Abstract
Based on the hypothesis that genetic modification of freshly isolated alveolar macrophages (AM) with the granulocyte-macrophage colony-stimulating factor (GM-CSF) cDNA would induce AM to proliferate, this study focuses on the ability of adenoviral (Ad) vectors to transfer and efficiently express the murine (m) GM-CSF cDNA in murine AM with consequent expansion in the number of AM in vitro and in vivo. To demonstrate that an Ad vector can effectively transfer and express genes in AM, murine AM recovered by bronchoalveolar lavage from the lung of Balb/c mice were infected with an Ad vector coding for green fluorescent protein (GFP) in vitro and expressed GFP in a dose-dependent fashion. Infection of AM with an Ad vector containing an expression cassette coding for mGM-CSF led to GM-CSF expression and to AM proliferation in vitro. When AM infected with AdGFP were returned to the respiratory tract of syngeneic recipient mice, GFP-expressing cells could still be recovered by bronchoalveolar lavage 2 weeks later. In vitro infection of AM with AdmGM-CSF and subsequent transplantation of the genetically modified AM to the lungs of syngeneic recipients led to GM-CSF expression in vivo. Strikingly, the AM recovered by lavage 5 weeks after transplantation demonstrated an increased rate of proliferation, and the total number of alveolar macrophages was 1.9-fold greater than controls. Importantly, the increase in the numbers of AM was selective (ie, other inflammatory cell numbers were unchanged), and there was no modification to the lung architecture. Thus, it is feasible to genetically modify AM with Ad vectors and to use this strategy to modify the behavior of AM in vivo. Based on the importance of AM in the primary defense of the respiratory epithelial surface, this strategy may be useful in enhancing pulmonary defenses in immunodeficiency states.
THE ALVEOLAR macrophage (AM), the pulmonary representative of the mononuclear phagocyte system, plays a central role in defending the lungs against inhaled organisms.1-3 In the normal human lung, there are approximately 50 AM on the epithelial surface of each alveolus and additional AM in the alveolar interstitium.4 Under normal circumstances, this is sufficient to provide a major defense of the respiratory epithelium. However, in circumstances in which the challenge of inhaled organisms is overwhelming or in which there is dysfunction in the AM, the lung is at increased risk for infection.5-11
Based on these considerations, one strategy to augment the defenses in the lung is to increase the number of AM on the respiratory epithelial surface. Theoretically, this could be accomplished by attracting more circulating macrophage precursors to the lung or by enhancing proliferation of AM in the alveoli. In regard to the second strategy, although AM are usually considered to be terminally differentiated, there is evidence that 1% to 2% of human and rodent AM proliferate within a 24-hour period,12-14 and in vitro studies have shown that AM proliferate in response to granulocyte-macrophage colony-stimulating factor (GM-CSF).15 16
With this background, we hypothesized that, by genetically modifying AM to express GM-CSF, the GM-CSF would stimulate proliferation of the AM population, thus expanding the number of AM. The data demonstrate that E1−, E3− Ad vectors can transfer and express transgenes to murine AM in vitro, including the marker gene coding for the jellyfish (Aquoriea victoria) green fluorescent protein (GFP), as well as the murine (m) GM-CSF cDNA. With transfer of the mGM-CSF cDNA, not only do AM express GM-CSF and proliferate in vitro, but also transplantation of the mGM-CSF cDNA-modified AM to the respiratory epithelial surface of syngeneic mice results in in vivo production of GM-CSF and selective expansion in number of AM in the lung.
MATERIALS AND METHODS
Adenovirus vectors.
The recombinant adenovirus vectors AdGFP, AdNull, and AdmGM-CSF used in this study are E1a−, partial E1b−, and partial E3−, based on the Ad5 genome, with the expression cassette in the E1 position. The expression casette includes the cytomegalovirus early/intermediate enhancer promoter (CMV), an artificial splice signal, the cDNA, and an SV40 stop/poly (A) signal. The AdGFP vector carries the humanized form of the gene coding for the jellyfish Aquoriea victoria green fluorescent protein,17 and the AdmGM-CSF vector contains the murine GM-CSF cDNA.18 The AdNull vector is identical to the AdGFP and AdmGM-CSF vectors, except that it lacks a cDNA in the expression cassette.19 The vectors were propagated, purified, and stored at −70°C, as previously described.20,21Titers of viral preparations were determined by plaque assay using 293 cells.22
Bronchoalveolar lavage.
The source of AM for this study was Balb/c mice (Charles River Laboratories, Wilmington, MA). The animals were of either sex, were 6 to 8 weeks old, and weighed 15 to 20 g. After anesthesia by intraperitoneal injection of a mixture of ketamine (60 mg/kg) and xylazine (5 mg/kg), the animals were bled via aortic incision, the trachea was exposed by midline incision, and the lungs were lavaged 10× with warm phosphate-buffered saline, pH 7.4 (PBS), via a 24-gauge catheter. The lavage fluid was filtered through one layer of gauze, centrifuged (400g for 10 minutes), and washed three times in PBS. Cells were resuspended in RPMI medium supplemented with 10% fetal calf serum, 50 U/mL penicillin, and 50 μg/mL streptomycin and plated on coverslip dishes23 or kept in teflon chambers (Savillex, Minneapolis, MN) for infection in suspension. Macrophage content (always >95%) was determined by modified Giemsa stain on cytospin preparations. Cell viability (always >95%) was determined by trypan blue exclusion. After 3 hours, the macrophages were washed with PBS to remove nonadherent cells. The typical yield of AM from one mouse was 3 × 105 cells.
Adenovirus transfer of the GFP gene to AM.
AM were infected with the AdGFP vector at 10, 50, and 200 multiplicity of infection (moi) for 90 minutes in serum-free RPMI. For morphologic studies, the cells were infected after adherence on coverslip dishes; for quantitative studies, the cells were infected in suspension in teflon chambers to prevent adherence. Infection with AdNull (200 moi) was used as a control. Thereafter, complete medium (RPMI supplemented with 10% fetal calf serum) was added after washing once with PBS. After 48 hours of infection, the adherent cells were fixed with 4% paraformaldehyde and nuclei were counterstained with 4′-6′-diamidiomo-2-phenyl indole (DAPI; Molecular Probes, Eugene, OR) and then analyzed for GFP expression by fluorescence microscopy. Cell-associated autofluorescence, commonly found in phagocytic cells, was distinguished from specific green fluorescent signal by superimposing the red channel and green channels. The autofluorescence appeared yellow (red and green), whereas specific GFP fluorescence remained green. Alternatively, cells were cultured in suspension in teflon chambers, washed, resuspended in PBS and 0.1% bovine serum albumin, and immediately evaluated for GFP expression by flow cytometry.
Transplantation of AdGFP-modified AM.
To evaluate the in vivo persistence of expression of the GFP transgene transferred to the AM, AM were infected in vitro with AdGFP at 100 moi in suspension, washed with PBS after a 90-minute infection period, and maintained in RPMI 10% fetal calf serum for 4 hours. Then the cells were washed 4 times with PBS and resuspended in PBS at 4 × 106/mL. The cells modified with the GFP gene were then slowly (over 1 minute in a total volume of 50 μL) administered (2 × 105 cells) to anesthetized naive syngeneic mice via the intratracheal route. As a control, AM infected with AdNull were handled in the identical manner. The animals were then killed after 2 and 14 days, and the cells on the respiratory epithelial surface were recovered by lavage. The lavaged cells were washed with PBS, and 3 × 104 cells were plated on coverslip dishes and, after 3 hours of adherence, fixed and analyzed by fluorescence microscopy, as described above. The remaining cells were analyzed for GFP expression by flow cytometry, as described above.
