Abstract
GATA-1 is a transcription factor required for development of erythroid cells. The expression of GATA-1 is tightly restricted to the hematopoietic lineage. Using transgene constructs containing zebrafish GATA-1 genomic sequences and the green fluorescent protein (GFP) reporter gene, we previously showed that a 5.6-kb enhancer/promoter fragment is sufficient to direct erythroid-specific expression of the GFP. In this study, we used enhancer/promoter fragments containing various deletion and point mutations to further characterize the cis-acting elements controlling tissue-specific GATA-1 expression. We report here the identification of distinct cis-acting elements that cooperate to confer on GATA-1 its hematopoietic expression pattern. A CACCC box, located 142 bp upstream of the translation start codon, is critical for the initiation of GATA-1 expression. A distal double GATA element is required for maintaining and enhancing the hematopoietic expression of GATA-1. The erythroid-specific activity of the GATA-1 promoter is also enhanced by a 49-bp sequence element located 218 bp upstream of the CACCC element and a CCAAT box adjacent to the double GATA motif. Finally, the hematopoietic specificity of the GATA-1 promoter is secured by a negative cis-acting element that inhibits expression in the notochord.
EXPRESSION OF THE transcription factor GATA-1 is restricted to hematopoietic cells, including erythroid progenitors, erythrocytes, megakaryocytes, mast cells, and eosinophils.1-3 Mouse embryonic stem cells with a disrupted GATA-1 gene fail to give rise to mature red blood cells,4indicating that GATA-1 is an essential regulator of the specification of progenitor cells to an erythroid fate. Differentiation assays in vitro have shown that murine GATA-1- embryonic stem cells could differentiate into erythroid precursors, but undergo cell-cycle arrest and death at the proerythroblast stage.5,6 This suggests that GATA-1’s function is to prevent apoptosis of erythroid precursors.7 Selective loss of GATA-1 expression in megakaryocytes of mutant mice resulted in a reduction in the number of platelets and produced hyperproliferation of megakaryocytes, indicating a role for GATA-1 in regulating these cell types.8
Given the importance of GATA-1 in specifying the erythroid lineage, defining the mechanisms underlying its tissue-specific expression has been a central issue in the blood field. Analyses of GATA-1 promoter activity in vitro have shown that GATA motifs are required for high levels of GATA-1 expression in erythroid cell lines.9-12These data suggest that GATA-1 expression is maintained by an autoregulatory mechanism during erythroid cell development. However, these analyses fail to account for the molecular mechanisms that control the initial expression of GATA-1. In addition, recent experiments have shown that expression of a lacZ reporter gene under the control of a GATA-1 promoter can be expressed in a mouse lacking the GATA-1 gene,13 suggesting that other GATA factors may be involved in the regulation of GATA-1 expression.
GATA-1 was identified through the isolation of factors that bound to a DNA sequence motif containing the core element WGATAR that is common to virtually all promoters of genes that are expressed specifically in erythroid cells.2,14 Similarly, the identification of cis-acting sequence elements required for tissue-specific expression of GATA-1 may lead to the isolation of upstream factors that regulate GATA-1 expression. The zebrafish model has several advantages for in vivo identification of cis-acting elements required for enhancer/promoter activities. By microinjecting DNA constructs containing tissue-specific promoters ligated to the green fluorescent protein (GFP) reporter gene, one can continuously observe the dynamic expression patterns of GFP in living transparent embryos.15Because hundreds of embryos can be microinjected within a single day, the transient expression of multiple constructs can be analyzed in a short amount of time. In addition, germline transgenic fish can be obtained from the microinjected embryos and used for further examination of stable expression patterns. Using this approach, we previously showed that a 5.6-kb enhancer/promoter fragment of GATA-1 is sufficient to direct erythroid-specific expression of the GFP in both transient and stable germline transgenic zebrafish.16 In this study, we further define the individual cis-acting elements required for hematopoietic expression of the GATA-1 gene. Our results show that, in conjunction with the positive cis-elements, a negative cis-element is used to repress nonspecific expression of the GATA-1 gene, thereby confining expression to hematopoietic cells.
MATERIALS AND METHODS
Generation of constructs.
Plasmid G1-GM2, which contains 5,552 bp of 5′ flanking sequence of the zebrafish GATA-1 gene linked to GM2, a modified GFP,16 was used as our basal construct. For mapping the distal control region, constructs 4967GM2, 4847GM2, 4776GM2, 4742GM2, 4683GM2, 4648GM2, 4623GM2, 4271GM2, 3590GM2, and 2564GM2 were generated by polymerase chain reaction (PCR) using SP6 primer specific for vector sequences in combination with specific primers P4967, P4847, P4776, P4742, P4683, P4648, P4623, P4271, P3590, and P2564, respectively. The numbers included in the primer names refer to the positions of their first 5′ base in the GATA-1 genomic sequence; position +1 denotes the translation start codon. Each specific primer consists of 22 to 30 nucleotides. Constructs 4648m1GM2, 4648m2GM2, and 4648m3GM2 were generated by PCR using mutant primers P4648m1 (5′-ACTCCAATCTAGCCAGCTTCTTATCA-3′), P4648m2 (5′-ACTCCAATCTAGATAGCTTCTTCCCA-3′), and P4648m3 (5′-ACTCGCCTCTAGATAGCTTCTTATCA-3′), respectively. Each mutant primer contains 2 to 3 altered bases (underlined). G1-GM2 was used as template in the above PCR reactions. PCR reactions were performed using the Expand High Fidelity PCR System (Boehringer Mannheim, Indianapolis, IN) for 25 cycles (94°C, 30 seconds; 55 to 65°C, 30 seconds; 68°C, 2 to 5 minutes). These PCR products were gel-purified and used directly for microinjections without subcloning.
