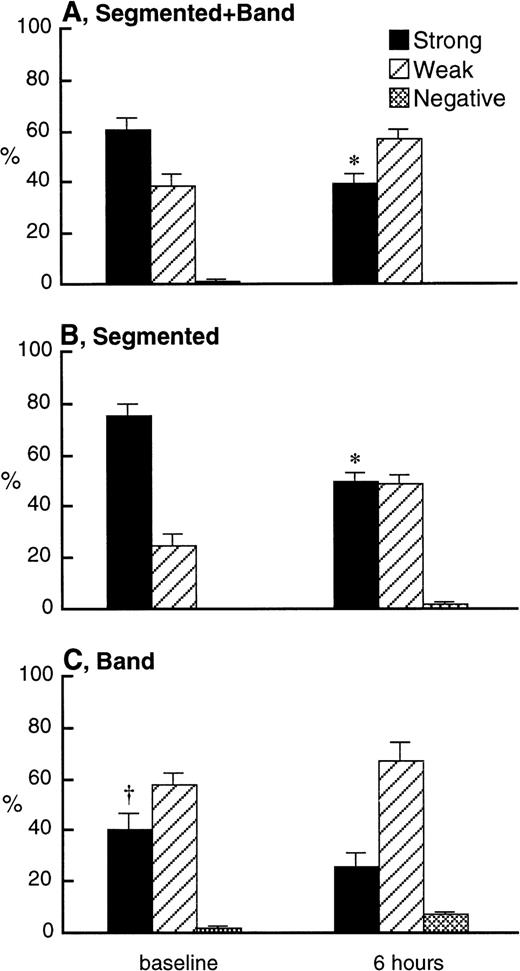
L-selectin on PMN in the bone marrow was evaluated 6 hours after dexamethasone treatment because the maximum decrease in L-selectin expression on circulating PMN occurs 12 hours after treatment. The results obtained by combining the segmented and band PMN (Fig 8A) showed that L-selectin expression was decreased 6 hours after dexamethasone treatment. This was due to reduction in L-selectin expression on the segmented PMN (Fig 8B), with little or no change in band cell L-selectin expression (Fig 8C). Comparison of Fig 8B and C also shows that, in the bone marrow, the band cells expressed lower levels of L-selectin than segmented PMN.
L-selectin expression on combination of band and segmented PMN (A), segmented PMN (B), and band PMN (C) in the bone marrow 6 hours after IV injection of 2.0 mg/kg dexamethasone (n = 4) using the immunocytochemical grading system (see Materials and Methods). Values are expressed as the means ± SEM. The percentage of mature (band + segmented) PMN with strong expression of L-selectin decreased after dexamethasone treatment. Similarly, the percentage of segmented PMN with strong expression of L-selectin was decreased by dexamethasone treatment, but not the percentage of band PMN. The percentage of band PMN with strong expression of L-selectin was smaller than that of segmented PMN at baseline. *P < .05 compared with baseline; †P < .01 compared with segmented PMN at baseline; Dex, dexamethasone.
L-selectin expression on combination of band and segmented PMN (A), segmented PMN (B), and band PMN (C) in the bone marrow 6 hours after IV injection of 2.0 mg/kg dexamethasone (n = 4) using the immunocytochemical grading system (see Materials and Methods). Values are expressed as the means ± SEM. The percentage of mature (band + segmented) PMN with strong expression of L-selectin decreased after dexamethasone treatment. Similarly, the percentage of segmented PMN with strong expression of L-selectin was decreased by dexamethasone treatment, but not the percentage of band PMN. The percentage of band PMN with strong expression of L-selectin was smaller than that of segmented PMN at baseline. *P < .05 compared with baseline; †P < .01 compared with segmented PMN at baseline; Dex, dexamethasone.
DISCUSSION
This study confirms the previous reports showing that glucocorticoids decrease the L-selectin expression on circulating PMN. It extends these observations by demonstrating that this decrease was due to a decrease in L-selectin expression on PMN in the bone marrow and not in the circulation. This glucocorticoid-induced decrease in L-selectin expression on PMN in the maturation pool of the bone marrow suggests that modulation of L-selectin expression on PMN in the bone marrow may be an important mechanism of glucocorticoid-induced reduction in neutrophilic inflammation.