Adenovirus transfer of the mGM-CSF cDNA to AM.
To analyze whether AM infected with AdmGM-CSF in vitro produced mGM-CSF, AM (2 × 105 cells per dish) were infected with AdmGM-CSF (moi 200) for 90 minutes at 37°C. After culturing for 2 days, supernatant was collected and mGM-CSF levels in the medium were quantified by double sandwich enzyme-linked immunoabsorbent assay (R&D, Minneapolis, MN) following the manufacturer’s instructions. To assess the effects of Ad-mediated transfer of the mGM-CSF cDNA on AM proliferation, AM were plated on coverslip dishes using Cell Locator coverslips (Eppendoff, Inc, Hamburg, Germany) and infected with AdmGM-CSF at 10, 50, 200, and 400 moi, as described above. After infection, the cells were counted daily; a minimum of 200 cells were counted on a defined field on the coverslip dishes. For analysis of the proportion of cells with mitotic nuclei, the cells were fixed with 4% paraformaldehyde and stained with DAPI.
Transplantation of AdmGM-CSF–modified AM.
To evaluate the consequences of transplanting mGM-CSF cDNA-modified AM to syngeneic mice, AM were infected in suspension in vitro for 90 minutes with AdmGM-CSF at 100 moi as described above for AdGFP. After washing 4×, 2 × 105 cells were transferred to anesthetized naive mice via the intratracheal route. As a control, AdNull-infected AM (100 moi) were transferred in the identical manner. As an additional control, 60 ng of recombinant mGM-CSF was administered intratracheally to Balb/c mice; this dose represents 4× the 24-hour in vitro production by 2 × 105 AM infected with 100 moi AdmGM-CSF in vitro and is used to control for the unlikely possibility that the mGM-CSF was produced in vitro and simply transplanted to the lungs with the AM. After 1 and 5 weeks, the mice underwent lavage. The lavage fluid was centrifuged and the cell-free supernatant was analyzed for mGM-CSF expression by enzyme-linked immunosorbent assay (ELISA). Serum was collected and also assessed for mGM-CSF levels. The cells were resuspended in RPMI and the cell content of the lavage was determined by counting with a Neubauer hemocytometer (Fisher Scientific, Springfield, MA). For analysis of the types of cells present in the lavage, cytospin samples of the lavaged cells were stained by modified Giemsa stain. For analysis of cell proliferation, 2 × 104 cells were plated in a 96-well plate. After 1 hour of adherence and thorough washing with PBS, the cells were labeled with [3H]thymidine for 24 and 72 hours. The cells were detached with 0.5 mg/mL trypsin-0.5 mmol/L ethylenediamine acetic acid and transferred to a filter membrane with a cell harvester, and the amount of [3H]thymidine incorporated was determined using a β-counter. From the remaining cells, total DNA was extracted using QuiAMP DNA extraction columns (Quiagen, Santa Clarita, CA) and amplified by polymerase chain reaction (PCR) using primers for the adenovirus region E4 (sense primer, ACCGCCCGCAGCATAA; antisense primer, TGAGGGGTCGCCACTT), mGM-CSF–specific transgene (sense primer, TTGCCTTTCTCTCCACAGGTGT, covering the CMV promoter region in the Ad vector; antisense primer, TTGGTGAAATTGCCCCGTAGA, covering the left side of the GM-CSF cDNA) and Hydrogen-Potassium ATPase (HK-ATPase; sense primer, TCCTTCATGGACCGTGGG; antisense primer, CTGCAAAGCTCTCCTGGAAGA) as genomic control. The PCR settings were 94°C for 5 minutes, 40 cycles of 94°C for 30 seconds, 58°C for 30 seconds, 72°C for 30 seconds, and a final elongation step at 72°C for 5 minutes. The PCR products were separated on a 1.6% agarose gel and analyzed by ethidium bromide staining.
Histological evaluation of lung.
To determine if morphological changes were induced by transplanting mGM-CSF cDNA-modified AM to the lung of syngeneic mice, AM were infected in vitro with AdmGM-CSF or AdNull at 100 moi and transferred to the lungs of syngeneic mice as described above. After 1 and 5 weeks, the animals were killed and the lungs were fixed with 10% formalin. Sections were stained with hemotoxilin-eosin and evaluated by light microscopy.
Statistical evaluation.
All data are presented as the mean ± standard error of the mean; statistical evaluations were performed using the two-tailed Student’st-test.
RESULTS
AdGFP expression in AM.
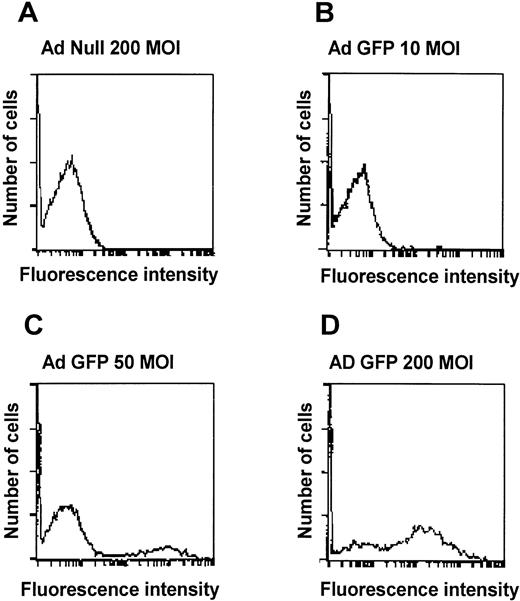
To evaluate if an Ad vector can transfer genes to AM, AM were infected in vitro with AdGFP using either adherent AM or AM in suspension. Fluorescence microscopy of adherent cells infected with AdGFP showed a dose-dependent, homogenous green fluorescence, characteristic for GFP expression (Fig 1). AdNull-infected cells showed no GFP expression. Flow cytometry analysis also showed a dose-dependent increase in the numbers of cells expressing GFP 48 hours after infection. At 200 moi, 65% of the cells expressed the transgene, compared with 19% at 50 moi and 6% at 10 moi (Fig 2). From these data, we conclude that Ad vectors can transfer to and express genes in AM in a dose-dependent manner and that it is possible to use Ad vectors to transfer genes to the majority of freshly isolated AM.
GFP expression in murine alveolar macrophages in vitro. Shown is fluorescence microscopy of adherent alveolar marophages 48 hours after infection with AdGFP at various moi. Infection with AdNull is used as control. GFP fluorescence is shown in green. Nuclei are counterstained with DAPI (blue). Autofluorescent intracellular infections appear yellow-orange. (A) AdNull, 200 moi. (B) AdGFP, 10 moi. (C) AdGFP, 50 moi. (D) AdGFP, 200 moi. Bar = 50 μm.
GFP expression in murine alveolar macrophages in vitro. Shown is fluorescence microscopy of adherent alveolar marophages 48 hours after infection with AdGFP at various moi. Infection with AdNull is used as control. GFP fluorescence is shown in green. Nuclei are counterstained with DAPI (blue). Autofluorescent intracellular infections appear yellow-orange. (A) AdNull, 200 moi. (B) AdGFP, 10 moi. (C) AdGFP, 50 moi. (D) AdGFP, 200 moi. Bar = 50 μm.
Quantitative assessment of Ad vector-mediated GFP expression in murine alveolar macrophages in vitro. Shown is flow cytometry analysis of alveolar macrophages infected for 48 hours in suspension with AdGFP at 10, 50, and 200 moi. Infection withAdNull is used as control. Shown on the ordinate is cell number and on the abscissa is shown the intensity of GFP. (A) AdNull, 200 moi. (B) AdGFP, 10 moi. (C) AdGFP, 50 moi. (D) AdGFP, 200 moi.
Quantitative assessment of Ad vector-mediated GFP expression in murine alveolar macrophages in vitro. Shown is flow cytometry analysis of alveolar macrophages infected for 48 hours in suspension with AdGFP at 10, 50, and 200 moi. Infection withAdNull is used as control. Shown on the ordinate is cell number and on the abscissa is shown the intensity of GFP. (A) AdNull, 200 moi. (B) AdGFP, 10 moi. (C) AdGFP, 50 moi. (D) AdGFP, 200 moi.
AdmGM-CSF induces AM proliferation in vitro.
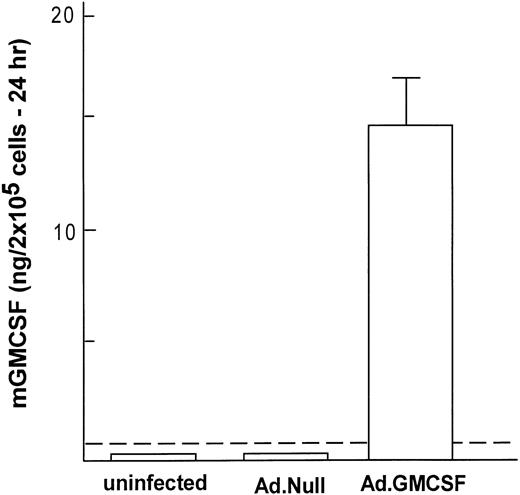
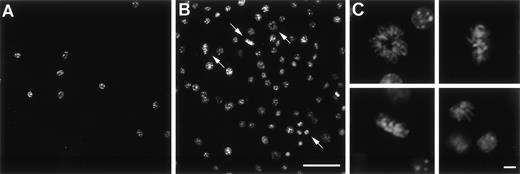
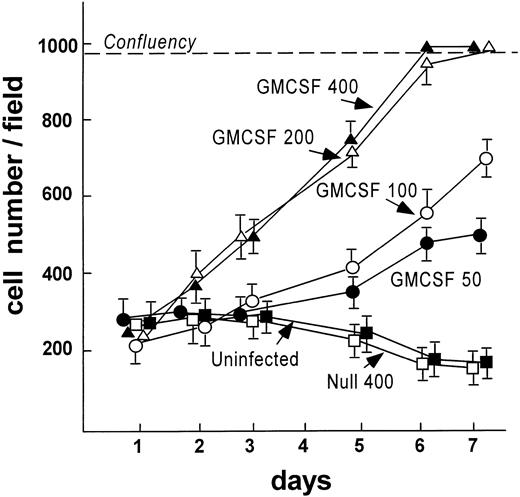
To analyze if Ad vector-mediated transfer of the mGM-CSF cDNA to AM induced AM proliferation, AM were infected in vitro with AdmGM-CSF at 200 moi. Murine GM-CSF was detectable in the supernatant of AdmGM-CSF–infected cells, but not AdNull-infected cells 48 hours after infection (Fig 3). Fluorescence microscopy of DAPI-stained nuclei showed an increase in cell number and an increase in the number of mitotic figures (2.3% ± 0.4% for AdmGM-CSF–infected cells at 48 hours, compared with less than 0.5% for AdNull-infected cells (P < .001; Fig 4). The number of AM increased over 7 days in a dose-dependent fashion; at 7 days, the AM infected with ≥50 moi had severalfold more AM in the dish compared with AdNull-infected cells or noninfected cells (P < .01, all comparisons, 7 days; Fig 5). Thus, in vitro genetic modification of AM by Ad-mediated transfer of the mGM-CSF cDNA leads to GM-CSF production and induces proliferation of the AM.
Genetic modification of murine alveolar macrophages to express murine GM-CSF. Shown is expression of mGM-CSF for 24 hours after in vitro infection with AdmGM-CSF or AdNull (control) compared with uninfected AM. The dashed line represents the sensitivity of the assay. The data represent the mean ± SEM from 4 samples measured by ELISA.
Genetic modification of murine alveolar macrophages to express murine GM-CSF. Shown is expression of mGM-CSF for 24 hours after in vitro infection with AdmGM-CSF or AdNull (control) compared with uninfected AM. The dashed line represents the sensitivity of the assay. The data represent the mean ± SEM from 4 samples measured by ELISA.
In vitro proliferation of murine AM after infection withAdmGM-CSF. AM were plated on cell locator dishes and infected with AdmGM-CSF or AdNull, each at 200 moi. The AM were fixed 96 hours later, and the nuclei were stained with DAPI and evaluated using fluorescence microscopy. (A) AdNull. (B) AdmGM-CSF; note the marked increase in the number of cells. Arrows indicate mitotic figures. (C) AdmGM-CSF, mitotic figures from (B) shown at higher magnification. Bar = 50 μm for (A) and (B); bar = 5 μm for (C).
In vitro proliferation of murine AM after infection withAdmGM-CSF. AM were plated on cell locator dishes and infected with AdmGM-CSF or AdNull, each at 200 moi. The AM were fixed 96 hours later, and the nuclei were stained with DAPI and evaluated using fluorescence microscopy. (A) AdNull. (B) AdmGM-CSF; note the marked increase in the number of cells. Arrows indicate mitotic figures. (C) AdmGM-CSF, mitotic figures from (B) shown at higher magnification. Bar = 50 μm for (A) and (B); bar = 5 μm for (C).
AdmGM-CSF–induced proliferation of murine AM in vitro. AM were plated on cell locator dishes and infected with AdmGM-CSF or AdNull at various moi as described for Fig 4. Shown is the cell number per high power field over a 7-day period. The dashed line represents confluency of the cells on the plate.
AdmGM-CSF–induced proliferation of murine AM in vitro. AM were plated on cell locator dishes and infected with AdmGM-CSF or AdNull at various moi as described for Fig 4. Shown is the cell number per high power field over a 7-day period. The dashed line represents confluency of the cells on the plate.
Transplantation of genetically modified AM.
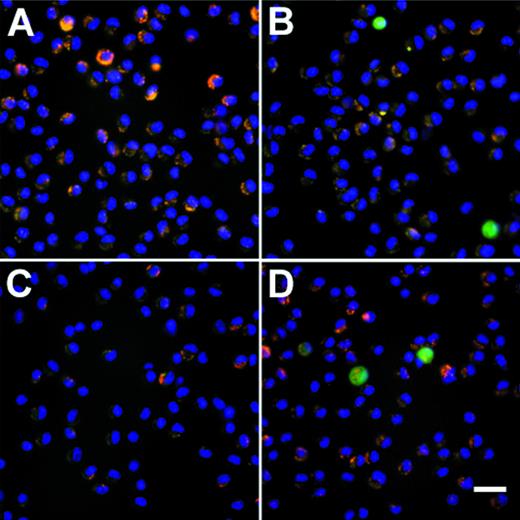
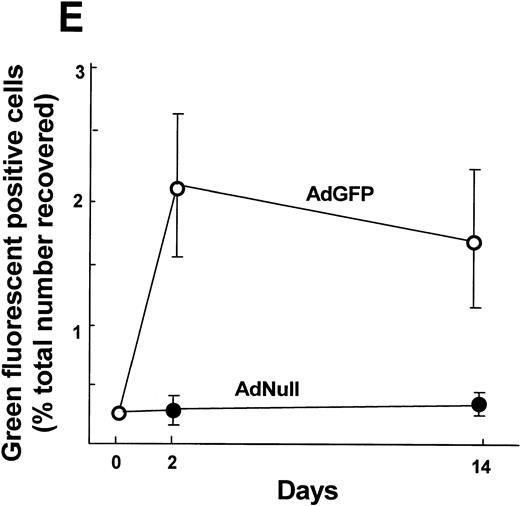
To demonstrate that AM modified by an Ad vector retain their in vitro genetic alteration when the modified AM were transplanted to the lung of syngeneic mice, AM modified by AdGFP or AdNull were transplanted to the lungs, and the mice were lavaged over time to recover AM. Assessment by immunofluorescence microscopy demonstrated GFP-positive AM at 2 and 14 days after transplantation in the AdGFP group (Fig 6A through D). Quantification of the proportion of GFP-positive cells by flow cytometry showed that GFP-expressing cells could be recovered at 2 days (2.0% ± 0.5% of total cells recovered) and 14 days (1.7% ± 0.4%) in the AdGFP group after transplantation, whereas none of the AdNull group animals showed GFP-positive cells (Fig 6E).
Persistance of genetically modified AM after in vivo transfer of alveolar macrophages that have been modified in vitro with AdGFP or AdNull. After infection (200 moi, 90 minutes at 37°C), the cells were washed and 2 × 105 cells were transplanted to the lungs of syngeneic recipient mice via the intratracheal route. AM were recovered by lavage after 2 and 14 days, and adherent cells were evaluated by fluorescence microscopy. GFP fluorescence is shown in green. Nuclei are counterstained with DAPI (blue) and autofluorescence appears yellow-orange. (A) AM recovered 2 days after transfer of AdNull-infected cells to the lung. (B) Same as (A) but AdGFP-infected AM. (C) AdNull, 14 days. (D) AdGFP, 14 days. Bar = 50 μm. Cells recovered from naive mice showed only autofluorescence (not shown). (E) Quantitative assessment of the number of genetically modified AM. Shown is the percentage of cells expressing green fluorescence, reflecting AM expression of GFP.
Persistance of genetically modified AM after in vivo transfer of alveolar macrophages that have been modified in vitro with AdGFP or AdNull. After infection (200 moi, 90 minutes at 37°C), the cells were washed and 2 × 105 cells were transplanted to the lungs of syngeneic recipient mice via the intratracheal route. AM were recovered by lavage after 2 and 14 days, and adherent cells were evaluated by fluorescence microscopy. GFP fluorescence is shown in green. Nuclei are counterstained with DAPI (blue) and autofluorescence appears yellow-orange. (A) AM recovered 2 days after transfer of AdNull-infected cells to the lung. (B) Same as (A) but AdGFP-infected AM. (C) AdNull, 14 days. (D) AdGFP, 14 days. Bar = 50 μm. Cells recovered from naive mice showed only autofluorescence (not shown). (E) Quantitative assessment of the number of genetically modified AM. Shown is the percentage of cells expressing green fluorescence, reflecting AM expression of GFP.
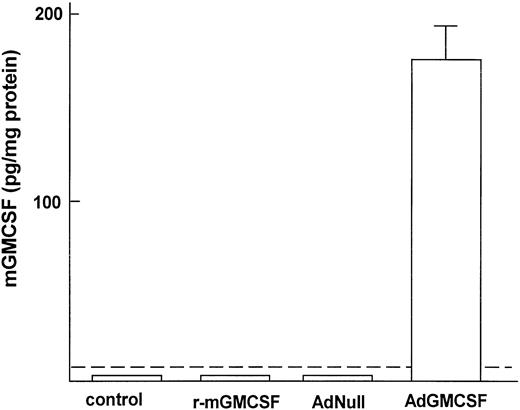
To analyze if transfer of AM infected in vitro with AdmGM-CSF can be used to induce AM proliferation in the lung in vivo, AM were infected in vitro with AdmGM-CSF and, after 24 hours, the cells were transplanted to the epithelial surface of lungs of syngeneic mice. Analysis of lavage fluid 7 days after transplantation showed increased mGM-CSF levels in the lavage of the mice that received AM modified with AdmGM-CSF, but not in mice receiving AdNull (Fig 7). In contrast, for mice receiving recombinant mGM-CSF (60 ng, the amount equivalent to 4-day in vitro production by 2 × 105 cells), no mGM-CSF could be detected 7 days later (Fig 7). Serum GM-CSF levels were undetectable in all groups (not shown). Consistent with the data demonstrating that in vitro AdmGM-CSF infection of AM induced increased proliferation of AM in vitro, recovery of AM genetically modified in vitro with AdmGM-CSF to express the mGM-CSF cDNA demonstrated that the AM were continuing to proliferate at an increased rate (Fig 8A). In this regard, AM recovered from the lungs by lavage at 7 days after transplantation of naive AM showed low levels of [3H]thymidine incorporation, as did AM recovered from the lungs 7 days after transplantation of AM infected with AdNull (Fig 8A). In marked contrast, transplantation of AM genetically modified ex vivo 7 days previously with AdmGM-CSF showed a significant increase in [3H]thymidine incorporation at 24 hours (P < .001 compared with all other groups) and 72 hours (P < .0001 compared with all other groups) of in vitro culture after recovery. In comparison, mice receiving 60 ng recombinant mGM-CSF showed no increase in proliferation (P > .5 compared with naive mice; P< .0001 compared with AM modified with AdmGM-CSF). To analyze if the AM recovered 5 weeks after transfer were still proliferating, the cells recovered by lavage were analyzed by [3H]thymidine incorporation in vitro (Fig 8B). The cells recovered from the AM GM-CSF group still showed increased proliferation after 24 and 72 hours of labeling (P < .01 at 24 hours and P < .0001 at 72 hours compared with AM Null). Interestingly, at 5 weeks, GM-CSF levels were not detectable in the lavage fluid (not shown), suggesting that the increased proliferation of the AM at 5 weeks was secondary to local, likely intracellular, stimulation of the AM by mGM-CSF.
Quantification of mGM-CSF in lavage fluid after transfer of genetically modified AM to the lung. Alveolar macrophages were infected in vitro with AdmGM-CSF or AdNull at 100 moi for 90 minutes and washed, and 2 × 105 AM were transplanted by the intratracheal route to the lungs of syngeneic mice. As a control, a parallel group of animals received 60 ng recombinant mGM-CSF (r-mGM-CSF). Quantification of mGM-CSF in lavage fluid was performed by ELISA 1 week after administration. The data are expressed as picograms of mGM-CSF in lavage fluid referenced to milligrams of total protein in the recovered fluid. The dashed line represents the lower limit of detection of the assay.
Quantification of mGM-CSF in lavage fluid after transfer of genetically modified AM to the lung. Alveolar macrophages were infected in vitro with AdmGM-CSF or AdNull at 100 moi for 90 minutes and washed, and 2 × 105 AM were transplanted by the intratracheal route to the lungs of syngeneic mice. As a control, a parallel group of animals received 60 ng recombinant mGM-CSF (r-mGM-CSF). Quantification of mGM-CSF in lavage fluid was performed by ELISA 1 week after administration. The data are expressed as picograms of mGM-CSF in lavage fluid referenced to milligrams of total protein in the recovered fluid. The dashed line represents the lower limit of detection of the assay.
Proliferation of AM after in vitro infection of AM by AdmGM-CSF and subsequent transplant of the genetically modified AM to the lung. Shown is in vitro proliferation of AM recovered by lavage 1 and 5 weeks after AM transfer. (A) Proliferation in vitro of AM recovered 1 week after transplantation. Shown is in vitro assessment of [3H]thymidine uptake over 24 and 72 hours for AM recovered from naive mice, from mice receiving intratracheal recombinant mGM-CSF (60 ng), from mice receiving AM modified with AdNull (AM Null), and from mice receiving transplanted AM modified with AdmGM-CSF (AM GM-CSF). (B) Proliferation in vitro of AM recovered by lavage 5 weeks after AM transfer. Shown is in vitro assessment of [3H]thymidine uptake over 24 and 72 hours for AM recovered from mice receiving transplanted AM modified with AdNull (AM Null) or AM modified with AdmGM-CSF (AM GM-CSF). Note that the ordinates of (A) and (B) are different; the assays performed at 1 week (A) and 5 weeks (B) are performed at different times with fresh cells; thus, the overall extent of [3H]thymidine uptake cannot be compared between the two panels. (C) Presence of Ad genome in AM 1 and 5 weeks after AM transfer. DNA was analyzed using PCR with primers to amplify Ad genome (E4), Ad genome plus mGM-CSF transgene (Ad-GM-CSF), and HK-ATPase as control. Shown is ethidium bromide staining of amplified DNA from naive control, AM Null at 1 and 5 weeks, and AM GM-CSF 1 and 5 weeks after AM transfer.
Proliferation of AM after in vitro infection of AM by AdmGM-CSF and subsequent transplant of the genetically modified AM to the lung. Shown is in vitro proliferation of AM recovered by lavage 1 and 5 weeks after AM transfer. (A) Proliferation in vitro of AM recovered 1 week after transplantation. Shown is in vitro assessment of [3H]thymidine uptake over 24 and 72 hours for AM recovered from naive mice, from mice receiving intratracheal recombinant mGM-CSF (60 ng), from mice receiving AM modified with AdNull (AM Null), and from mice receiving transplanted AM modified with AdmGM-CSF (AM GM-CSF). (B) Proliferation in vitro of AM recovered by lavage 5 weeks after AM transfer. Shown is in vitro assessment of [3H]thymidine uptake over 24 and 72 hours for AM recovered from mice receiving transplanted AM modified with AdNull (AM Null) or AM modified with AdmGM-CSF (AM GM-CSF). Note that the ordinates of (A) and (B) are different; the assays performed at 1 week (A) and 5 weeks (B) are performed at different times with fresh cells; thus, the overall extent of [3H]thymidine uptake cannot be compared between the two panels. (C) Presence of Ad genome in AM 1 and 5 weeks after AM transfer. DNA was analyzed using PCR with primers to amplify Ad genome (E4), Ad genome plus mGM-CSF transgene (Ad-GM-CSF), and HK-ATPase as control. Shown is ethidium bromide staining of amplified DNA from naive control, AM Null at 1 and 5 weeks, and AM GM-CSF 1 and 5 weeks after AM transfer.
To analyze if Ad genome is present in the cells recovered by lavage, the DNA of the cells was analyzed by PCR using Ad- and transgene-specific primers. AM infected with Ad Null showed Ad-specific E4 signal at 1 and 5 weeks; AM infected with AdmGM-CSF demonstrated E4- as well as AdGM-CSF–specific bands. Control cells showed no Ad signal. The genomic control (HK-ATPase) was present in all samples.
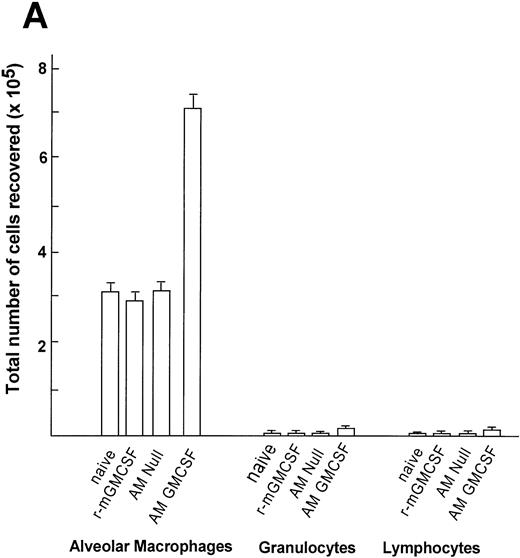
Quantification of the cells recovered in the lavage showed an increased number of alveolar macrophages in the group transplanted with AM genetically modified with AdmGM-CSF compared with the other groups (Fig 9A). At 7 days, the number of AM recovered from the mice receiving transplants of AM modified with the mGM-CSF cDNA was greater than twofold higher compared with the AM Null group, the recombinant mGM-CSF group, and the naive group (P< .0001, all comparisons AdmGM-CSF–modified AM to all other groups). The absolute cell number recovered in the lavage of the animals who had received 60 ng recombinant mGM-CSF (the amount calculated to be produced by 2 × 105 cells infected in vitro over 4 days) showed no increase in cell number in vivo (P > .5 compared with naive mice). The analysis of the differential cell count demonstrated that there was negligible increase in the number of lymphocytes and neutrophils in the group receiving transplants of AM modified by AdmGM-CSF, but the percentage of AM in the lavage remained higher than 95%, so that the increase in absolute cell number was dominated by an increase in the number of AM. No eosinophils or basophils were recovered in any group.
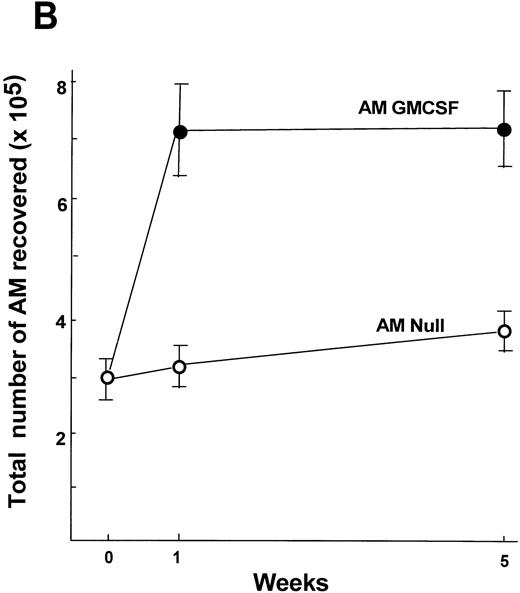
Time course of expansion in numbers of alveolar macrophages after in vitro infection of alveolar macrophages by AdmGM-CSF and subsequent transfer of the genetically modified AM to the lung. Alveolar macrophages were infected in vitro with AdmGM-CSF (AM GM-CSF) or AdNull (AM Null) at 100 moi. After 90 minutes, the cells were washed and 2 × 105 AM were transplanted by the intratracheal route to the lungs of syngeneic mice. (A) Total number of cells recovered by lavage 7 days after the administration of genetically modified AM. Shown are total numbers of AM, granulocytes, and lymphocytes. The granulocytes were greater than 99% neutrophils. (B) Number of AM recovered by lavage 1 and 5 weeks after administration of AM genetically modified in vitro with AdmGM-CSF compared with AdNull or naive mice. The data regarding the number of AM from 1 week are the same as the AM data from (A). The data represent the mean ± standard error of the mean for 4 to 6 mice for each data point.
Time course of expansion in numbers of alveolar macrophages after in vitro infection of alveolar macrophages by AdmGM-CSF and subsequent transfer of the genetically modified AM to the lung. Alveolar macrophages were infected in vitro with AdmGM-CSF (AM GM-CSF) or AdNull (AM Null) at 100 moi. After 90 minutes, the cells were washed and 2 × 105 AM were transplanted by the intratracheal route to the lungs of syngeneic mice. (A) Total number of cells recovered by lavage 7 days after the administration of genetically modified AM. Shown are total numbers of AM, granulocytes, and lymphocytes. The granulocytes were greater than 99% neutrophils. (B) Number of AM recovered by lavage 1 and 5 weeks after administration of AM genetically modified in vitro with AdmGM-CSF compared with AdNull or naive mice. The data regarding the number of AM from 1 week are the same as the AM data from (A). The data represent the mean ± standard error of the mean for 4 to 6 mice for each data point.
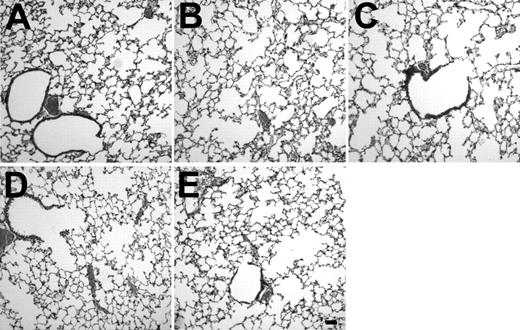
Analysis of the number of AM recovered by lavage 5 weeks after transplantation demonstrated that the number of AM was still elevated in the AM GM-CSF group compared with the AM Null group (1.9-fold,P < .01; Fig 9B). The minor increase in the number of AM in the AM Null group compared with day 7 is most likely due to increase in body weight and age over that period. The histological analysis of lung sections from animals receiving AM modified with AdNull or AdmGM-CSF demonstrated no abnormalities, including no accumulation of inflammatory cells or fibrosis 1 and 5 weeks after AM transfer (Fig 10).
Lung histology after in vitro infection of AM by AdmGM-CSF or AdNull and subsequent transfer of the genetically modified AM to the lung. Shown is light microscopy of hematoxylin/eosin-stained sections of formalin fixed lung 1 and 5 weeks after AM transfer. (A) Naive mice. (B) Mice receiving AM modified with AdNull 1 week previously. (C) Mice receiving AM modified with AdmGM-CSF 1 week previously. (D) Similar to (B), but after 5 weeks. (E) Similar to (C), but after 5 weeks. Bar = 100 μm.
Lung histology after in vitro infection of AM by AdmGM-CSF or AdNull and subsequent transfer of the genetically modified AM to the lung. Shown is light microscopy of hematoxylin/eosin-stained sections of formalin fixed lung 1 and 5 weeks after AM transfer. (A) Naive mice. (B) Mice receiving AM modified with AdNull 1 week previously. (C) Mice receiving AM modified with AdmGM-CSF 1 week previously. (D) Similar to (B), but after 5 weeks. (E) Similar to (C), but after 5 weeks. Bar = 100 μm.
DISCUSSION
The ability of the lung to defend itself against inhaled organisms depends, to a significant degree, on the number and function of alveolar macrophages in the respiratory epithelial surface.1-3 The AM is a member of mononuclear phagocyte system and, as such, is derived from the monocytic lineage of bone marrow cells.1,2 The number of AM on the respiratory epithelial surface results from a balance of three processes: monocytes recruited from the blood, AM proliferating in situ, and AM dying or leaving the lung via the mucociliary escalator or the interstitial lymphatics.1-3 Although a small proportion of AM do proliferate in situ,12,13,24 the vast majority of AM are normally derived from blood monocytes recruited to the lung. The present study shows that this balance can be significantly altered by modifying the genetic repertoire of the AM using an Ad vector to transfer and express the cDNA coding for mGM-CSF, a mediator known to mediate AM proliferation.15,16 In this regard, despite the general concept that AM are terminally differentiated and the challenges in genetically modifying AM, the data demonstrate that Ad vectors can transfer and express genes in AM and that the AM remain genetically modified when transplanted to the lungs of syngeneic mice. Importantly, in vitro genetic modification of AM with the mGM-CSF cDNA and transplantation of the mGM-CSF cDNA-modified AM to the respiratory epithelial surface of syngeneic mice result in enhanced proliferation of the AM in situ in the lung, with an associated increase in AM number for at least 5 weeks, with Ad genome still detectable at 5 weeks. Finally, unlike the marked lung morphologic changes and recruitment of multiple inflammatory cell types associated with lung epithelial expression of mGM-CSF,25 the selective genetic modification of AM with the mGM-CSF cDNA results in selective enhancement of AM numbers, with no associated pulmonary pathology.
Gene transfer to AM.
The ability to transfer and express a gene in cells in vitro and in vivo is a powerful tool to help understand the biology of the transferred gene and the target cell and can be used as a therapeutic strategy in which the gene product functions to modify the pathologic processes within, or external to, the modified cells.26,27Gene transfer is conventionally accomplished using a vector, a physical or biologic carrier that transfers the gene of interest into the cell and to the site where it will be expressed, usually the nucleus. With the armamentarium of nonviral and viral vectors available, most cell types can be genetically modified. One exception is the AM, cells that likely impose a hurdle to efficient gene transfer through their highly efficient capacity to scavenge and eliminate foreign material1-3; ie, the AM likely destroys the vector and gene before the gene can be translocated and expressed. To circumvent this hurdle, we hypothesized that adenovirus vectors, the most efficient of the gene transfer vectors in regard to entering cells and trafficking to the nucleus,27,28 may be able to transfer and express genes in AM by virtue of the efficiency of Ad breaking out of endosomes before endosome-lysosome fusion.29
Various approaches to gene transfer to mononuclear phagocytes, including AM, have been attempted. The nonviral approaches include Fc-receptor-mediated endocytosis for AM targets in vitro,30glycosylated polylysine complexes for monocyte-derived macrophages in vitro,31 and mannosylated polylysine complexes to target spleen and liver macrophages in a murine model in vivo.32Terminally differentiated tissue macrophages are not good targets for murine Maloney leukemia virus derived retroviral vectors because of the requirement of this vector for proliferation of the target cells for effective gene transfer33; thus, the use of retrovirus vectors relevant to mononuclear phagocytes has focused primarily on targeting proliferating stem cells of the monocytic lineage.34,35 In vitro gene transfer to monocyte-derived macrophages has been reported for a replication-deficient herpes simplex-derived vector36 and an autonomous parvovirus vector37 and to AM with an adeno-associated vector.38 Ad vectors have been used to transfer genes into monocyte-derived macrophages.39-43 In general, the efficiency of gene transfer to macrophages is much lower than gene transfer to other cell types.
The concept of using Ad vectors to infect AM is based on the knowledge that wild-type Ad can persist in monocytic cell lines for more than 1 year,44 and wild-type Ad infection has been described in murine, porcine, canine, and bovine AM.45-48 Although we have been successful in transferring genes to a high proportion of naive AM in vitro, it does require a high multiplicity of infection. The reason for this is unclear; it may be because of the efficiency of AM in scavenging and destroying microorganisms and possibly because of a deficiency of subgroup C Ad receptors on the AM surface. In regard to the scavenging function of AM, tissue macrophages, including AM, have been shown to impose a hurdle for Ad-mediated gene transfer to the respiratory epithelium in vivo.49-52 In regard to Ad receptors, AM do not appear to have an abundance of the appropriate receptors for subgroup C Ad,53 and upregulating integrins relevant for Ad internalization has resulted in improved gene transfer efficiency in monocyte-derived macrophages.40,42Interestingly, despite the high moi required to achieve high efficiency of gene transfer to AM, the Ad does not appear to significantly injure AM, as it does with high moi in some cell types.54 55
Effects of GM-CSF transfer to AM.
GM-CSF is a potent cytokine and growth factor with a variety of functions. It is normally expressed by macrophages, T cells, mast cells, endothelial cells, and fibroblasts in response to immune and inflammatory stimuli.56 GM-CSF acts in a paracrine fashion and usually does not appear in the circulation at detectable levels.56 Among the biological activities that have been associated with GM-CSF relevant to macrophages in vitro are proliferation, cytokine expression, increased parasite killing, and tumor cell killing.56-61 Systemic overexpression of GM-CSF in vivo is associated with hematopoiesis, eosinophilia, and the development of a myeloproliferative syndrome56,62; whereas GM-CSF knock-out mice developed severe lung pathology similar to alveolar proteinosis.63 Transgenic animals overexpressing GM-CSF developed accumulations of macrophages in the eyes, muscle, peritoneal, and pleural cavities.64 In contrast, transgeneic mice overexpressing GM-CSF specifically in lung epithelial cells resulted in hyperplasia of alveolar type II epithelial cells.65 Recombinant GM-CSF has been shown to induce proliferation of AM in vitro.15,16 In vivo recombinant GM-CSF does not increase AM number in the lung after subcutaneous daily injections66 or intratracheal administration67in mice, whereas administration of aerosolized GM-CSF to the lungs of nonhuman primates resulted in an increased number of macrophages and neutrophils in the lung.68
With this background, our study demonstrates that transplantation to the lung of AM genetically modified to express mGM-CSF leads to AM proliferation in vivo in the respiratory tract, whereas administration of recombinant GM-CSF did not result in increased AM number. Expression of GM-CSF within the AM seems more efficient than administration of the recombinant protein due to the site of expression and the continuous production, although we did not directly compare the potency of recombinant GM-CSF, which has been previously shown to induce AM proliferation,15,16 and AdGM-CSF on AM proliferation in vitro. Interestingly, the transplantation of AM genetically modified with the mGM-CSF cDNA to the murine lung has different consequences than the delivery of the mGM-CSF cDNA to the respiratory epithelium of rats.25 In this context, our observation that transplantation of mGM-CSF cDNA modified AM leads to enhanced AM proliferation and expansion of AM without accumulation of other inflammatory cell types and no associated lung pathology differs markedly from the observation of Xing et al25 that Ad vector-mediated transfer of the GM-CSF cDNA to the respiratory epithelium results in accumulation of eosinophils in addition to AM and the associated development of fibrosis. Because the mGM-CSF cDNA was transferred to the AM in our study using an Ad vector similar in design to that used by Xing et al25 to transfer the GM-CSF cDNA to the respiratory epithelium, the marked differences in the results suggest that expression of the GM-CSF cDNA may display different biologic properties depending on the cell in which it is expressed. Alternatively, the transfer of mGM-CSF to the respiratory epithelium may result in the overproduction of GM-CSF leading to systemic effects (as we have observed in Ad-vector mediated transfer of the thrombopoietin cDNA to the respiratory epithelium69), because the concentration of GM-CSF in the lavage fluid reported by Xing et al was between 100 and 20,000 pg/mL compared with 54 pg/mL in our study. GM-CSF–producing macrophages could be taken up into the pulmonary interstitium and traffic to the regional lymph nodes. This could theoretically influence the numbers of macrophages in the lung, but this is unlikely, because there would be no specific mechanism where this would increase new alveolar macrophages in the lung. Because the number of AM in the lung after AM GM-CSF transfer doubled but the recovered AM demonstrated an eightfold increase in thymidine incorporation in vitro, the possibility exists that the half life of the GM-CSF–transduced AM could be decreased if the in vitro proliferation reflects the in vivo behavior accurately. If the number of cells expressing GM-CSF would have been similar to that seen with the GFP-transduced AM, where about 2% of the recovered cells showed clear transgene expression, the number of AM would have theoretically increased by 12% to 16% after 1 week, based on our in vitro studies. However, the evaluation of GFP expression by fluorescence microscopy of freshly isolated cells most likely underestimates the number of transgene expressing cells, because weakly positive cells will not be detected due to the relative high autofluorescence; furthermore, the in vivo doubling behavior of AM might be different than in vitro. Systemic overexpression of the mGM-CSF cDNA in mice resulted in increased hematopoiesis and eosinophilia and leads to the development of a myeloproliferative syndrome62; ie, marked overexpression of GM-CSF might be potentially dangerous and outweigh the potential benefits of selective, locally produced GM-CSF.
Potential usefulness of genetic manipulation of AM.
Alveolar macrophages represent an interesting target for genetic modification due to their role in the innate and adaptive immune response, importance in combating infectious organisms, and role in inflammatory disorders as well as being a site for replication of a variety of infectious agents, such as human immunodeficiency virus (HIV).1-3 For example, GM-CSF cDNA-modified AM might be useful in enhancing pulmonary host defense in states of GM-CSF deficiency such as pulmonary alveolar proteinosis and possibly for anticancer strategies.56-62 Using Ad-mediated modification of AM could also be easily accomplished using the cDNAs for other cytokines that may be beneficial to increase pulmonary host defense potential, such as interferon-γ.70-73 An additional benefit of the ex vivo/in vivo strategy used in the present study is that it had relatively long-lasting effects compared with direct Ad vector gene transfer to the lung. It is of interest, therefore, that actively proliferating macrophages could still be recovered 5 weeks after AM transfer, a time period when Ad vector gene expression after direct administration of an Ad vector to the respiratory tract decreases to undetectable levels in Balb/c mice.74 Finally, genetic manipulation of AM and the subsequent in vivo transplant of the genetically modified cells might not only be beneficial for potential therapeutic applications, but might also be a useful tool to study the physiologic and pathophysiologic role of various mediators in the respiratory tract.
ACKNOWLEDGMENT
The authors thank B. Ferris and R. Ramaligan in our laboratory for helpful advice, Berns Gansbacher (University of Munich, Munich, Germany) for the mGM-CSF cDNA, and N. Mohamed in help for preparing this manuscript.
Supported in part by the National Institutes of Health (NIH)/National Heart, Lung and Blood Institute (Grants No. P01 5-P01-HL51746, 1-P01-HL59312, and HL51746-03); the Cystic Fibrosis Foundation (Bethesda, MD); the Will Rogers Memorial Fund (White Plains, NY); and GenVec, Inc (Rockville, MD). M.A.S.M. is supported by the Gar Reichman Fund of the Cancer Research Institue (New York, NY) and NIH/Cancer Center Support Grant No. CA-08748.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Ronald G. Crystal, MD, The New York Hospital-Cornell Medical Center, 520 E 70th St, ST 505, New York, NY 10021; e-mail: geneticmedicine@mail.med.cornell.edu.

![Fig. 8. Proliferation of AM after in vitro infection of AM by AdmGM-CSF and subsequent transplant of the genetically modified AM to the lung. Shown is in vitro proliferation of AM recovered by lavage 1 and 5 weeks after AM transfer. (A) Proliferation in vitro of AM recovered 1 week after transplantation. Shown is in vitro assessment of [3H]thymidine uptake over 24 and 72 hours for AM recovered from naive mice, from mice receiving intratracheal recombinant mGM-CSF (60 ng), from mice receiving AM modified with AdNull (AM Null), and from mice receiving transplanted AM modified with AdmGM-CSF (AM GM-CSF). (B) Proliferation in vitro of AM recovered by lavage 5 weeks after AM transfer. Shown is in vitro assessment of [3H]thymidine uptake over 24 and 72 hours for AM recovered from mice receiving transplanted AM modified with AdNull (AM Null) or AM modified with AdmGM-CSF (AM GM-CSF). Note that the ordinates of (A) and (B) are different; the assays performed at 1 week (A) and 5 weeks (B) are performed at different times with fresh cells; thus, the overall extent of [3H]thymidine uptake cannot be compared between the two panels. (C) Presence of Ad genome in AM 1 and 5 weeks after AM transfer. DNA was analyzed using PCR with primers to amplify Ad genome (E4), Ad genome plus mGM-CSF transgene (Ad-GM-CSF), and HK-ATPase as control. Shown is ethidium bromide staining of amplified DNA from naive control, AM Null at 1 and 5 weeks, and AM GM-CSF 1 and 5 weeks after AM transfer.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/2/10.1182_blood.v93.2.655/4/m_blod40202008ax.jpeg?Expires=1767839257&Signature=d-GkGeEHhpJUojisK31b6MBWb7TycGu4BMbvZUk7JrtiR9IMvQLBNeej0kLZl7cm5T3UwmHgpbazvq~QGD1Hvfhbs6qn01FnaZ1-AZecfFgyPOl-6qFtNJrhcdqAYvuTwaR9ktIKfZYms8BmGWxmVrK1mUceu8jakmaehBb-sQNxPllCdU4n1mLcGKp7uL0viBLUAWXNudW~HlAGtQnMMU3M~ua5wURQHQlZgpZixPEAl5KJ-zjrqv1seC-ebAbDGHbaMYSf8RkC2T5Pw5Cmk83Ik70nsugyLKFSI8NBiHV4E8TiJmVCJao9YIBA6WSMgTXM1qJoFgZABkWoWPorbw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 8. Proliferation of AM after in vitro infection of AM by AdmGM-CSF and subsequent transplant of the genetically modified AM to the lung. Shown is in vitro proliferation of AM recovered by lavage 1 and 5 weeks after AM transfer. (A) Proliferation in vitro of AM recovered 1 week after transplantation. Shown is in vitro assessment of [3H]thymidine uptake over 24 and 72 hours for AM recovered from naive mice, from mice receiving intratracheal recombinant mGM-CSF (60 ng), from mice receiving AM modified with AdNull (AM Null), and from mice receiving transplanted AM modified with AdmGM-CSF (AM GM-CSF). (B) Proliferation in vitro of AM recovered by lavage 5 weeks after AM transfer. Shown is in vitro assessment of [3H]thymidine uptake over 24 and 72 hours for AM recovered from mice receiving transplanted AM modified with AdNull (AM Null) or AM modified with AdmGM-CSF (AM GM-CSF). Note that the ordinates of (A) and (B) are different; the assays performed at 1 week (A) and 5 weeks (B) are performed at different times with fresh cells; thus, the overall extent of [3H]thymidine uptake cannot be compared between the two panels. (C) Presence of Ad genome in AM 1 and 5 weeks after AM transfer. DNA was analyzed using PCR with primers to amplify Ad genome (E4), Ad genome plus mGM-CSF transgene (Ad-GM-CSF), and HK-ATPase as control. Shown is ethidium bromide staining of amplified DNA from naive control, AM Null at 1 and 5 weeks, and AM GM-CSF 1 and 5 weeks after AM transfer.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/2/10.1182_blood.v93.2.655/4/m_blod40202008bx.jpeg?Expires=1767839257&Signature=OP23X6cbTMCvqYzoAemoCqVVwfRZxJJxFND2w7cj13UX4L1xWsDgVDC-ZXlp1cbECT3atmnVyJxdmZbVnGQAsx6XYVpRpMH3sbHKhoFsXOXtxuNAVn9wQzfkhrxZSoRH8ZIuQckh3fAYzXKWWIQwi0UHhlbc3OKqXhqxj85e-g41QxxqE84JkG515rQT-MQ5Gk2hEbBwVv7vUes-sRZ2j0NWZkCIKXssP7Rvcb4Ap5uP~g4dYp2bYWq0nI6pDYuPo9g6ycsk43qsly7P5st0rZq686Plr82K657Ex~h6~m7jbDVpGE9m9ZwiWYVHooDOxRWKxkV8RWOO1AuVBxlWog__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 8. Proliferation of AM after in vitro infection of AM by AdmGM-CSF and subsequent transplant of the genetically modified AM to the lung. Shown is in vitro proliferation of AM recovered by lavage 1 and 5 weeks after AM transfer. (A) Proliferation in vitro of AM recovered 1 week after transplantation. Shown is in vitro assessment of [3H]thymidine uptake over 24 and 72 hours for AM recovered from naive mice, from mice receiving intratracheal recombinant mGM-CSF (60 ng), from mice receiving AM modified with AdNull (AM Null), and from mice receiving transplanted AM modified with AdmGM-CSF (AM GM-CSF). (B) Proliferation in vitro of AM recovered by lavage 5 weeks after AM transfer. Shown is in vitro assessment of [3H]thymidine uptake over 24 and 72 hours for AM recovered from mice receiving transplanted AM modified with AdNull (AM Null) or AM modified with AdmGM-CSF (AM GM-CSF). Note that the ordinates of (A) and (B) are different; the assays performed at 1 week (A) and 5 weeks (B) are performed at different times with fresh cells; thus, the overall extent of [3H]thymidine uptake cannot be compared between the two panels. (C) Presence of Ad genome in AM 1 and 5 weeks after AM transfer. DNA was analyzed using PCR with primers to amplify Ad genome (E4), Ad genome plus mGM-CSF transgene (Ad-GM-CSF), and HK-ATPase as control. Shown is ethidium bromide staining of amplified DNA from naive control, AM Null at 1 and 5 weeks, and AM GM-CSF 1 and 5 weeks after AM transfer.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/2/10.1182_blood.v93.2.655/4/m_blod40202008cw.jpeg?Expires=1767839257&Signature=vWMhKBJV8RhqrF7t1yyZAE0XifLSHQkKNcUa2Ns2ei6mba~RFpYXHVPVED-ZMXMSIq1jId5q-ouf8KaHUfxR3Aa52s3ySkhffOu-Xfb89LDel0PAz4dSQ5wDQsN-Y3m2UQwsGvy785UalpCi86mjm-Z46aHaVkhXGgiMsO4cPV6zHuyLCJzAQ3S7mQvEwtJryhO1Jwnrk2gEO3ZnlH8bJMibgrxZOdsND1NVXAyz2YmBXruo3vEOHTFm7CUDdQp3AVnIdmzKsQzv9b-H~lWxYS-YpDmw62Ni6KQx12BW88ROOTeK5vU39oMSnvqEa~gh3QimiUzkWlprnsyn4~Iw3w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)