To generate construct G1m-GM2, three DNA fragments (A-C) derived from construct G1-GM2 were ligated together. Fragment A, containing the distal region immediately upstream from the double GATA motif (see Results), was amplified by PCR using a T7 primer and the specific primer (5′-TGGGGTACCTAGATTGGAGTGGGAGGTTGGG-3′) and was digested with SalI/KpnI. Fragment B, containing the proximal region immediately downstream from the double GATA motif, was amplified by PCR using an SP6 primer and the specific primer (5′-TGGGGTACCACAGTTCAGCAGCAGCGCACA-3′) and was digested with KpnI/BamHI. Fragment C was produced by deleting the 5.6-kb promoter region from G1-GM2 by digesting withSalI/BamHI. For microinjections, construct G1m-GM2 was linearized with XhoI.
In XeX-GM2, the GM2 gene was driven by a 450-bp Xenopuselongation factor (EF) 1α enhancer/promoter sequence.17 The 150-bp enhancer was removed byXhoI/SphI digestion to generate Xs-GM2. To generate construct G1DE-Xs-GM2, a 2,589-bp XhoI/ClaI (blunt-ended) fragment (from -2968 to -5552) containing the distal positive control region of the zebrafish GATA-1 promoter was ligated to Xs-GM2. Before microinjection, this construct was linearized withXhoI. Linearized Xs-GM2 was used as control for microinjection.
To identify proximal regulatory elements, a series of deletion constructs with varying sizes of the middle region of the zebrafish GATA-1 promoter was generated by PCR using G1-GM2 as template. These constructs are DE139, DE168, DE191, DE259, DE367, DE421, DE468, DE501, DE613, DE764, and DE1776, in which DE represents the -5552/-4256 distal positive control region and the numbers represent the length of the remaining proximal region (upstream from the translation start codon). A pair of primers, RP and Pn, were used to generate each construct. Primer RP (5′-ATGAATTCCATTGAGCGTACTGTAATAT-3′) is complementary to the sense strand, contains an EcoRI site (underlined), and was used in all of the PCR reactions. The Pn primers are complementary to the antisense strand and each one was used in a specific PCR reaction. The PCR products were treated with 10 U Klenow fragment of DNA polymerase at 37°C for 1 hour, purified by electrophoresis, allowed to self-ligate, and used to transform bacterial cells. The same strategy was used to generate constructs DE168m1, DE168m2, and DE168m3, in which primers containing base replacements were used in the PCR reactions. The three corresponding mutant primers are P168m1 (5′-CCAACCCCAAGTACCCCAACCCCACCCAT-3′), P168m2 (5′-CCAAAAAAAAGTACCCTTTCCCCACCCAT-3′), and P168m3 (5′-CCAAAAAAAAGTACCCCAACCCTTTCCAT-3′) (modified bases are underlined). The promoter/GFP inserts of these constructs were amplified using primers P4967 and SP6 to remove the vector sequence and a 585-bp unnecessary 5′ distal region of the promoter. The PCR products were purified and directly used for microinjection unless otherwise stated. These PCR reactions were performed using the Expand High Fidelity PCR System (Boehringer Mannheim) for 25 cycles (94°C, 30 seconds; 62°C, 30 seconds; 68°C, 3 minutes).
Microinjection of zebrafish embryos.
For microinjections, the digested DNA or PCR fragments were purified using GENECLEAN III Kit (Bio 101 Inc, Vista, CA), and resuspended in 5 mmol/L Tris, 0.5 mmol/L EDTA, 0.1 mol/L KCl at a final concentration of 50 μg/mL. Approximately 0.125% tetramethyl-rhodamine dextran was included in the DNA preparation as a microinjection control. Fertilized eggs from wild-type zebrafish were dechorionated by pronase treatment and microinjected at 1-cell stage.15 Each construct was microinjected independently three to eight times to generate sufficient numbers of surviving embryos for observation.
Fluorescent microscopic observation.
The microinjected embryos were examined for GFP expression at various developmental stages under a fluorescein isothiocyanate (FITC) filter on a Zeiss microscope (Germany). Live embryos were anesthetized using tricaine as described previously.15Embryos were considered positive for GFP expression if they had more than five GFP-positive cells in the early hematopoietic tissue, the intermediate cell mass (ICM), and later in the circulating blood. The percentage of blood-specific, GFP-positive embryos after microinjections was calculated to evaluate the GATA-1 promoter/enhancer activities of the constructs. Data obtained from independent microinjections with the same construct were pooled.
Transgenic fish expressing GFP were identified through fluorescent microscopic observation. The microinjected founder fish were mated to wild-type fish, and their progeny were observed for GFP expression. The founder fish that produced GFP positive eggs were considered transgenic and used to breed into homozygotes.
RESULTS
Positive erythroid-specific elements are present in the distal 5′ flanking region.
We previously generated a construct, G1-GM2 (Fig 1A), by ligating a modified GFP gene (GM2) to a 5.6 kb zebrafish GATA-1 genomic fragment upstream of the translation start codon.16 Transgenic zebrafish carrying the G1-GM2 transgene have GFP expression in hematopoietic progenitors and erythrocytes. The expression pattern of GFP recapitulated that of the GATA-1 gene as shown by RNA in situ hybridization,18suggesting that the 5.6-kb putative GATA-1 promoter/enhancer contains all of the regulatory elements necessary for GATA-1 expression in the erythroid lineage. To facilitate the identification of regulatory elements, we sequenced the entire 5.6 kb promoter/enhancer. Sequence alignment analysis using computer software (DNA STAR, Madison, WI) failed to show any highly conserved sequences between the 5′ flanking region of the zebrafish GATA-1 gene and those of mouse and human. A search of the transcription factor database using MatInspector V2.119 showed hundreds of potential transcription factor binding sites within this 5.6 kb sequence, including four double GATA motifs, a type of GATA site that appears to be important for expression of many erythroid-specific genes.9-12,20 21 To determine which potential transcription factor binding sites are functionally important, a systematic deletion analysis was performed.
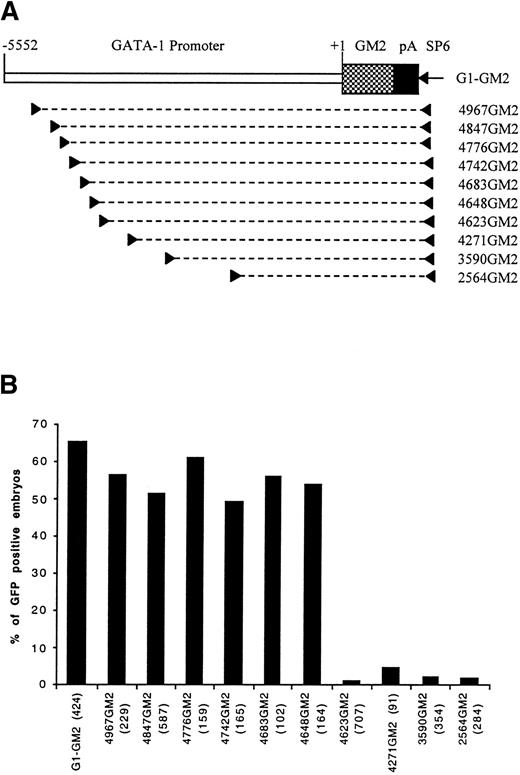
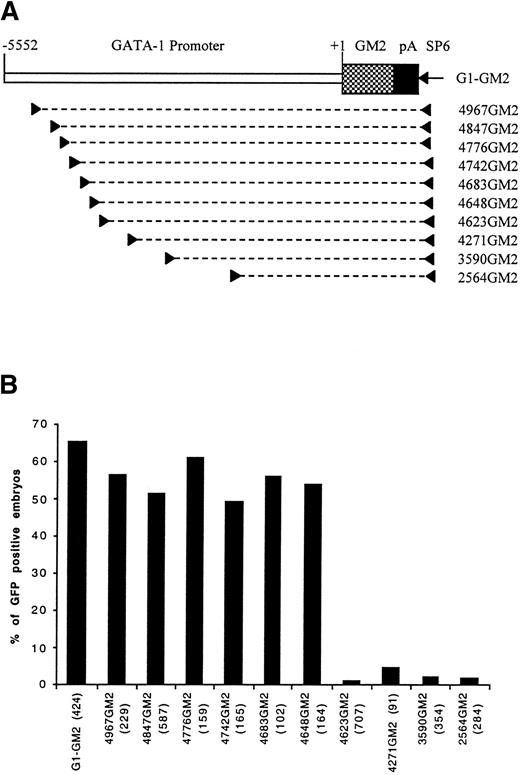
Identification of a distal control region by PCR dissection of GATA-1 promoter. (A) A map of construct G1-GM2 is shown. To generate deletion constructs, a specific primer (left arrowhead) and SP6 primer (right arrowhead) were used to amplify a portion of G1-GM2, as denoted by the broken line. (B) The percentages of GFP-positive 48-hour embryos obtained after microinjection of these constructs are shown, with the number of embryos observed for each construct indicated in parentheses.
Identification of a distal control region by PCR dissection of GATA-1 promoter. (A) A map of construct G1-GM2 is shown. To generate deletion constructs, a specific primer (left arrowhead) and SP6 primer (right arrowhead) were used to amplify a portion of G1-GM2, as denoted by the broken line. (B) The percentages of GFP-positive 48-hour embryos obtained after microinjection of these constructs are shown, with the number of embryos observed for each construct indicated in parentheses.