Several studies have shown that dexamethasone decreases L-selectin expression on circulating PMN.14-17 This decrease becomes detectable 8 hours after dexamethasone treatment in bovine and human14,16 and 12 hours after treatment in the rabbits reported here. Circulating activating stimuli such as complement fragments or endotoxin decrease L-selectin expression on PMN rapidly (within 1 hour),9 23 unlike the delayed response produced by dexamethasone. These reports suggested the hypothesis that dexamethasone might reduce L-selectin expression on circulating PMN by influencing PMN precursors in the bone marrow. This was supported by our flow cytometry data showing two distinct cell populations 24 hours after dexamethasone treatment (Fig 2). The population expressing high levels of L-selectin (right-sided peak) has a similar fluorescence intensity as the baseline cells, indicating that at 24 hours after dexamethasone, the bone marrow has escaped from the influence of the dexamethasone and has resumed production of PMN with normal levels of L-selectin expression.
The effect of dexamethasone on circulating PMN was examined by administrating BrdU to donor rabbits and transferring the BrdU-labeled PMN to recipients in whole blood. A previous report from our laboratory established that the mature circulating PMN spontaneously shed L-selectin as they age in the circulation,10 and the present results show that glucocorticoids slow this process (Fig 5). This implies that the effect of dexamethasone on the peripheral blood PMN does not account for the observed decrease in L-selectin expression on PMN after dexamethasone treatment.
Pulse labeling of the dividing PMN in the bone marrow allows us to determine L-selectin expression on younger PMN recently released from the bone marrow into the circulation. The BrdU-labeled PMN observed in the circulation at 60 and 72 hours after labeling shows that dexamethasone decreased the L-selectin expression on the PMN entering the circulation from the bone marrow (Fig 7). These results support the hypothesis that dexamethasone promotes the release of PMN with low levels of L-selectin from the bone marrow. It is also unlikely that dexamethasone induced L-selectin shedding from these PMN, because incubation of circulating PMN with dexamethasone in vitro does not alter their L-selectin expression.20,24 25 Furthermore, the activation-sensitive surface receptor CD18 did not change on the PMN after dexamethasone treatment. Therefore, we conclude that the reduction in L-selectin expression is the result of downregulation rather than activation-associated shedding of L-selectin.
The decrease in L-selectin expression on mature PMN (bands and segmented cells) in the maturation pool of the bone marrow 6 hours after dexamethasone treatment further supports the concept that glucocorticoids downregulate L-selectin expression of bone marrow cells. Interestingly, the effect was more pronounced on segmented PMN than on band cells. The difference of L-selectin expression between segmented and band cells (Fig 8B and C) at baseline suggests significant production of L-selectin at this stage of PMN maturation in the bone marrow. This supports work by Lund-Johansen and Terstappen4 and our laboratory7 that L-selectin increased on PMN as they mature in the postmitotic pool of the bone marrow and extend it by showing that a large increase occurs between the band and segmented stages of PMN maturation.
Several factors could explain our results. Glucocorticoids could decrease the transcription of the L-selectin mRNA in maturing PMN. Glucocorticoids have been shown to inhibit gene transcription by inhibiting the binding of the Ap-1 transcription factor33and decreasing NF-κB activity.34,35 The limited sequence data available for the L-selectin promoter region indicates that it does not contain an Ap-1 or NF-κB DNA-binding site36 and suggests that glucocorticoids may not directly affect L-selectin gene transcription in this way. Glucocorticoids could also indirectly affect the expression of L-selectin on PMN in the bone marrow. Glucocorticoids might also influence the expression of L-selectin by enhancing the transcription of IκBα gene and protein synthesis that would inhibit the translocation of NF-κB to the nucleus.34 35 This effect could reduce cytokine production and indirectly alter L-selectin expression on PMN in the bone marrow.
In summary, the results of this study demonstrate that dexamethasone slows the spontaneous decrease of L-selectin on aging PMN in the circulation, decreases L-selectin expression on PMN recently released from the bone marrow into the circulation, and decreases L-selectin expression on mature PMN (band and segmented) in the bone marrow. It also shows that glucocorticoids decrease the L-selectin expression on circulating PMN primarily by downregulating the L-selectin expression on PMN in the maturation pool of the bone marrow. Because L-selectin is critically important in the early stages of PMN recruitment, we speculate that this is a key mechanism through which glucocorticoids regulate neutrophilic influx into inflammatory sites.
ACKNOWLEDGMENT
The authors thank Beth Whalen, Corinne Rocchini, Daniela Zamfir, and Jennifer Hards for technical supports; Stuart Green for photography; and Yulia D’yachkova for statistical analysis.
Supported by Grant No. 4219 from the Medical Research Council of Canada.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Stephan F. van Eeden, MD, PhD, Pulmonary Research Laboratory, University of British Columbia, St Paul’s Hospital, 1081 Burrard St, Vancouver, BC, Canada V6Z1Y6; e-mail:svaneeden@prl.pulmonary.ubc.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal