Abstract
In the present study, we investigated the effects of stem cell factor (SCF) and/or thrombopoietin (TPO) on the cell production by cord blood CD34+ cells using a serum-deprived liquid culture system. Although SCF alone supported a modest production of neutrophilic cells and a remarkable generation of mast cells, the addition of TPO to the culture containing SCF caused an apparent generation of neutrophilic cells, identified by immunocytochemical staining and flow cytometric analysis. The significant production of neutrophilic cells by SCF and TPO was persistently observed from 2 weeks to 2 to 3 months of culture. The interaction between SCF and TPO on the neutrophilic cell generation was greater than the combined effects of SCF with granulocyte colony-stimulating factor (G-CSF) or granulocyte-macrophage colony-stimulating factor (GM-CSF). The addition of neutralizing antibody against G-CSF or GM-CSF did not influence the SCF + TPO-dependent neutrophilic cell production. A single-cell culture study showed that not only CD34+CD38+ c-kit+ cells but also CD34+CD38−c-kit+ cells were responsible for the neutrophilic cell generation. In clonal cell cultures, GM progenitors as well as erythroid progenitors and multipotential progenitors expanded in the cultures supplemented with SCF and TPO. The neutrophilic cells grown by SCF + TPO were at myeloblast to band cell stages, and scarcely matured to segmented neutrophils. In addition, the cells generated by SCF + TPO were stained with monoclonal antibodies against myeloperoxidase, elastase, lactoferrin, and CD11b, but they had negligible levels of alkaline phosphatase (ALP) and CD35. The replating of the CD34−c-kit−/low CD15+ cells grown by SCF + TPO into a culture containing SCF + G-CSF permitted both the terminal maturation into segmented cells and the appearance of ALP and CD35. These results indicate the existence of a G-CSF/GM-CSF–independent system of neutrophilic cell production.
A HUGE NUMBER OF CIRCULATING neutrophils are supplied daily from a small number of hematopoietic stem cells to maintain homeostasis. In cases of infection this neutrophil production is rapidly increased. It is generally held that this differentiation pathway is regulated in part by growth factors including granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin (IL)-3, and stem cell factor (SCF). Although there is increasing evidence that G-CSF plays an important role as an endogenous regulator of neutrophil production,1,2 the existence of G-CSF–independent mechanisms of granulopoiesis is also shown on the basis of the results obtained using G-CSF or G-CSF receptor-deficient mice.2 3
Thrombopoietin (TPO), c-mpl ligand, was cloned by several independent groups as a growth factor for the megakaryocyte-platelet lineage.4-8 Treatment with TPO was shown to accelerate platelet, red blood cell, and neutrophil recovery in myelosuppressed mice, indicating the in vivo effects of TPO on multiple cell lineages.9 Kobayashi et al10 reported that TPO synergizes with SCF and/or IL-3 in support of the formation of GM colonies as well as multilineage colonies and erythroid colonies. In addition, TPO was shown to augment the expansion efficiency for various types of hematopoietic progenitors including GM progenitors in the presence of several cytokines including SCF in a liquid suspension culture system.11,12 Our recent study showed that the addition of TPO to cultures containing SCF or SCF + GM-CSF caused a significant increase in the production of GM colony-forming cells by primitive hematopoietic progenitors of patients with juvenile chronic myelogenous leukemia.13 These lines of evidence prompted us to examine the possibility of neutrophil production induced by SCF + TPO. For this purpose, serum-deprived liquid cultures were initiated with CD34+ cord blood cells, and were maintained with the repeated addition of TPO and/or SCF.
MATERIALS AND METHODS
Factors and antibodies.
Human recombinant TPO, SCF, IL-3, GM-CSF, and erythropoietin (EPO) were provided by Kirin Brewery Co, Ltd (Takasaki, Japan). Human recombinant G-CSF and rabbit antihuman G-CSF polyclonal antibody were generously provided by Chugai Pharmaceutical Co (Tokyo, Japan). A 1:10,000 dilution of antihuman G-CSF antibody neutralized the activity of 100 pg of G-CSF.14 A mouse monoclonal antibody (MoAb) for GM-CSF (Ab-1; Oncogene Science, Uniondale, NY) was used at 2 μg/mL, as reported previously.15
For the flow-cytometric analysis, MoAbs for CD34 (8G12, fluorescein isothiocyanate, FITC; allophycocyanin, APC), CD2 (S5.2, FITC), CD14 (MφP9, FITC), CD38 (HB7, APC), and c-kit (104D2, phycoerythrin, PE) were purchased from Becton Dickinson Immunocytometry Systems (Mountain View, CA); the MoAbs for CD15 (80H5, FITC) and CD19 (J4.119, FITC) were from Immunotech S.A. (Marseilles, France), and the MoAb for CD41b (TP80, FITC) was from Nichirei (Tokyo, Japan). The MoAb for glycophorin A (GPA, JC159, FITC) was from Dako (Glostrup, Denmark).
For the immunocytochemical analysis, purified MoAbs for human myeloperoxidase (MPO, CLB-MPO-1) and CD2 (T11) were purchased from Immunotech S.A. The MoAb for human lactoferrin (2B8) was from Advanced Immunochemical (Long Beach, CA); MoAbs for CD11b (2LPM19c), CD14 (Tük 4), CD15 (C3D-1), CD19 (HD37), GPA (JC159), CD35 (To5) and human elastase (NP57) were from Dako; MoAbs for human eosinophil peroxidase (MAB1087) and mast cell tryptase (MAB1222) were from Chemicon International Inc (Temecula, CA).
Cell preparation.
Cord blood samples were aspirated in heparinized plastic syringes from the umbilical vein at normal delivery. Fully informed consent was obtained from the mothers of all neonates before harvesting the specimens. Mononuclear cells (MNCs) were separated by density centrifugation over Ficoll-Plaque (Pharmacia, Fine Chemicals, Piscataway, NJ), washed twice, and suspended in Ca2+- and Mg2+-free phosphate-buffered saline (PBS) containing 1 mmol/L EDTA-2Na, and 2.5% fetal bovine serum (FBS, Hyclone, Logan, UT). After treatment with Silica (Immuno-Biological Laboratories, Fujioka, Japan) for 30 minutes at 37°C, CD34+ cells were enriched using a Dynal CD34 Progenitor Cell Selection System (Dynal A.S., Oslo, Norway). In brief, 1.0 × 107 cells were mixed with the same number of polystyrene beads coated with a MoAb specific for CD34 (Dynabeads M-450 CD34), and incubated for 30 minutes at 4°C. Bead-rosetted cells were separated by a magnet. For the detachment of the beads from the cells, affinity-purified polyclonal antibodies against the Fab portion of anti-CD34 antibody (Detach-a-Bead CD34) were added, and incubation was performed for 45 minutes at room temperature. The detached beads were removed by the magnet, and the cells were collected as CD34+ cells. More than 90% of the isolated cells were CD34+, as determined by FACScan flow cytometry (Becton Dickinson).
Serum-deprived liquid culture.
Serum-deprived liquid cultures were performed in a 24-well culture plate (#3047; Becton Dickinson) using a modification of the technique described previously.16 Two × 104CD34+ cells were cultured in each well containing 2 mL α-medium (Flow Laboratories Inc, Rockville, MD) supplemented with 1% deionized bovine serum albumin (BSA; Sigma Chemical Co, St Louis, MO), 600 μg/mL fully iron-saturated human transferrin (approximately 98% pure; Sigma), 16 μg/mL soybean lecithin (Sigma), 9.6 μg/mL cholesterol (Nakalai Chemicals Ltd, Tokyo, Japan), and 10 ng/mL of TPO, 10 ng/mL of G-CSF, 10 ng/mL of GM-CSF, and 10 ng/mL of SCF, alone or in combination. The plates were incubated at 37°C in a humidified atmosphere flushed with a mixture of 5% CO2, 5% O2, and 90% N2. Half of the cells and culture medium was replaced weekly with fresh medium containing growth factor(s). The number of viable cells was determined by a trypan-blue exclusion test using hemocytometers, and the cells were processed for immunocytochemical staining and flow cytometric analysis.
Clonal cell culture.
Clonal cell cultures were performed in 35-mm Lux suspension culture dishes (#171099; Nunc, Naperville, IL) by the technique described previously.17 The culture consisted of 1 to 5 × 103/mL cells, α-medium, 0.9% methylcellulose (Shinetsu Chemical Co, Tokyo, Japan), 1% deionized BSA, 30% FBS, 100 U/mL of IL-3, 10 ng/mL of GM-CSF, 10 ng/mL of G-CSF, 10 ng/mL of SCF, and 2 U/mL of EPO. Dishes were incubated at 37°C in a humidified atmosphere flushed with a 5% CO2. On day 14, GM colonies, erythroid bursts, and mixed erythroid colonies consisting of erythroid cells and cells of other than erythroid lineage were scored in situ on an inverted microscope according to the criteria described previously.18 19
Serum-deprived single-cell culture.
To elucidate which subpopulations of CD34+ cells generated neutrophilic cells under stimulation with TPO + SCF, two-step sorting was performed, as described previously.13 Cord blood MNCs were incubated with 20 μL FITC-conjugated anti-CD34 MoAb, 5 μL APC-conjugated anti-CD38 MoAb and 20 μL PE-conjugated anti–c-kit MoAb for 30 minutes at 4°C. As negative controls, cells were stained with FITC-, APC-, and PE-conjugated mouse IgG1 (Becton Dickinson). After two washes, CD34+CD38+ c-kit+ cells, CD34+CD38+c-kit- cells, CD34+CD38-c-kit+ cells, and CD34+ CD38-c-kit- cells were individually sorted in 5 mL tubes by a FACStarplus flow cytometer (Becton Dickinson). The cells in each group were then resorted into the individual wells of a 96-well U-bottomed tissue culture plate (#3077; Becton Dickinson) containing 100 μL of the serum-deprived culture medium supplemented with SCF and/or TPO, using the FACStarplus flow cytometer equipped with an automatic cell deposition unit. Ninety-nine percent of the wells contained a single cell on the first day of culture. The plates were incubated at 37°C in a humidified atmosphere flushed with a mixture of 5% CO2, 5% O2, and 90% N2. After 3 weeks, colonies of more than 30 cells were scored in situ on an inverted microscope, and the constituent cells of colonies were identified on cytocentrifuged preparations stained with May-Grünwald-Giemsa.
Flow cytometric analysis and cell sorting.
For the analysis of surface markers on the cultured cells, 1 to 2 × 106 cells were collected in plastic tubes and incubated with appropriately diluted FITC- or PE-MoAb, as described previously.13 15 The cells were washed twice, after which their surface markers were analyzed with the FACScan flow cytometer, using the Lysis 2 software program (Becton Dickinson). Viable cells were gated according to their forward light-scatter characteristics (FSC) and side-scatter characteristics (SSC). The proportion of positive cells was determined by comparison to cells stained with FITC- or PE-conjugated mouse isotype-matched Ig.
For the identification of the maturation stage of neutrophilic cells grown by SCF + TPO, the cultured cells at 3 weeks (1 × 106) were incubated with 5 μL APC-conjugated anti-CD34 MoAb, 20 μL PE-conjugated anti–c-kit MoAb, and 20 μL FITC-conjugated anti-CD15 MoAb for 30 minutes at 4°C. As negative controls, cells were stained with APC-, PE-, and FITC-conjugated mouse isotype-matched Ig (Becton Dickinson and Immunotech S.A.). After two washes, CD34− c-kit−/lowCD15+cells were sorted by the FACStarplus flow cytometer.
Cytochemical staining.
Cultured cells were spread on glass slides using a Cytospin II (Shandon Southern, Sewickly, PA), and stained with May-Grünwald-Giemsa, Sudan black B, Biebrich scarlet, or toluidine blue. Cytochemical reactions with peroxidase (POX), α-naphthyl butyrate esterase, acid phosphatase, and alkaline phosphatase (ALP) were performed by the conventional methods.20
Immunocytochemical staining.
Reactions with mouse MoAbs against MPO, elastase, lactoferrin, CD2, CD11b, CD14, CD15, CD19, CD35, GPA, eosinophil POX, and tryptase were detected using the ALP-anti-ALP method (Dako APAAP Kit System; Dako Corp, Carpinteria, CA), as described previously.21 The isotype mouse MoAb was used as a control. Briefly, cytocentrifuged samples were fixed with Carnoy’s fluid, washed with PBS, and preincubated with normal rabbit serum to saturate the Fc receptors on the cell surface. After being washed with PBS three times, the samples were reacted with mouse MoAb for 30 minutes at room temperature in a humidified chamber. After three more washes with PBS, the samples were reacted with rabbit antimouse IgG antibody, washed three times, and successively reacted with the calf intestinal ALP-mouse monoclonal anti-ALP complex. Finally, ALP activity was detected with naphthol AS-MX phosphate, Fast Red TR, and levamisole to inhibit nonspecific ALP activity. The specimens were counterstained with hematoxylin. Three hundred cells were examined.
Statistical analysis.
All experiments were performed at least three times and were shown to be reproducible. Values are expressed as mean ± SD. One-way analysis of variance, followed by post hoc contrasts with Bonferroni limitation, was employed for more than four independent groups.
RESULTS
Production of neutrophilic cells by combination of SCF and TPO from CD34+ cord blood cells in long-term serum-deprived liquid cultures.
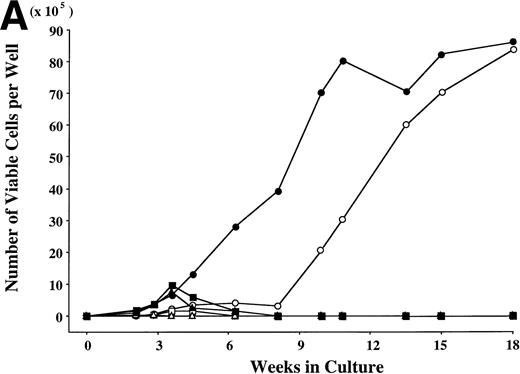
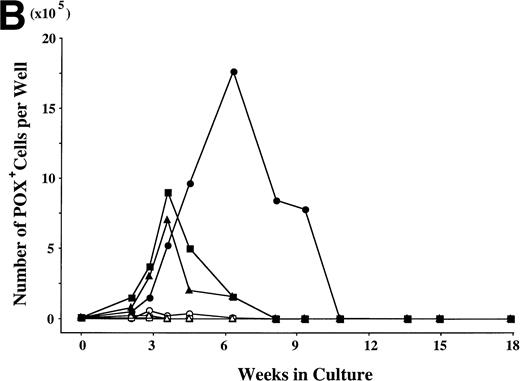
To examine the effects of SCF and TPO, alone or in combination, on the cell production by hematopoietic progenitors, we initiated serum-deprived liquid cultures with 2 × 104CD34+ cord blood cells per well, and maintained the cultures with the repeated addition of growth factor(s). G-CSF and GM-CSF were used as controls. The results are presented in Fig 1. In the presence of G-CSF, the total viable cell number increased to a maximum of twice the input quantity after 2 weeks. A large part of the progenies were neutrophils, and they did not survive beyond 4 weeks. Significant cell production was observed in a well containing GM-CSF (16-fold the input cell number after 4 weeks), and most of the cultured cells were eosinophils. In the case of TPO, the total number of viable cells increased until 2 weeks, with a peak of 18-fold the input quantity. More than 95% of the cultured cells were positive for CD41b, and the cells with other lineage-specific markers (CD2, CD19, CD11b, CD14, CD15, GPA) were at a negligible level, as reported previously.16 The number of viable cells decreased after 2 weeks. SCF exerted a modest stimulatory effect on the cell growth until 8 weeks. Subsequently, the apparent cell proliferation was observed. After 4 weeks, most of the progenies reacted with antitryptase MoAb, indicating their mast cell property. In addition, less than 10% of POX+ cells were generated from 4 weeks to 7 weeks, as shown in Fig 2. The combination of SCF + G-CSF or SCF + GM-CSF caused a synergistic increase in the numbers of total viable cells and POX+cells, in particular at 4 weeks. These results are consistent with previous results.22 However, there were no viable cells under stimulation with SCF + G-CSF or SCF + GM-CSF after 8 weeks. The combination of SCF and TPO exerted a significant interaction on the cell production from 4 weeks to 10 weeks, compared with each factor alone. Neutrophil-like cells positive for POX (Fig 3) first appeared after 2 weeks of the culture with SCF + TPO, and accounted for approximately 60% to 80% of the cells generated between 4 weeks and 7 weeks. The production of POX+ cells supported by SCF + TPO at 7 weeks was at a twofold greater level, compared with the results obtained by the other two-factor combinations at 4 weeks. There was a decrease in the POX+ cell generation accompanied by an increase in mast cell growth after 10 weeks.
Combination of SCF and TPO stimulates neutrophilic cell production by CD34+ cord blood cells in serum-deprived liquid culture. (A) CD34+ cord blood cells (2 × 104) were plated per well containing serum-deprived liquid culture medium supplemented with SCF, TPO, G-CSF, or GM-CSF, alone or in combination. Half of the cells and culture medium were replaced weekly with fresh medium containing growth factor(s). Numbers of viable cells were serially counted. (B) The cultured cells were processed for staining with POX. The results shown are from one representative experiment of three. Similar results were obtained in the other two experiments. SCF (○), TPO (◊), G-CSF (▵), GM-CSF (□), SCF + TPO (•), SCF + G-CSF (▴), SCF + GM-CSF (▪).
Combination of SCF and TPO stimulates neutrophilic cell production by CD34+ cord blood cells in serum-deprived liquid culture. (A) CD34+ cord blood cells (2 × 104) were plated per well containing serum-deprived liquid culture medium supplemented with SCF, TPO, G-CSF, or GM-CSF, alone or in combination. Half of the cells and culture medium were replaced weekly with fresh medium containing growth factor(s). Numbers of viable cells were serially counted. (B) The cultured cells were processed for staining with POX. The results shown are from one representative experiment of three. Similar results were obtained in the other two experiments. SCF (○), TPO (◊), G-CSF (▵), GM-CSF (□), SCF + TPO (•), SCF + G-CSF (▴), SCF + GM-CSF (▪).
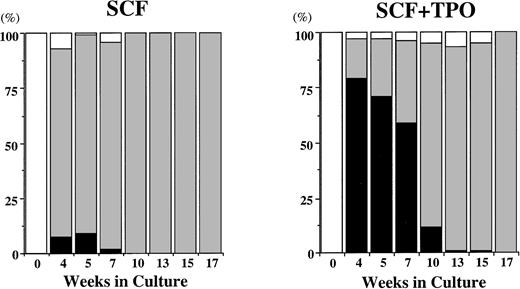
Time course of relative frequency of POX+cells and tryptase+ cells grown by SCF, or SCF + TPO. The cells were identified on cytocentrifuged preparations stained with POX or with a MoAb against tryptase. POX+ cells (black bars), tryptase+ cells (gray bars), others (white bars).