Four deletion constructs, 4967GM2, 4271GM2, 3590GM2, and 2564GM2, containing the GM2 gene under the control of variable lengths of the GATA-1 promoter/enhancer region were generated (Fig 1A) and used to microinject zebrafish embryos. When the construct, 4967GM2, was microinjected, approximately 60% of the microinjected embryos had GFP expression in embryonic circulating blood cells at 48 hours postfertilization. This result was similar to those obtained with the original G1-GM2 construct (Fig 1B). However, in embryos microinjected with constructs 4271GM2, 3590GM2, or 2564GM2, GFP-positive circulating blood cells were nearly absent (Fig 1B). Because these three constructs share a deletion of a 696-bp sequence extending from -4967 to -4271, we concluded that this region contains positive control elements necessary for the expression of zebrafish GATA-1 in early embryonic circulating blood cells.
To precisely map the 696-bp distal positive control region, we generated six more constructs, 4847GM2, 4776GM2, 4742GM2, 4683GM2, 4648GM2, and 4623GM2 (Fig 1A), with progressive deletions in the 696-bp sequence. After microinjection with construct 4623GM2, only seven of the 707 observed embryos had a few circulating GFP-positive blood cells. In contrast, microinjection with any of the other five constructs resulted in approximately 50% of embryos expressing GFP in circulating blood cells (Fig 1B). This suggests that a 26-bp sequence from -4648 to -4623 positively regulates hematopoietic expression of GATA-1 gene.
A double GATA motif in the distal positive control region is the key regulatory element.
The 26-bp distal positive control region has a sequence of ACTCCAATCTAGATAGCTTCTTATCA. A search for potential transcription factor binding sites19showed that this sequence contains two consensus GATA motifs (in bold) and a CCAAT box element (underlined). The 5′ GATA motif between -4635 and -4638 is separated by 6 bp from an inverted 3′ GATA motif (TATC) between -4624 and -4627. Clusters of GATA motifs are found in humans,10 mouse,10,12 and chicken9,11 GATA-1 promoters and in other erythroid-specific promoters20,21 and are believed to be important regulatory elements. The CCAAT box element is present in the regulatory regions of many genes and often upregulates gene transcription.22-26 Therefore, deletions and mutations were generated in the GATA and CCAAT elements to investigate their roles in GATA-1 expression in embryonic blood cells.
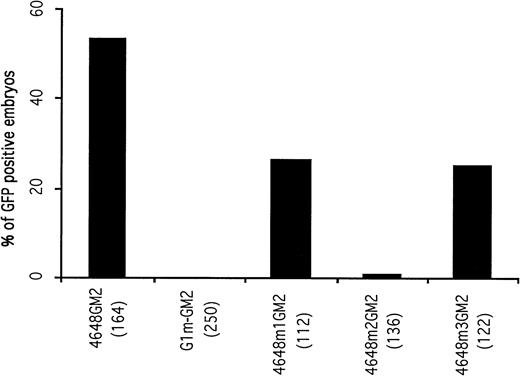
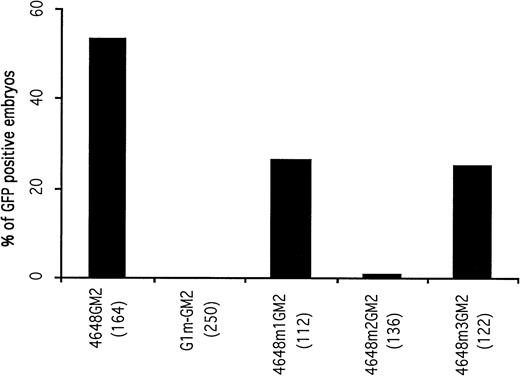
A 14-bp fragment including the double GATA motifs and extending from -4624 to -4637 was deleted from the construct G1-GM2 to generate a new construct, G1m-GM2. A total of 250 embryos microinjected with G1m-GM2 were examined. The microinjected embryos lacked circulating GFP-positive blood cells at 48 hours postfertilization (Fig 2), although GFP expression was observed in some of the microinjected embryos before the 20 somite stage (discussed below). This suggests that the double GATA motif is a cis-acting element essential for the maintenance and enhancement of GATA-1 expression in circulating blood cells.
Mutational analysis of the distal control elements. The percentages of GFP-positive, 48-hour embryos obtained after microinjection of deletion constructs are presented. The result obtained with the 4648GM2 construct is shown again as a control. Deletion of the double GATA motif within the distal control region (construct G1m-GM2) completely abolished GFP expression in circulating blood cells of microinjected embryos.
Mutational analysis of the distal control elements. The percentages of GFP-positive, 48-hour embryos obtained after microinjection of deletion constructs are presented. The result obtained with the 4648GM2 construct is shown again as a control. Deletion of the double GATA motif within the distal control region (construct G1m-GM2) completely abolished GFP expression in circulating blood cells of microinjected embryos.
To address whether the two GATA motifs are equally important for GATA-1 expression in blood cells, two constructs containing mutations in each GATA site were generated. Construct 4648m1GM2 had altered bases in the 5′ GATA motif, while 4648m2GM2 contained mutations in the 3′ inverted GATA motif. Of 136 embryos microinjected with the construct 4648m2GM2, only one embryo contained circulating GFP-positive blood cells. When construct 4648m1GM2 was microinjected, 26.7% of the embryos had GFP expression in circulating blood cells, which was approximately half the number seen using the parent construct 4648GM2 (Fig 2). These results indicate that the 5′ GATA motif is less important than the 3′ inverted GATA motif in maintaining erythroid-specific expression of GATA-1.
To determine if the CCAAT box was required for hematopoietic expression of GATA-1, a mutation construct (4648m3GM2) with CGCCT instead of CCAAT was generated. After microinjection with this construct, 25.4% of the embryos had GFP expression in circulating blood cells. Furthermore, the number of GFP-positive blood cells in the embryo was significantly less than those of embryos microinjected with its parent construct, 4648GM2. These results imply that the CCAAT motif has the ability to enhance the hematopoietic expression of GATA-1.
Activity of distal positive control elements requires its own minimal promoter.
To determine whether activity of the GATA-1 distal positive control elements was context dependent, construct G1DE-Xs-GM2 was generated by ligating a 2,589-bp region from -2968 to -5552 containing the GATA-1 distal control elements (GATA-1 motifs and CCAAT box) to theXenopus elongation factor 1α minimal promoter Xs-GM2.17 Microinjection of Xs-GM2 showed that GFP was expressed in various tissues, including muscle, enveloping layer cells, notochord, and melanocytes (data not shown). Of 283 embryos microinjected with Xs-GM2, however, only two had GFP-expressing blood cells. Similarly, only three of 408 embryos had circulating GFP-positive blood cells after microinjection withG1DE-Xs-GM2. This result indicates that the distal control elements of the GATA-1 requires its own proximal lineage-specific cis-acting elements to exert full activity. As described below, a CACCC box in the proximal region of the GATA-1 promoter is absolutely required for hematopoietic transcription of GATA-1. The same element is not present in the Xenopus elongation factor 1α minimal promoter, which may explain why the G1DE-Xs-GM2 was unable to confer high-level expression of the reporter gene GFP in hematopoietic tissues.
A proximal CACCC box is essential for the expression of GATA-1.
To identify potential cis-acting elements in the proximal region of GATA-1 promoter, we generated a series of constructs that had the distal positive control region, extending from -4256 to -5552, ligated to variable lengths of its downstream sequence followed by the reporter gene GM2 (Fig 3A). These constructs were used to microinject one-cell zebrafish embryos.
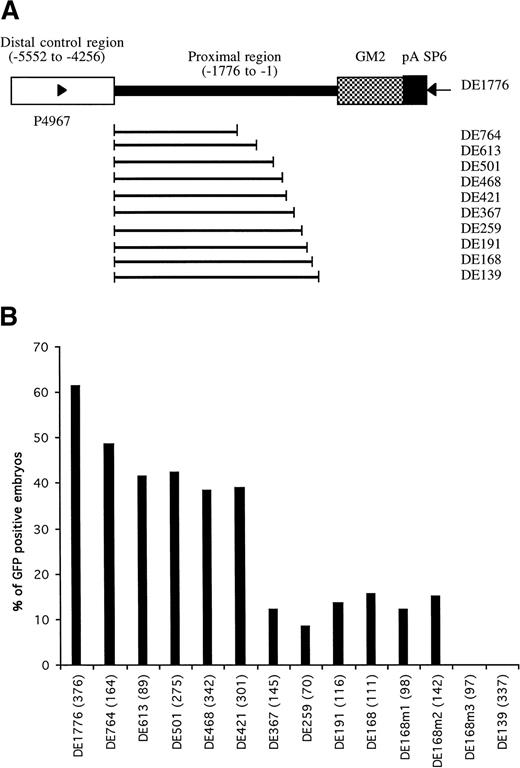
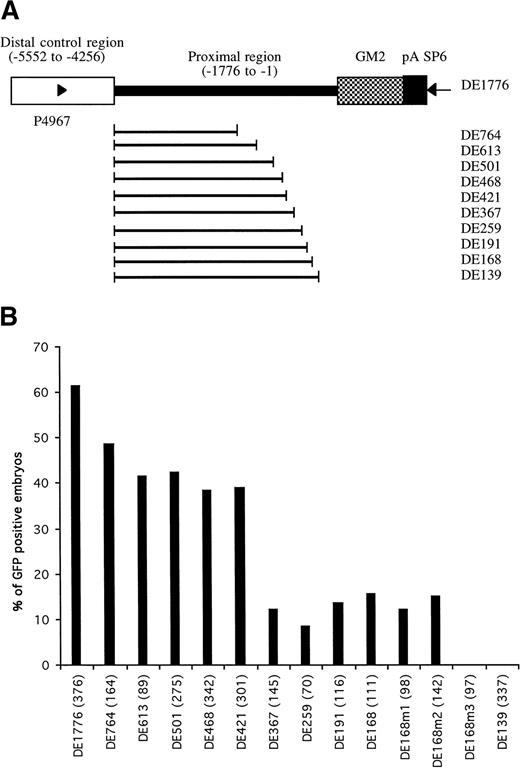
Identification of proximal cis-elements in GATA-1 promoter/enhancer region. (A) Parent construct DE1776 was generated by deleting a sequence between -4257 and -1775 of the GATA-1 promoter from plasmid G1-GM2. Other constructs were generated by deleting variable lengths of sequence between the distal control region and translation start codon. Deleted regions are indicated by the bold line. Primers P4967 and SP6 (arrowheads) were used to amplify the region in each construct required for microinjection (detailed in Materials and Methods). (B) The percentages of GFP-positive 48-hour embryos obtained after microinjection of these constructs are shown, with the number of embryos observed for each construct indicated in parentheses.
Identification of proximal cis-elements in GATA-1 promoter/enhancer region. (A) Parent construct DE1776 was generated by deleting a sequence between -4257 and -1775 of the GATA-1 promoter from plasmid G1-GM2. Other constructs were generated by deleting variable lengths of sequence between the distal control region and translation start codon. Deleted regions are indicated by the bold line. Primers P4967 and SP6 (arrowheads) were used to amplify the region in each construct required for microinjection (detailed in Materials and Methods). (B) The percentages of GFP-positive 48-hour embryos obtained after microinjection of these constructs are shown, with the number of embryos observed for each construct indicated in parentheses.
When constructs retaining a proximal promoter region of at least 168 bp (construct DE168) were microinjected, more than 10% of the embryos showed GFP expression in circulating blood cells (Fig 3B). When the retained proximal region was shortened to 139 bp (construct DE139), no GFP-positive cells were seen in the microinjected embryos. This suggested that the region between -168 to -139 contained important regulatory elements for the hematopoietic expression of zebrafish GATA-1. This 29-bp region has a sequence of CCAAAAAAAAGTACCCCAACCCCACCCAT and is rich in purines at the 5′ end and rich in pyrimidines at the 3′ end. The pyrimidine-rich region contains a potential CACCC box (in bold) that has been shown to play a role in transcriptional regulation of many erythroid genes.9,10,12 27-30 To address the potential role of the CACCC box in the regulation of GATA-1 expression, base mutations were introduced into the CACCC box and adjacent regions, using the construct DE168 as a template (see Materials and Methods). Changes of CAC to TTT in the CACCC box (construct DE168m3) completely eliminated the expression of GFP in the circulating blood cells, whereas the other two mutations outside the CACCC box (DE168m1 and DE168m2) did not affect GFP expression (Fig 3B). This suggests that the CACCC box, located from -146 to -142 in the GATA-1 locus, is absolutely necessary for GATA-1 expression in the hematopoietic lineage.
Initiation of GATA-1 expression requires the proximal CACCC box but not the distal double GATA element.
The experiments described above show that both the distal double GATA motif and the proximal CACCC motif are required for zebrafish GATA-1 expression in circulating blood cells of 48-hour embryos. As in mouse,12,13,31 however, the question of which motif is responsible for the initiation of GATA-1 expression is still unresolved. In zebrafish, GATA-1 expression in hematopoietic progenitor cells starts at approximately the one somite stage.16 18Thus, an earlier observation of microinjected embryos should allow the identification of cis-elements that play a role in the initiation of GATA-1 expression. Embryos microinjected with the construct G1m-GM2, which contained nearly the entire promoter/enhancer region except the distal double GATA motif, did not show GFP expression in circulating blood cells in 48-hour embryos. However, GFP expression was detected at earlier developmental stages, ie, at the 2 to 20 somite stages (Fig 4B). Microinjection of other constructs lacking the double GATA motif, but containing the proximal CACCC box, also produced GFP expression in embryos at earlier stages, but it was not maintained beyond 20 hours after fertilization. This suggests that, although the distal double GATA motif is required to promote and maintain the level of GATA-1 expression, it is not essential for the initiation of GATA-1 transcription. In contrast, when embryos were microinjected either with construct DE139, which lacked the proximal CACCC box, or with construct DE168m3, which had mutations in the CACCC box, no GFP expression was observed at the earlier developmental stages (Fig 4C). This shows that the CACCC box is absolutely required for the initiation of GATA-1 expression.
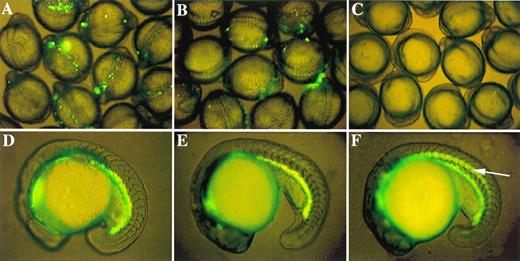
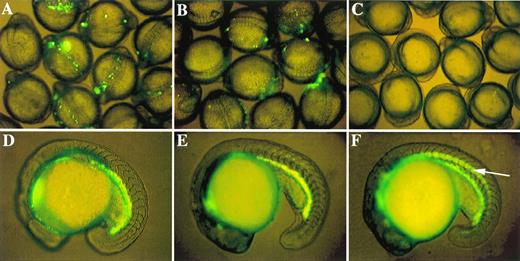
Role of proximal CACCC box in initiation of GATA-1 expression and existence of a negative control element. GFP expression patterns in 9 to 12 somite embryos microinjected with three critical GATA-1/GFP linearized constructs: (A) G1-GM2 produces strong GFP expression; (B) G1m-GM2 produces weak GFP expression; and (C) DE139 shows no GFP expression at all. Zebrafish embryos at the 18- to 19-hour stages transgenic for G1-GM2 (D) or DE1776 (E) show similar patterns of GFP expression. GFP is expressed in the notochord (indicated by an arrow in F) of embryos transgenic for DE468, although GFP is also present in abundance in the hematopoietic intermediate cell mass. Similar results were observed in germline fish transgenic for DE421 (data not shown).
Role of proximal CACCC box in initiation of GATA-1 expression and existence of a negative control element. GFP expression patterns in 9 to 12 somite embryos microinjected with three critical GATA-1/GFP linearized constructs: (A) G1-GM2 produces strong GFP expression; (B) G1m-GM2 produces weak GFP expression; and (C) DE139 shows no GFP expression at all. Zebrafish embryos at the 18- to 19-hour stages transgenic for G1-GM2 (D) or DE1776 (E) show similar patterns of GFP expression. GFP is expressed in the notochord (indicated by an arrow in F) of embryos transgenic for DE468, although GFP is also present in abundance in the hematopoietic intermediate cell mass. Similar results were observed in germline fish transgenic for DE421 (data not shown).
Other positive and negative regulatory elements are required for GATA-1 expression.
We noted that constructs containing both the distal double GATA motif and the proximal CACCC box, but with varying lengths of the region between them, have different enhancer/promoter activity (Fig 3). Microinjection of construct DE1776, containing 1,776 bp of proximal region, resulted in 61.4% of embryos expressing GFP in circulating blood cells. This result was similar to that obtained with the full-length construct G1-GM2. However, microinjection of construct DE367, containing only 367 bp upstream from the translation start codon, produced only 12.1% of microinjected embryos expressing GFP in blood cells. This is significantly less than that obtained with the other six constructs (DE1776, DE764, DE613, DE501, DE468, and DE421). In addition, less than 5% of the GFP positive embryos had more than 10 GFP positive blood cells. This is also significantly lower than that obtained with the other constructs. These results suggest that the 49-bp region extending from -421 to -366 is a proximal positive control region that can increase the blood-specific expression of GATA-1. This region contains potential binding sites for transcription factors such as C/EBPB, AP1, and OCT1, as shown by analysis with MatInspector V2.1.19
When microinjected with G1-GM2, approximately 6% of the microinjected embryos showed nonspecific expression of GFP in notochord, muscle, skin, and other types of cells. Although embryos microinjected with constructs containing deletions between -1777 to -468 continued to express GFP in blood cells, approximately 40% of the microinjected embryos exhibited GFP expression in the notochord (data not shown). This result suggests that a negative cis-acting element may be required to repress nonhematopoietic expression of zebrafish GATA-1 gene.
Essential elements of the GATA-1 promoter are confirmed by analyzing germline transgenic zebrafish.
By observing GFP expression in progeny of the microinjected founder fish, we identified several transgenic zebrafish lines that harbor different GATA-1/GFP constructs, as described above. One transgenic line derived from construct DE1776 had strong GFP expression in hematopoietic progenitors (Fig 4E) and circulating erythrocytes. This pattern is indistinguishable from that of the G1-GM2 germline transgenic zebrafish (Fig 4D).16 This observation confirms that the 2,750-bp between position -4257 to -1775 (deleted in construct DE1776) is not required for proper expression of GATA-1. We have also obtained transgenic germline fish from constructs DE468 and DE421. Consistent with results from the transient expression studies, both lines have a hematopoietic GFP expression pattern that is identical to G1-GM2 transgenic lines (Figs 4F and D). In addition, both DE468 and DE421 transgenic lines have GFP expression in the notochord. This further validates the transient assay results suggesting that negative cis-acting elements play a role in conferring hematopoietic expression of the zebrafish GATA-1 gene.
DISCUSSION
The transcription factor GATA-1 plays an important role in hematopoietic development by regulating the expression of downstream hematopoietic genes.16,32 33 Similarly, the expression of GATA-1 itself must be regulated by other lineage-specific transcription factors. Characterization of the cis-acting elements that control the expression of GATA-1 gene should lead to the identification of factors that act upstream of GATA-1.
To date, much of the knowledge concerning regulation of GATA-1 gene expression has been obtained from studies of the mouse and chicken promoters. Transient transfection assays in cultured cells have shown that a double GATA motif, located upstream of the first exon, is required for full promoter activity of the mouse GATA-1 gene.10,12 In addition, it has been shown that mutations in a CACCC box between the double GATA motif and the first exon can reduce this promoter activity.12 Recent studies in transgenic mice have shown that the activation of GATA-1 gene expression in primitive or definitive erythroid cells is controlled by different regulatory sequences.12,13,31 For instance, a transgene with a short proximal mouse GATA-1 promoter could only express infrequently in definitive erythroid cells.13 The inclusion of an upstream sequence not only increased the expression frequency in definitive erythroid cells, but also activated the expression of the transgene in primitive erythroid cells. However, the specific sequence motifs in that upstream region have not been identified. So far, the implication of CACCC boxes in the initiation of GATA-1 expression has not been shown in the above studies.
Using transgene constructs containing zebrafish GATA-1 genomic sequences and the GFP reporter gene, we previously demonstrated that a 5.6-kb enhancer/promoter fragment is sufficient to direct erythroid-specific expression of the GFP.16 In this study, we have identified individual cis-acting elements that are required for the erythroid-specific expression of the zebrafish GATA-1 gene (Fig 5). We have found that a CACCC box in the proximal region between -146 and -142 is critical for initiating zebrafish GATA-1 expression, whereas a double GATA motif in the distal region between -4635 and -4627 is necessary for enhancing and maintaining hematopoietic expression of the GATA-1 gene. These two regulatory elements cooperate with other positive and negative elements to confer hematopoietic transcription of the GATA-1 gene.
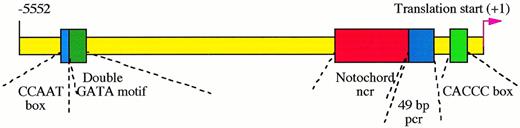
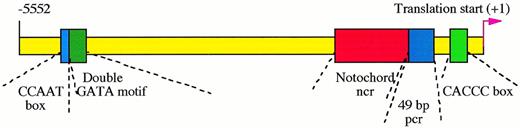
Cis-regulatory elements in zebrafish GATA-1 genomic locus. The proximal CACCC box element at position -146 is absolutely required for the initiation of GATA-1 gene expression in hematopoietic cells, while the distal double GATA motif between -4635 and -4627 is necessary for enhancing and maintaining this expression. The CCAAT box at -4643 and another 49-bp positive control region (pcr) between -421 and -366 strengthen the GATA-1 expression. The expression of GATA-1 in the notochord, a nonhematopoietic tissue, is repressed by a negative control region (ncr) located between -1776 to -468.
Cis-regulatory elements in zebrafish GATA-1 genomic locus. The proximal CACCC box element at position -146 is absolutely required for the initiation of GATA-1 gene expression in hematopoietic cells, while the distal double GATA motif between -4635 and -4627 is necessary for enhancing and maintaining this expression. The CCAAT box at -4643 and another 49-bp positive control region (pcr) between -421 and -366 strengthen the GATA-1 expression. The expression of GATA-1 in the notochord, a nonhematopoietic tissue, is repressed by a negative control region (ncr) located between -1776 to -468.
Cis-regulatory sequence elements mediate transcription specificity and activation by binding to specific proteins. Our studies suggest that factors binding to the CACCC box could be critical for the initiation of GATA-1 expression during embryonic development. The erythroid Kruppel-like factor (EKLF) is the first identified hematopoietic-specific transcription factor that binds to the CACCC box.34 However, it is unlikely that the EKLF is required for GATA-1 expression because GATA-1 is still able to express in murine EKLF-/- embryos.35 BKLF, a second erythroid CACCC-box-binding transcription factor, shows a high-affinity with many CACCC motifs present in the promoters of erythroid-specific genes including GATA-1 in an in vitro assay.36 Whether BKLF or other unidentified erythroid Kruppel-like factors can bind to the CACCC box in vivo and activate the expression of GATA-1 in zebrafish remains to be determined.
Studies in other species showed that a functional GATA motif in the proximal region of GATA-1 promoters was able to be bound by GATA-1.10-12 Based on such observations, a positive autoregulatory mechanism was proposed for increasing and maintaining GATA-1 expression during differentiation and cellular maturation. McDevitt et al (1997) showed that GATA-1 is not required for activation and maintenance of GATA-1 gene expression because a GATA-1/lacZ transgene could express in a GATA-1-background.13 Our studies establish an essential role for the double GATA cis-acting element in maintaining hematopoietic expression of GATA-1. However, which member of the GATA family does this is yet to be identified.
Although the CCAAT box was reported to be involved in blood-specific gene expression,22,24-26,37 its importance in GATA-1 expression has not been determined. We show that a mutation in the CCAAT box immediately upstream of the distal double GATA site significantly reduces GATA-1 expression. Considering the short distance between the CCAAT box and the double GATA motif, factors binding to the CCAAT box might function through interactions with a GATA factor to influence the transcription level of the GATA-1 gene. This kind of interaction may involve multiple factors as suggested by the study of Wadman et al,38 in which an erythroid specific DNA-binding complex including TAL1, E47, GATA-1, and Ldb1/NLI proteins was shown to interact with closely linked GATA and CAGGTG sites.
To the best of our knowledge, this study represents the first report describing a negative regulatory mechanism for blood-specific gene expression. The expression of GATA-1 in the notochord is apparently suppressed through an interaction between a negative regulatory element in the GATA-1 promoter and notochord-derived negative factors. This type of regulation has been reported to repress the expression of neuron-specific genes in nonneuronal tissues.39 40
We identified the cis-acting elements important for GATA-1 promoter activity through a transient, whole zebrafish embryonic reporter gene assay. The data obtained by transient assays have been confirmed by expression patterns obtained in germline transgenic zebrafish. By ligating essential elements of the zebrafish GATA-1 promoter, we generated stable transgenic zebrafish with GFP expression comparable to that obtained by using a full-length promoter. These results validate the zebrafish as a whole embryo system for the efficient identification of those cis-acting elements playing critical roles in modulating the expression of developmentally regulated genes.
ACKNOWLEDGMENT
We thank Jason R. Jessen, Scott Marty, Billie Moore, and Han Wang for helpful discussions and comments on the manuscript.
Supported by grants from the American Heart Association of Georgia and the National Institutes of Health (to S.L.). S.L. is a recipient of the American Society of Hematology Scholar Award.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Shuo Lin, PhD, Institute of Molecular Medicine, Medical College of Georgia, Augusta, GA 30912; e-mail: slin@mail.mcg.edu.