Time course of relative frequency of POX+cells and tryptase+ cells grown by SCF, or SCF + TPO. The cells were identified on cytocentrifuged preparations stained with POX or with a MoAb against tryptase. POX+ cells (black bars), tryptase+ cells (gray bars), others (white bars).
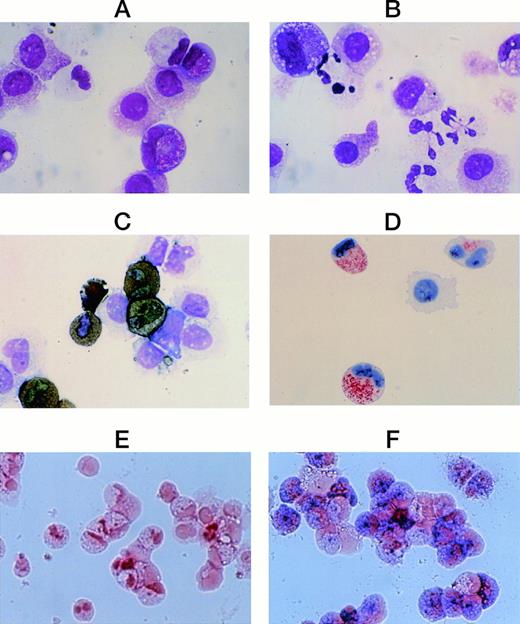
Cytological characteristics of neutrophilic cells grown by SCF + TPO from CD34+ cord blood cells. Staining of cultured cells grown by SCF + TPO at 8 weeks with May-Grünwald-Giemsa (A); with POX (C); with a MoAb for MPO (D), and with ALP (E). The cells generated by SCF + TPO at 8 weeks were harvested and recultured with SCF + TPO + G-CSF. After 1 week, the cells were processed for staining with May-Grünwald-Giemsa (B), and with ALP (F). Original magnification: ×1,000 (A-D), ×400 (E,F).
Cytological characteristics of neutrophilic cells grown by SCF + TPO from CD34+ cord blood cells. Staining of cultured cells grown by SCF + TPO at 8 weeks with May-Grünwald-Giemsa (A); with POX (C); with a MoAb for MPO (D), and with ALP (E). The cells generated by SCF + TPO at 8 weeks were harvested and recultured with SCF + TPO + G-CSF. After 1 week, the cells were processed for staining with May-Grünwald-Giemsa (B), and with ALP (F). Original magnification: ×1,000 (A-D), ×400 (E,F).
There are four possibilities for POX+-cell lineages: neutrophilic, eosinophilic, basophilic, and monocytic series. The POX+ cells grown by SCF + TPO were negative for Biebrich scarlet, eosinophil POX, toluidine-blue and α-naphthyl butyrate esterase staining. On the other hand, they reacted with anti-MPO MoAb, acid phosphatase and Sudan black B, but not ALP, as presented in Fig 3.
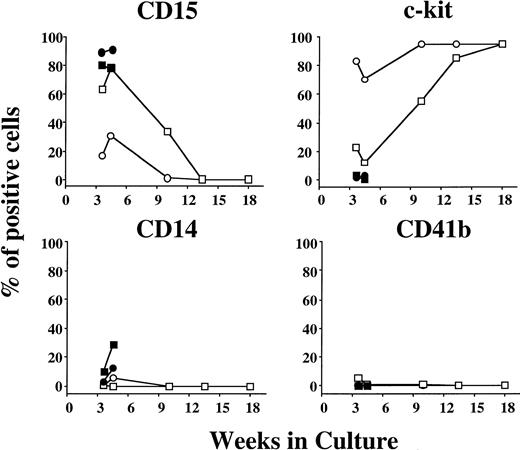
The flow cytometric analysis showed that under stimulation with SCF, approximately 70% to 80% of the cells grown at 3 to 4 weeks of the culture were positive for c-kit, and most of the remaining cells reacted with anti-CD15 MoAb, as shown in Fig 4. After 9 weeks, the frequency of c-kit+ cells was higher than 95%. The percentages of CD34+, GPA+, CD2+, or CD19+ cells were less than 1% at 3 to 4, 10, 13, and 18 weeks. On the other hand, the relative number of CD15+cells grown by SCF + TPO was significantly higher between 3 weeks and 10 weeks in comparison with the value obtained by SCF alone, whereas the percentages of CD14+ and CD41b+ cells were at a negligible or low level. Most of the cultured cells were positive for c-kit after 13 weeks. The frequency of CD34+, GPA+, CD2+, or CD19+ cells was less than 1% at various time points up to 18 weeks except that 2% to 3% of the cultured cells expressed CD34 at 3 to 4 weeks of the culture. In the presence of SCF + G-CSF or SCF + GM-CSF, a large portion of the cultured cells was positive for CD15, and c-kit+ cells were virtually negative at 3 to 4 weeks. These results indicate the significant neutrophilic production by the combination of SCF and TPO from CD34+ cord blood cells.
Time course of surface marker expression on cultured cells grown by SCF, SCF + TPO, SCF + G-CSF, or SCF + GM-CSF from CD34+ cord blood cells. Surface marker expression was determined by flow cytometry. SCF (○), SCF + TPO (□), SCF + G-CSF (•), SCF + GM-CSF (▪).
Time course of surface marker expression on cultured cells grown by SCF, SCF + TPO, SCF + G-CSF, or SCF + GM-CSF from CD34+ cord blood cells. Surface marker expression was determined by flow cytometry. SCF (○), SCF + TPO (□), SCF + G-CSF (•), SCF + GM-CSF (▪).
It is possible that the endogenous secretion of G-CSF or GM-CSF contributes to the SCF + TPO-dependent neutrophil production, because of the cytokine liberation by mast cells.23 However, neither G-CSF nor GM-CSF was detected in the supernatants of the 8 weeks- and 12 weeks-cultured cells grown by SCF alone or SCF + TPO. Moreover, the addition of a 1:10,000 dilution of anti–G-CSF and 2 μg/mL of anti–GM-CSF neutralizing antibodies to the cultures containing SCF + TPO did not influence the neutrophilic cell production until 4 weeks (data not shown).
Maturation stage of neutrophilic cells grown by SCF + TPO.
For the examination of neutrophil-associated proteins in the neutrophilic cells grown by SCF + TPO, the cultured cells generated by SCF + TPO at 3 weeks were harvested and recultured with SCF + TPO, SCF + G-CSF, or SCF + GM-CSF for another week. As presented in Table 1 (Experiment 1), the cultured neutrophilic cells in the presence of SCF + G-CSF contained MPO, elastase, lactoferrin, CD11b, CD35, and ALP. In the case of SCF + GM-CSF, all of the neutrophil-associated proteins except ALP were detectable. In the cultures with SCF + TPO, in contrast, the neutrophilic cells contained MPO, elastase, lactoferrin, and CD11b, but not CD35 and ALP. To examine the possibility that SCF + TPO exerted inhibitory effects on the expressions of CD35 and ALP, neutrophilic cells grown by SCF + TPO were harvested at 8 weeks and recultured with SCF + TPO, SCF + TPO + G-CSF, or SCF + TPO + GM-CSF for 1 week. As presented in Table 1 (Experiment 2), similar results were obtained. Thus, the neutrophilic cells generated by SCF + TPO appeared to be defective in CD35 and ALP.
Neutrophil-Associated Proteins in Neutrophilic Cells Grown by Various Combinations of Growth Factors From CD34+ Cord Blood Cells in Serum-Deprived Liquid Culture
| Growth Factors . | MPO . | Elastase . | Lactoferrin . | CD11b . | CD35 . | ALP . |
|---|---|---|---|---|---|---|
| Exp. 1 (at 3 weeks) | ||||||
| SCF + TPO | + | + | + | + | − | − |
| SCF + G-CSF | + | + | + | + | + | + |
| SCF + GM-CSF | + | + | + | + | + | − |
| Exp. 2 (at 8 weeks) | ||||||
| SCF + TPO | + | + | + | + | − | − |
| SCF + TPO + G-CSF | + | + | + | + | + | + |
| SCF + TPO + GM-CSF | + | + | + | + | + | − |
| Growth Factors . | MPO . | Elastase . | Lactoferrin . | CD11b . | CD35 . | ALP . |
|---|---|---|---|---|---|---|
| Exp. 1 (at 3 weeks) | ||||||
| SCF + TPO | + | + | + | + | − | − |
| SCF + G-CSF | + | + | + | + | + | + |
| SCF + GM-CSF | + | + | + | + | + | − |
| Exp. 2 (at 8 weeks) | ||||||
| SCF + TPO | + | + | + | + | − | − |
| SCF + TPO + G-CSF | + | + | + | + | + | + |
| SCF + TPO + GM-CSF | + | + | + | + | + | − |
For the examination of neutrophil-associated proteins in neutrophilic cells grown by SCF + TPO from CD34+ cord blood cells, the cells generated at 3 weeks or at 8 weeks in culture were harvested and recultured with various combinations of growth factors. After 1 week, the cells were processed for staining with MoAbs for MPO, elastase, lactoferrin, CD11b, and CD35 or with ALP. The results shown are from one representative experiment of three. Similar results were obtained in the other two experiments.
Next, we examined the direct effects of the two-factor combinations on neutrophilic maturation. For this purpose, we sorted CD34−c-kit−/lowCD15+cells grown by SCF + TPO at 3 weeks using the FACStarplusflow cytometer as shown in Fig 5, and replated them into cultures containing SCF + TPO, SCF + G-CSF, or SCF + GM-CSF. The results are presented in Table2. More than 95% of the sorted cells were viable and positive for POX. They were composed of myeloblasts, promyelocytes, myelocytes, metamyelocytes, and band cells, but not segmented cells. An obvious transition from promyelocytes/myelocytes to metamyelocytes/band cells was observed 24 hours after the culture containing SCF + TPO. However, segmented cells barely appeared even 72 hours after the culture. The cultured cells were virtually negative for CD35 and ALP throughout the culture. In the case of SCF + G-CSF, terminal maturation into band cells and segmented cells became apparent after 48 hours. The frequency of cells positive for CD35 and ALP corresponded to that of segmented cells. In the presence of SCF + GM-CSF, there was an intermediate differentiation into segmented cells, as compared with the other two-factor combinations. In accordance with the results shown in Table1, the cells in the culture containing SCF + GM-CSF reacted with anti-CD35 MoAb, but not ALP.
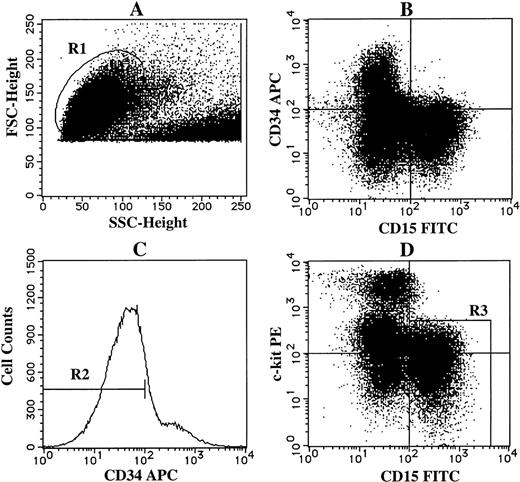
Sorting of CD34−c-kit−/lowCD15+ cells grown by SCF + TPO from CD34+cord blood cells. The cultured cells grown by SCF + TPO at 3 weeks were stained with APC-conjugated anti-CD34 MoAb, PE-conjugated anti–c-kit MoAb and FITC-conjugated anti-CD15 MoAb. As negative controls, APC-, PE-, and FITC-conjugated mouse isotype-matched Ig were used. (A) The viable cell region (R1) was gated on the basis of FSC and SSC. (B) CD34 and CD15 expressions of the cells in the R1 region. (C) The gate (R2) was set on CD34− cells. (D) The expressions of c-kit and CD15 on these cells were then examined. The cells in the R3 region were sorted as CD34−c-kit−/low CD15+cells.
Sorting of CD34−c-kit−/lowCD15+ cells grown by SCF + TPO from CD34+cord blood cells. The cultured cells grown by SCF + TPO at 3 weeks were stained with APC-conjugated anti-CD34 MoAb, PE-conjugated anti–c-kit MoAb and FITC-conjugated anti-CD15 MoAb. As negative controls, APC-, PE-, and FITC-conjugated mouse isotype-matched Ig were used. (A) The viable cell region (R1) was gated on the basis of FSC and SSC. (B) CD34 and CD15 expressions of the cells in the R1 region. (C) The gate (R2) was set on CD34− cells. (D) The expressions of c-kit and CD15 on these cells were then examined. The cells in the R3 region were sorted as CD34−c-kit−/low CD15+cells.
Maturation Stage of Neutrophilic Cells Grown by SCF + TPO
| . | Differential Counts (%) . | Positive Cells (%) . | ||||||
|---|---|---|---|---|---|---|---|---|
| MB . | PMC . | MC . | MMC . | BC . | Segm . | CD35 . | ALP . | |
| At the beginning of culture | 0.9 | 12.2 | 59.1 | 14.8 | 13.0 | 0 | 0 | 0 |
| SCF + TPO | ||||||||
| 12 hours | 0.8 | 12.3 | 41.5 | 23.1 | 22.3 | 0 | 0 | 0 |
| 24 hours | 0.6 | 1.9 | 29.9 | 36.4 | 30.8 | 0.4 | 0 | 0 |
| 48 hours | 0 | 0.7 | 33.3 | 35.0 | 30.3 | 0.7 | 0 | 0 |
| 72 hours | 0 | 0.7 | 33.8 | 33.8 | 31.0 | 0.7 | 0 | 0 |
| SCF + G-CSF | ||||||||
| 12 hours | 0.6 | 4.3 | 39.0 | 39.0 | 17.1 | 0 | 0 | 0 |
| 24 hours | 0 | 3.2 | 44.2 | 35.9 | 16.0 | 0.7 | 0 | 0 |
| 48 hours | 0 | 0.6 | 30.1 | 34.6 | 30.7 | 4.0 | 4 | 4 |
| 72 hours | 0 | 1.2 | 23.4 | 40.1 | 25.7 | 9.6 | 8 | 9 |
| SCF + GM-CSF | ||||||||
| 12 hours | 0 | 4.6 | 62.1 | 25.6 | 7.7 | 0 | 0 | 0 |
| 24 hours | 0 | 0 | 30.3 | 48.7 | 20.0 | 1.0 | 2 | 0 |
| 48 hours | 0 | 0 | 9.3 | 53.5 | 33.0 | 4.2 | 3 | 0 |
| 72 hours | 0 | 0 | 8.2 | 55.1 | 31.6 | 5.1 | 6 | 0 |
| . | Differential Counts (%) . | Positive Cells (%) . | ||||||
|---|---|---|---|---|---|---|---|---|
| MB . | PMC . | MC . | MMC . | BC . | Segm . | CD35 . | ALP . | |
| At the beginning of culture | 0.9 | 12.2 | 59.1 | 14.8 | 13.0 | 0 | 0 | 0 |
| SCF + TPO | ||||||||
| 12 hours | 0.8 | 12.3 | 41.5 | 23.1 | 22.3 | 0 | 0 | 0 |
| 24 hours | 0.6 | 1.9 | 29.9 | 36.4 | 30.8 | 0.4 | 0 | 0 |
| 48 hours | 0 | 0.7 | 33.3 | 35.0 | 30.3 | 0.7 | 0 | 0 |
| 72 hours | 0 | 0.7 | 33.8 | 33.8 | 31.0 | 0.7 | 0 | 0 |
| SCF + G-CSF | ||||||||
| 12 hours | 0.6 | 4.3 | 39.0 | 39.0 | 17.1 | 0 | 0 | 0 |
| 24 hours | 0 | 3.2 | 44.2 | 35.9 | 16.0 | 0.7 | 0 | 0 |
| 48 hours | 0 | 0.6 | 30.1 | 34.6 | 30.7 | 4.0 | 4 | 4 |
| 72 hours | 0 | 1.2 | 23.4 | 40.1 | 25.7 | 9.6 | 8 | 9 |
| SCF + GM-CSF | ||||||||
| 12 hours | 0 | 4.6 | 62.1 | 25.6 | 7.7 | 0 | 0 | 0 |
| 24 hours | 0 | 0 | 30.3 | 48.7 | 20.0 | 1.0 | 2 | 0 |
| 48 hours | 0 | 0 | 9.3 | 53.5 | 33.0 | 4.2 | 3 | 0 |
| 72 hours | 0 | 0 | 8.2 | 55.1 | 31.6 | 5.1 | 6 | 0 |
CD34−c-kit−/lowCD15+ cells grown by SCF + TPO at 3 weeks were sorted by flow cytometry, and replated at 2,000 cells per well containing SCF + TPO, SCF + G-CSF, or SCF + GM-CSF, as described in Materials and Methods. The cells were serially harvested, and processed for staining with May-Grünwald Giemsa, ALP, or a MoAb for CD35. The results shown are from one representative experiment of three. Similar results were obtained in the other two experiments.
Abbreviations: MB, myeloblasts; PMC, promyelocytes; MC, myelocytes; MMC, metamyelocytes; BC, band cells; Segm, segmented cells; ALP, alkaline phosphatase.
Production of neutrophilic cells by both CD34+CD38+c-kit+ cells and CD34+CD38-c-kit+ cells under stimulation with SCF plus TPO.
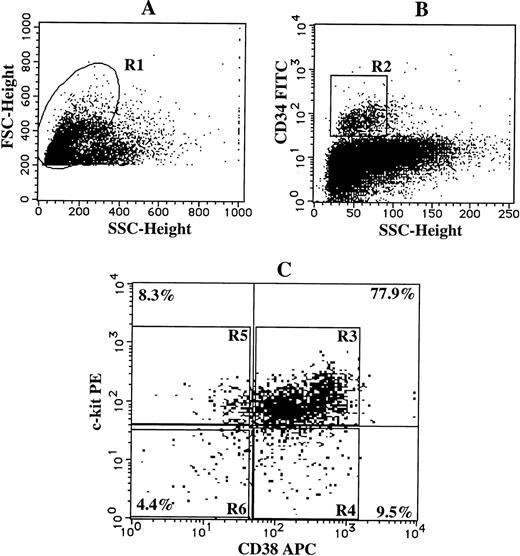
To determine which subpopulations of CD34+ cells were responsible for the SCF + TPO-dependent neutrophilic cell production, we divided CD34+ cells into 4 subsets on the basis of CD38 and c-kit expression: CD38+c-kit+, CD38+ c-kit-, CD38-c-kit+, and CD38-c-kit- cells, as presented in Fig 6. Single-cell sorting was performed in all the subsets by the FACStarplus flow cytometer, and each of the sorted cells was incubated with SCF or SCF + TPO for 3 weeks. The results are presented in Table 3. One-fourth of the CD34+CD38+c-kit+cells formed neutrophilic colonies or neutrophilic cell/mast cell colonies in response to SCF + TPO. In addition, CD34+CD38-c-kit+ cells also responded to the two-factor combination to generate progenies, 25% of which were of the neutrophilic or neutrophilic/mast cell lineage. However, the proliferative response of CD34+CD38+c-kit- cells and CD34+CD38-c-kit- cells was at a negligible level. Under stimulation with SCF alone, a small number of neutrophilic cell-containing colonies were grown only in the culture with CD34+CD38+c-kit+cells.
Single-cell sorting of four subpopulations of CD34+ cord blood cells. Cord blood MNCs were stained with FITC-conjugated anti-CD34 MoAb, APC-conjugated anti-CD38 MoAb, and PE-conjugated anti–c-kit MoAb. As negative controls, FITC-, APC-, and PE-conjugated mouse IgG1 were used. (A) The lymphoblastic region (R1) was gated on the basis of FSC and SSC. (B) The gate (R2) was set on CD34+ cells. (C) The expressions of CD38 and c-kit on CD34+cells were examined. The single-cells in the R3, R4, R5, or R6 region were sorted as CD34+CD38+c-kit+, CD34+CD38+c-kit-, CD34+CD38−c-kit+, or CD34+CD38− c-kit- cells, respectively, using an automatic cell deposition unit equipped with the FACStarplus flow cytometer, as described in Materials and Methods.
Single-cell sorting of four subpopulations of CD34+ cord blood cells. Cord blood MNCs were stained with FITC-conjugated anti-CD34 MoAb, APC-conjugated anti-CD38 MoAb, and PE-conjugated anti–c-kit MoAb. As negative controls, FITC-, APC-, and PE-conjugated mouse IgG1 were used. (A) The lymphoblastic region (R1) was gated on the basis of FSC and SSC. (B) The gate (R2) was set on CD34+ cells. (C) The expressions of CD38 and c-kit on CD34+cells were examined. The single-cells in the R3, R4, R5, or R6 region were sorted as CD34+CD38+c-kit+, CD34+CD38+c-kit-, CD34+CD38−c-kit+, or CD34+CD38− c-kit- cells, respectively, using an automatic cell deposition unit equipped with the FACStarplus flow cytometer, as described in Materials and Methods.
Production of Neutrophilic Cells by Both CD34+CD38+c-kit+ Cells and CD34+CD38−c-kit+ Cells Under Stimulation With SCF Plus TPO
| CD38/c-kit . | Stimuli . | Colony Type . | Total . | ||||
|---|---|---|---|---|---|---|---|
| n . | nm . | mast . | nmast . | undifferentiated . | |||
| +/+ | SCF | 0 | 2 | 6 | 2 | 44 | 54/96 |
| SCF + TPO | 7 | 0 | 16 | 18 | 41 | 82/96 | |
| +/− | SCF | 0 | 0 | 0 | 0 | 0 | 0/96 |
| SCF + TPO | 0 | 0 | 0 | 0 | 0 | 0/96 | |
| −/+ | SCF | 0 | 0 | 0 | 0 | 3 | 3/96 |
| SCF + TPO | 1 | 0 | 3 | 6 | 18 | 28/96 | |
| −/− | SCF | 0 | 0 | 0 | 0 | 1 | 1/96 |
| SCF + TPO | 0 | 0 | 0 | 0 | 1 | 1/96 | |
| CD38/c-kit . | Stimuli . | Colony Type . | Total . | ||||
|---|---|---|---|---|---|---|---|
| n . | nm . | mast . | nmast . | undifferentiated . | |||
| +/+ | SCF | 0 | 2 | 6 | 2 | 44 | 54/96 |
| SCF + TPO | 7 | 0 | 16 | 18 | 41 | 82/96 | |
| +/− | SCF | 0 | 0 | 0 | 0 | 0 | 0/96 |
| SCF + TPO | 0 | 0 | 0 | 0 | 0 | 0/96 | |
| −/+ | SCF | 0 | 0 | 0 | 0 | 3 | 3/96 |
| SCF + TPO | 1 | 0 | 3 | 6 | 18 | 28/96 | |
| −/− | SCF | 0 | 0 | 0 | 0 | 1 | 1/96 |
| SCF + TPO | 0 | 0 | 0 | 0 | 1 | 1/96 | |
Single-cell sorting was performed by two-step sorting. Cord blood CD34+CD38+c-kit+ cells, CD34+CD38+c-kit− cells, CD34+CD38−c-kit+ cells, and CD34+CD38−c-kit− cells were individually sorted in 5 mL-tubes by the FACStarplus flow cytometer. Then, cells in each group were resorted into the individual wells of a 96-well culture plate containing SCF or SCF + TPO. A half medium exchange was performed every week. After 3 weeks, the constituent cells in each well were identified under microscopic visualization and/or on the cytospin preparations stained with May-Grünwald Giemsa. The results shown are from one representative experiment of three. Similar results were obtained in the other two experiments.
Abbreviations: n, neutrophilic cells; m, macrophages; mast, mast cells; nmast, neutrophilic cells/mast cells; undifferentiated, undifferentiated cells which disintegrated without any terminal differentiation until 3 weeks.
Kinetics of hematopoietic progenitors in the culture containing SCF + TPO.
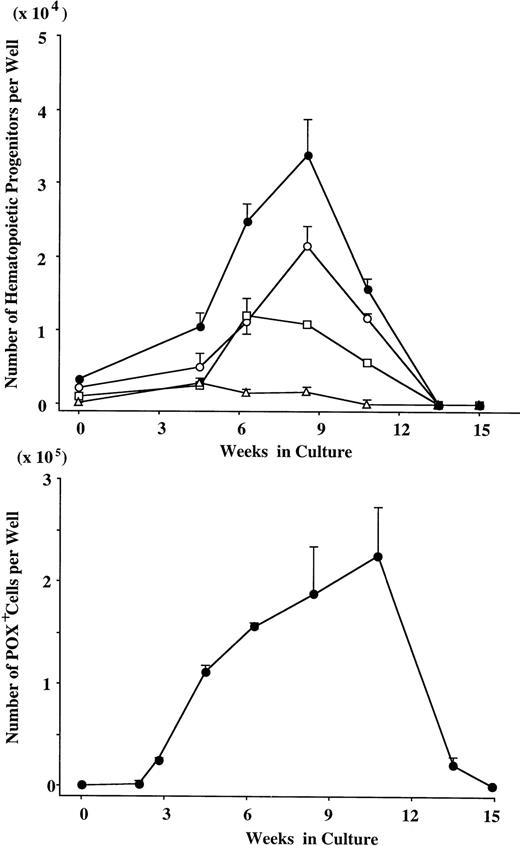
Finally, we examined the increase of the POX+ cell count and of colony-forming cells under stimulation with SCF + TPO. As presented in Fig 7, the significant POX+ cell generation was observed up to 10 weeks. The number of GM progenitors increased continuously for 8 weeks. Erythroid progenitors and multipotential progenitors were also generated, but the extent of expansion was inferior to that of GM progenitors. At 13 weeks of culture, the number of POX+ cells was rapidly decreased, and hematopoietic progenitors could not be detected.
Expansion of hematopoietic progenitors under stimulation with SCF + TPO. After numbers of viable cells were serially counted, the cultured cells were incubated in methylcellulose culture supplemented with IL-3, GM-CSF, G-CSF, SCF, and EPO. After 14 days, GM colonies, erythroid bursts, and mixed erythroid colonies were scored. The data are the mean ± SD of the number of colonies per well in triplicate suspension cultures. The results shown are from one representative experiment of three. Similar results were obtained in the other two experiments. GM colonies (○), erythroid bursts (□), mixed erythroid colonies (▵), and total colonies (•).
Expansion of hematopoietic progenitors under stimulation with SCF + TPO. After numbers of viable cells were serially counted, the cultured cells were incubated in methylcellulose culture supplemented with IL-3, GM-CSF, G-CSF, SCF, and EPO. After 14 days, GM colonies, erythroid bursts, and mixed erythroid colonies were scored. The data are the mean ± SD of the number of colonies per well in triplicate suspension cultures. The results shown are from one representative experiment of three. Similar results were obtained in the other two experiments. GM colonies (○), erythroid bursts (□), mixed erythroid colonies (▵), and total colonies (•).
DISCUSSION
In the present study, SCF alone induced a modest production of neutrophilic cells and a remarkable generation of mast cells by cord blood CD34+ cells after 4 weeks of culture. The SCF–dependent neutrophilic cell production is consistent with results reported previously.24,25 More than 95% of the cells grown by TPO were positive for CD41b, and cells of the neutrophilic lineage were at a negligible level as reported previously.16 In contrast, the combination of SCF and TPO significantly stimulated the generation of neutrophilic cells, identified by immunocytochemical staining and flow cytometric analysis, which continued up to 2 to 3 months of the culture. It is interesting that the effect of SCF + TPO on the neutrophilic cell generation was greater than that of SCF + G-CSF or SCF + GM-CSF. The possibility was ruled out that the SCF + TPO-dependent neutrophilic cell production resulted from the secretion of G-CSF and GM-CSF by concomitantly grown mast cells. In addition, no IL-1β, IL-3, or IL-6 was detected in the supernatants of the 8-week and 12-week cells grown by SCF alone or SCF + TPO (data not shown). The single-cell culture study showed that neutrophilic cells were generated by not only CD34+CD38+c-kit+ cells but also CD34+CD38-c-kit+ cells under stimulation with SCF + TPO, with no influence of nonhematopoietic cells or mediators other than the two factors. In the clonal-cell cultures, GM progenitors as well as erythroid progenitors and multipotential progenitors expanded in the culture supplemented with SCF and TPO. Taken together with the previous evidence described by Kimura et al26 that a combination of IL-3, SCF, and IL-6/soluble IL-6 receptor supports neutrophilic maturation without the presence of late-acting lineage-specific factors, our results indicate the existence of a G-CSF/GM-CSF–independent system of neutrophilic cell production. It is likely that the neutrophilic generation induced by SCF + TPO is conducted via the production of committed GM progenitors by primitive progenitors.
The neutrophilic cells grown by SCF + TPO were at myeloblast to band cell stages. However, there were scarcely any leukocytes at the polymorphonuclear stage. Although the cells generated by SCF + TPO were stained with MoAbs against MPO, elastase, lactoferrin, or CD11b, the specific contents of secretory vesicles such as ALP and CD35 were at negligible levels. It is well known that ALP is expressed on the external aspect of the plasma membrane of the fully differentiated neutrophils. Moreover, Kumar et al27 indicated that CD35-containing vesicles in polymorphonuclear leukocytes arise by the endocytic retrieval of proteins that are on the plasma membrane. The replating of CD34−c-kit−/lowCD15+ cells into the culture containing SCF + G-CSF permitted both the terminal maturation into segmented cells and the appearance of ALP and CD35. This is in agreement with the previous finding that G-CSF induces ALP in granulocytes from normal individuals.28 These results suggest that the maturation arrest at the band cell stage in the neutrophilic cells grown by SCF + TPO is not due to an intrinsic defect, but is the result of an insufficient potential of the two-factor combination for supporting the terminal neutrophilic maturation.
The number of mast cells was significantly higher in the cultures containing SCF + TPO than in the cultures containing SCF alone between 6 weeks and 10 weeks of the culture (Fig 1A and Fig 2). In this regard, the addition of TPO resulted in an increase in the colony formation supported by SCF from CD34+CD38+c-kit+ cells, or CD34+CD38−c-kit+ cells. In contrast, the mast cell growth was not influenced when TPO was added to the cultures containing SCF at the 15-week culture (data not shown). Thus, the increased level of mast cell generation in the presence of SCF + TPO may result from the two-factor combination-mediated expansion at the hematopoietic progenitor level, but not at the mast cell level.
The mast cell population kept expanding up to at least 4 months in the presence of SCF + TPO. This long-term generation of mast cells is consistent with the results described previously.29 30 On the other hand, the differentiation toward the neutrophilic lineage was apparently time limited. Based on the kinetic study of hematopoietic progenitors in the culture containing SCF + TPO, it may result from an exhaustion of hematopoietic progenitors belonging to the myelopoietic lineages.
Because the activation or suppression of one or more lineage- and developmental-specific transcription factors in part determines the developmental expression pattern of neutrophil-specific genes,31 the neutrophilic cells grown by SCF + TPO may be a useful model for studying the G-CSF– or GM-CSF–mediated regulation of genes expressed at late stages of neutrophilic maturation.
This work was supported by Grants-in-Aid No. 09670796 and 09770537 from the Ministry of Education of Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal