Abstract
The mi locus encodes a member of the basic-helix-loop-helix-leucine zipper protein family of transcription factors (hereafter called MITF). Mutant alleles of mi,Mior, and Miwh are deletion or point mutation of the basic domain by which MITF binds DNA. The basic domain also has nuclear localization potential. In the present study, we compared the mast cell abnormalities ofMior/Mior andMiwh/Miwh mice with those ofmi/mi mice, of which many have been described by us. The number of mast cells in the skin of Mior/Miorsuckling mice was remarkably decreased from that observed inmi/mi suckling mice, but the number was normal in the skin ofMiwh/Miwh suckling mice. The decrease in skin mast cells was more severe in the mi/mi embryos than inmi/mi suckling mice, but the magnitude of the decrease was comparable between Mior/Mior embryos and Mior/Mior suckling mice. The poor mRNA expression of granzyme B and tryptophan hydroxylase genes was observed in all cultured mast cells (CMCs) derived from the spleens ofMiwh/Miwh,Mior/Mior, and mi/mi mice. However, the poor expression of mouse mast cell protease-4 (MMCP-4), MMCP-5, and MMCP-6 was observed only inMior/Mior and mi/mi CMCs. MITF encoded by Miwh mutant allele (Miwh-MITF) showed deficient but demonstratable DNA binding, but mi-MITF and Mior-MITF did not show any DNA binding ability. Although Miwh-MITF and Mior-MITF showed normal nuclear localization potential, the potential was significantly impaired in mi-MITF. The rank order of mast cell abnormality (mi/mi >Mior/Mior >Miwh/Miwh) appears to be related to the functional abnormality of MITF encoded by each mutant gene.
THE mi LOCUS ENCODES a member of the basic-helix-loop-helix-leucine zipper (bHLH-Zip) protein family of transcription factors (hereafter called mi-transcription factor [MITF]).1,2 Spontaneous, chemical, radiation, and insertional mutageneses provided abundant mutant alleles at themi locus.3,4 More than 10 mutants are known to exist at the mi locus, and half of them have been characterized at the molecular level.5,6 The allele that has been studied most intensively is the mi mutant allele. The mi/mimice show depletion of pigment in both the hair and eyes, microphthalmia, and osteopetrosis.3,4 In addition, the number of mast cells decreases and their phenotype is abnormal inmi/mi mice.7-13 Although most mast cells in the skin of normal (+/+) mice are stained with berberine sulfate that binds heparin proteoglycan, few mast cells are berberine sulfate positive in the skin of mi/mi mice.11,14-17 Cultured mast cells (CMCs) derived from the spleen of mi/mi mice are deficient in the expression of various genes, such as the mouse mast cell protease-6 (MMCP-6),18 MMCP-5,19c-kit,20 p75 nerve growth factor receptor,21 granzyme B (Gr B),22 tryptophan hydroxylase (TPH),22 and integrin α4 subunit genes.23 MITF transactivates the genes whose expression is deficient in mi/mi CMCs.18-23
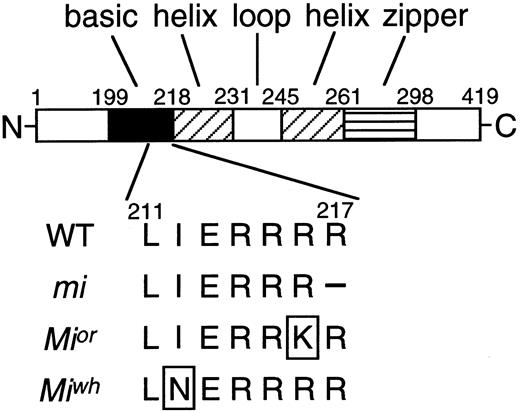
MITF encoded by the mi mutant allele (mi-MITF) deletes 1 of 4 consecutive arginines in the basic domain.1,5,6 Themi-MITF is defective in DNA binding ability and nuclear localization potential.24,25 Because of these two abnormalities, the mi-MITF does not transactivate target genes.18-23,25Mior andMiwh are other mutant alleles at the milocus.3,4 As in the case of mi-MITF, MITF encoded by the Mior or Miwh mutant allele (or-MITF or wh-MITF, respectively) also possesses a mutation at the basic domain.5,6 Arginine changes to lysine at codon 216 in or-MITF, whereas isoleucine changes to aspargine at codon 212 in wh-MITF. In contrast to the detailed molecular study of the mi-MITF, few studies have been performed regarding or-MITF or wh-MITF. The depletion of pigment and microphthalmia ofMior/Mior mice are not distinguishable from those of mi/mi mice, whereas the degree of osteopetrosis is milder in Mior/Mior mice than inmi/mi mice.3,4,26 The depletion of pigment ofMiwh/Miwh mice is not distinguishable from that of Mior/Mior andmi/mi mice, whereas the degree of microphthalmia is milder inMiwh/Miwh mice than inMior/Mior mice.3,4Moreover, osteopetrosis is not detectable inMiwh/Miwh mice.4Abnormalities of mast cells have not been studied inMior/Mior andMiwh/Miwh mice.
In the present study, we compared the number of mast cells in the skin of Mior/Mior andMiwh/Miwh mice with that in the skin ofmi/mi and +/+ mice. We also compared the phenotype of skin mast cells and that of CMCs derived from the spleen amongMior/Mior, Miwh/Miwh, mi/mi, and +/+ mice. The rank order of mast cell abnormalities was mi/mi >Mior/Mior >Miwh/Miwh; this rank order was the same as that of the severity observed in microphthalmia and osteopetrosis. The rank order appeared to be explained by the molecular abnormality of MITF.
MATERIALS AND METHODS
Mice.
A diagram of the mi locus with its theoretical functional domains and the location of the mutations investigated in this study is shown in Fig 1. The original stock of C57BL/6-mi/+ (mi/+) and C57BL/6-Miwh/+ (Miwh/+) mice was purchased from the Jackson Laboratory (Bar Harbor, ME), and the original stock of C57BL/6-Mior/+ (Mior/+) mice was kindly given by Dr L. Lamoreux (Texas A&M University, College Station, TX). They were maintained in our laboratory by consecutive backcrosses to our own inbred C57BL/6 colony (>15 generations at the time of the present experiment inmi/+ and Miwh/+ mice and >10 generations in Mior/+ mice). Female and male heterozygous mice were crossed together, and the resulting homozygous mice were selected by their white coat color.3 4 C57BL/6-+/+ (+/+) mice raised in our laboratory were used as a control.
A diagram of MITF with its theoretical functional domains and the location of the mutations investigated in this study. The mutation point at or-MITF or wh-MITF was boxed. The amino acids are numbered from the initiation codon. The mi-MITF deletes one of three arginines (amino acid 215 to 217). It is unclear which one has been deleted.
A diagram of MITF with its theoretical functional domains and the location of the mutations investigated in this study. The mutation point at or-MITF or wh-MITF was boxed. The amino acids are numbered from the initiation codon. The mi-MITF deletes one of three arginines (amino acid 215 to 217). It is unclear which one has been deleted.
Cells.
Pokeweed mitogen-stimulated spleen cell conditioned medium (PWM-SCM) was prepared according to the method described by Nakahata et al.27 PWM-SCM may contain interleukin-3 (IL-3), IL-4, IL-6, IL-9, IL-12, IL-13, granulocyte-macrophage colony-stimulating factor, macrophage colony-stimulating factor, tumor necrosis factor α, transforming growth factor β, interferon γ, and insulin growth factor 1.28 Mice of mi/mi,Mior/Mior,Miwh/Miwh, and control +/+ were used at 2 to 3 weeks of age to obtain CMCs. Mice were killed by decapitation after ether anesthesia and spleens were removed. Spleen cells were cultured in α-minimal essential medium (α-MEM; ICN Biomedicals, Costa Mesa, CA) supplemented with 10% PWM-SCM and 10% fetal calf serum (FCS; Nippon Bio-supp Center, Tokyo, Japan). Half of the medium was replaced every 5 days. The number of cells derived from the spleen of each mutant mice was 1 × 107 within 4 weeks. Greater than 95% of cells contained alcian blue-positive granules and were considered to be CMCs 4 weeks after initiation of the culture. Three independent cultures were examined for each mutant. The 293T cells were provided by Dr D. Baltimore (Rockefeller University, New York, NY) and were maintained in Dulbecco’s modification of Eagle’s medium (DMEM; Flow Laboratories, Irvine, UK) supplemented with 10% FCS.
Staining and counting of mast cells.
Pregnant mice on day 18 postcoitum (pc) were killed by decapitation after ether anesthesia, and embryos were removed, fixed in Carnoy’s solution, and embedded in paraffin. Twenty days after birth, mice were also killed by decapitation after ether anesthesia. Pieces of dorsal skin were removed, smoothed onto a piece of the filter paper to keep them flat, fixed in Carnoy’s solution, and embedded in paraffin. Sections of embryos and skin pieces were stained with alcian blue or with berberine sulfate. The staining method with alcian blue or berberine sulfate has been described previously.9 14-16Mast cells between the epithelium and panniculus carnosus were counted under the microscope, and the number was expressed as mast cells per centimeter of skin. Berberine sulfate-positive mast cells were counted under the fluorescent microscope, and the proportion of berberine sulfate-positive cells to alcian blue-positive cells was calculated.
In situ hybridization.
Skin pieces were removed from the back of 20-day-old mice, fixed in 4% paraformaldehyde in 0.1 mol/L phosphate buffer (PB; pH 7.4), and embedded in paraffin. The technique of in situ hybridization has been described in detail.29 To obtain the MMCP-4,30MMCP-6,31 and MC-CPA probes,32 single-stranded cDNA was generated from total RNA extracted from CMCs from +/+ mice using the lithium chloride-urea method.33 The specific cDNA of proteases was then amplified with specific primers for each protease by polymerase chain reaction (PCR).13 The cDNAs were subcloned into the EcoRV site of Bluescript KS (−) plasmid (pBS; Stratagene, La Jolla, CA) that contains T3 and T7 promoters to generate probes. The number of various protease mRNA-positive cells and that of alcian blue-positive cells were counted in the serial sections, and the proportion of various protease mRNA-positive cells to alcian blue-positive cells was calculated.
Northern blot analysis.
Each RNA sample was prepared from 1 × 107 of CMCs using the lithium chloride-urea method.33 Northern blot analysis was performed using MMCP-4,30MMCP-5,34 MMCP-6,31c-kit,35 TPH,22 Gr B,22 and GAPDH36 cDNAs labeled with α-[32P]-dCTP (DuPont/NEN Research Products, Boston, MA; 10 mCi/mL) by random oligonucleotide priming. After hybridization at 42°C, blots were washed to a final stringency of 0.2× SSC (1× SSC is 150 mmol/L NaCl and 15 mmol/L trisodium citrate, pH 7.4) and subjected to autoradiography.
Concentration of serotonin.
The concentration of serotonin was measured using high-performance liquid chromatography (HPLC) with electrochemical detection.37 Briefly, CMCs were collected, washed with phosphate-buffered saline (PBS), counted, and sonicated for 20 seconds in a sonicator (Tomy, Tokyo, Japan) in 1 mL of ice-cold 3% perchloric acid containing 5 mmol/L EDTA and 1 mmol/L sodium metabissulfate. The homogenate was centrifuged at 10,000g for 15 minutes at 4°C and the supernatant was applied directly to the HPLC column. The concentration of serotonin per 1.0 × 106 cells was calculated.
Flow cytometry.
CMCs were harvested and washed once with cold PBS containing 0.5% bovine serum albumin (BSA) and 0.1% sodium azide. The cells were incubated with rat anti–c-kit monoclonal antibody (MoAb) ACK238 39 (kindly given by Dr S.I. Nishikawa, Kyoto University, Kyoto, Japan) at 4°C for 30 minutes, rinsed, developed with fluorescein isothiocyanate (FITC)-conjugated rabbit antirat IgG, and then analyzed on a FACScan (Becton Dickinson, Los Angeles, CA).
Immunohistochemistry.
Freshly dissected tissue samples were covered with tissue-Tek OCT compound (an embedding medium to freeze tissues; Miles Inc, Elkhart, IN) and frozen in liquid nitrogen. Frozen sections (4 μm thick) were prepared with a cryostat. The sections were fixed with ice-cold methanol-acetone (1:1), washed in PBS (pH 7.5), and incubated with ACK2 MoAb at the concentration of 10 μg/mL in PBS overnight at 4°C. The sections were than washed five times with PBS and incubated with FITC-conjugated rabbit antirat IgG antibody (DAKO A/S, Glostrup, Denmark) for 40 minutes at 4°C, followed by extensive washing with PBS. The specimens were examined using a confocal laser scanning microscope (LSM-GM200; Olympus, Tokyo, Japan). After examination with the confocal laser scanning microscope, the specimens were washed and stained with alcian blue and nuclear fast red.
Evaluation of cellular response.
Cellular response was quantified by an 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT; Sigma Chemical, St Louis, MO) rapid colorimetric assay as described previously.40 41 Briefly, triplicate aliquots of cells (5 × 104) suspended in 100 μL of Cosmedium-001 (Cosmo Bio Co, Tokyo, Japan) were cultured in 96-well microtiter plates for 48 hours at 37°C with various concentrations of recombinant mouse stem cell factor (rmSCF). rmSCF was a generous gift of Kirin Brewery Co Ltd (Tokyo, Japan). MTT (10 μL of 5 mg/mL solution of MTT in PBS) was added to all wells for the final 4 hours of the culture. Acid isopropanol (100 μL of 0.04 N HCl in isopropanol) was added to all of the wells and mixed to dissolve the dark blue crystals. The optical density was then measured on a Multiskan MCC/340MKII (Labsystems, Helsinki, Finland) with a test wavelength of 540 nm and a reference wavelength of 620 nm.
Construction of expression plasmids and immunocytochemistry.
The pBS containing the whole coding region of +-MITF,mi-MITF, or-MITF, or wh-MITF was constructed in our laboratory18 (hereafter called pBS-+-MITF, pBS-mi-MITF, pBS-or-MITF, and pBS-wh-MITF, respectively). pEF-BOS expression vector was kindly provided by Dr S. Nagata42 (Osaka University, Osaka, Japan). The SmaI-HincII fragment of pBS-+-MITF, pBS-mi-MITF, pBS-or-MITF, or pBS-wh-MITF was introduced into the blunted Xba I site of pEF-BOS. The expression plasmid was transfected into 293T cells, and the overexpressed MITF protein was detected by anti-MITF polyclonal antibody as described previously.25 Briefly, the cells were fixed with 100% methanol, permealized by treatment with 0.2% Triton X-100 in PBS, and incubated with the rabbit anti-MITF polyclonal antibody. The specimens were washed with PBS and then incubated with the biotin-conjugated goat antirabbit IgG antibody (DAKO A/S). Immuno-reacted cells were visualized with streptavidin-biotin-peroxidase and 0.05% diaminobenzidine-0.02% hydroperoxide solution (DAKO A/S) according to the manufacturer’s instructions.
Electrophoretic gel mobility shift assay (EGMSA).
The production of the fusion protein containing glutathione-S-transferase (GST) and MITF was described previously.24 Oligonucleotides were labeled with α-[32P]-dCTP by filling 5′-overhangs and were used as probes of EGMSA. DNA-binding assays were performed in a 20 μL reaction mixture containing 10 mmol/L Tris-HCl (pH 8.0), 1 mmol/L ethylenediaminetetraacetic acid (EDTA), 75 mmol/L KCl, 1 mmol/L dithiothreitol, 4% Ficoll type 400, 50 ng of poly (dI-dC), 25 ng of labeled DNA probe, and 3.5 μg of GST-MITF fusion protein. After incubation at room temperature for 15 minutes, the reaction mixture was subjected to electrophoresis at 14 V/cm at 4°C on a 5% polyacrylamide gel in 0.25× TBE buffer (1× TBE is 90 mmol/L Tris-HCl, 64.6 mmol/L boric acid, and 2.5 mmol/L EDTA, pH 8.3). The polyacrylamide gels were dried on Whatman 3MM chromatography paper (Whatman International Ltd, Maidstone, UK) and subjected to autoradiography.
Dissociation assay.
Dissociation assay was performed according to Bousset et al.43 Reaction mixtures for the DNA-+-MITF and DNA-wh-MITF complexes were prepared as described above, and one sixth of each reaction mixture was electrophoresed. A 500-fold molar excess of nonlabeled oligonucleotide containing the CACATG motif was then added, and the reactions were sampled after further incubation times of 30, 60, 90, 120, and 150 seconds. The regions of the autoradiogram containing DNA-+-MITF or DNA-wh-MITF complex were scanned with a densitometer (Molecular Dynamics, Sunnyvale, CA), and the values were plotted against the incubation period.
RESULTS
The number of mast cells was examined in the skin of embryos and suckling mice of +/+, mi/mi,Mior/Mior, andMiwh/Miwh genotype. Histological sections of day-18 pc embryos and the skin pieces of 20-day-old mice were stained with alcian blue. In both day-18 pcMior/Mior embryos and 20-day-oldMior/Mior mice, the number of mast cells decreased to one third that of +/+ mice (Table 1). The number of mast cells in day-18 pc Mior/Mior embryos was greater than that of day-18 pc mi/mi embryos, whereas the number of mast cells in 20-day-old Mior/Mior mice was comparable to that of 20-day-old mi/mi mice. The number of mast cells was normal in day-18 pcMiwh/Miwh embryos and 20-day-oldMiwh/Miwh mice (Table 1).
Mature skin mast cells of +/+ mice contain heparin. Because mast cells stained with berberine sulfate are considered to contain heparin, proportion of berberne sulfate positive mast cells was used as an index of maturation. In day-18 pc +/+ embryos, approximately half of the skin mast cells were berberine sulfate positive, whereas only a few percent of mast cells were berberine sulfate positive in day-18 pcmi/mi and Mior/Mior embryos (Table 2). The proportion of berberine sulfate-positive mast cells in day-18 pc Miwh/Miwhembryos was comparable to that of day-18 pc +/+ embryos. In 20-day-old +/+ mice, most of skin mast cells were berberine sulfate positive, but the proportions of berberine sulfate positive mast cells remained 3% and 36% in 20-day-old mi/mi andMior/Mior mice, respectively. The proportions of berberine sulfate-positive mast cells in day-18 pcMiwh/Miwh embryos and 20-day-oldMiwh/Miwh mice was comparable to those of +/+ mice of the same age (Table 2).
The expression of mast cell-specific protease genes was analyzed by in situ hybridization in mast cells of the skin. In mi/mi andMior/Mior mice, the proportion of MMCP-4 or MMCP-6 mRNA+ mast cells decreased remarkably, but that of MMCP-5 or MC-CPA mRNA+ mast cells did not (Table 3). The skin mast cells ofMiwh/Miwh mice normally expressed all protease genes examined.
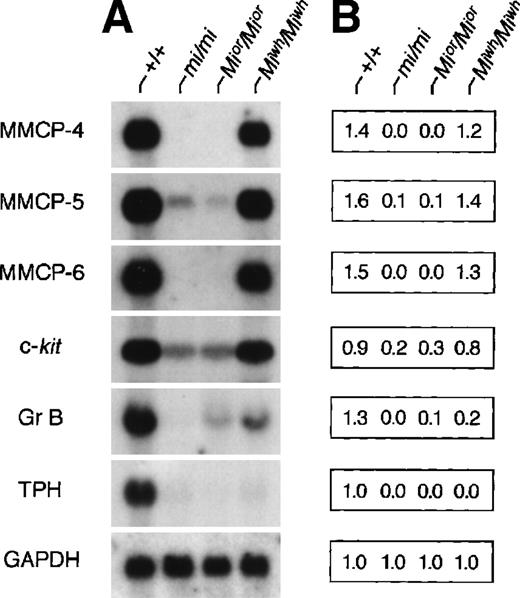
The expression of genes that had been demonstrated to be affected by MITF was examined in CMCs derived from the spleen of +/+,mi/mi, Mior/Mior, andMiwh/Miwh mice. As shown previously,18-22 the expression of all examined genes was hardly detectable in mi/mi CMCs. InMior/Mior CMCs, the expression of MMCP-4, MMCP-5, MMCP-6, and TPH genes was hardly detectable, whereas the expression of Gr B and c-kit genes of moderate degree was detectable (Fig 2). InMiwh/Miwh CMCs, expression levels of MMCP-4, MMCP-5, MMCP-6, and c-kit genes were comparable to those of +/+ CMCs, whereas the expression levels of Gr B and TPH genes decreased significantly (Fig 2). The expression levels of Gr B and TPH genes in Miwh/Miwh CMCs were comparable to those of Mior/Mior CMCs.
Expression of various genes in CMCs derived from +/+,mi/mi, Mior/Mior, andMiwh/Miwh mice. (A) The blot was hybridized with 32P-labeled cDNA probe of MMCP-4, MMCP-5, MMCP-6, c-kit, Gr B, TPH, or GAPDH. (B) Quantification of amount of each mRNA using densitometry. Three independent experiments were performed, and comparable results were obtained. A representative experiment is shown.
Expression of various genes in CMCs derived from +/+,mi/mi, Mior/Mior, andMiwh/Miwh mice. (A) The blot was hybridized with 32P-labeled cDNA probe of MMCP-4, MMCP-5, MMCP-6, c-kit, Gr B, TPH, or GAPDH. (B) Quantification of amount of each mRNA using densitometry. Three independent experiments were performed, and comparable results were obtained. A representative experiment is shown.
The expression of MMCP-5 gene was detectable in skin mast cells but not in CMCs of mi/mi and Mior/Miormice (Table 3 and Fig 2). We previously reported that the addition of SCF significantly increased the amount of MMCP-5 mRNA in mi/miCMCs and speculated that SCF synthesized by fibroblasts may induce the MMCP-5 expression in mi/mi skin mast cells.19 The different pattern of MMCP-5 expression between CMCs and skin mast cells of mi/mi and Mior/Mior mice may be attributable to the different concentration of SCF surrounding mast cells.
The serotonin content of +/+, mi/mi,Mior/Mior, andMiwh/Miwh CMCs was compared, because TPH is the rate-limiting enzyme of the serotonin synthesis.44 The serotonin content was significantly smaller in mi/mi, Mior/Mior, and Miwh/Miwh CMCs than in +/+ CMCs (Table4).
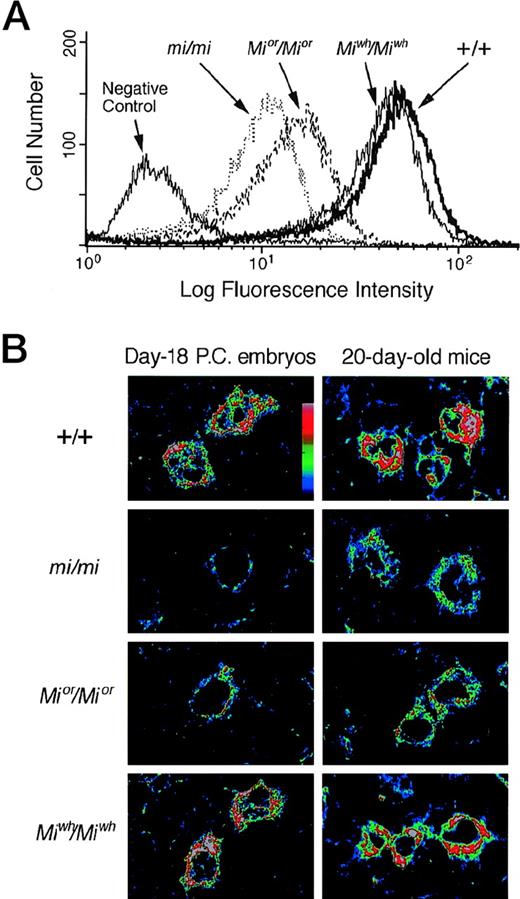
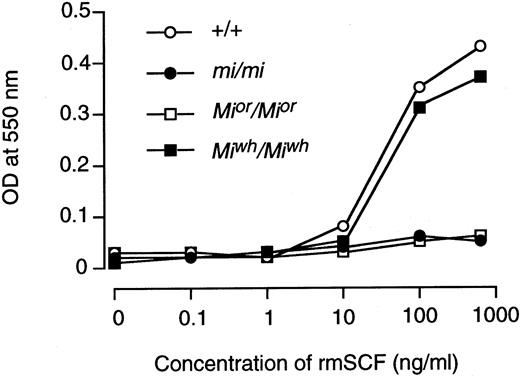
The expression of c-kit protein in CMCs was examined by flow cytometry. The expression level of c-kit protein decreased significantly in Mior/Mior andmi/mi CMCs, and the magnitude of decrease was more remarkable in mi/mi CMCs than in Mior/MiorCMCs (Fig 3A). The expression level of c-kit protein in Miwh/Miwh CMCs was comparable to that of +/+ CMCs (Fig 3A). Next, the expression level of c-kit protein was compared in the skin mast cells of day-18 pc embryos and 20-day-old mice by using the confocal laser scanning microscope. At least 20 fields were examined in three tissue samples. In skin mast cells of day-18 pc embryos, the expression level of c-kit protein decreased significantly inMior/Mior and mi/mi genotypes; the magnitude of decrease was more remarkable in mi/mi embryos than in Mior/Mior embryos (Fig 3B). The skin mast cells of day-18 pc Miwh/Miwhembryos expressed a level of c-kit protein comparable to that of +/+ embryos. In skin mast cells of 20-day-old mice, the expression level of c-kit protein decreased in theMior/Mior and mi/mi genotype (Fig 3B). The expression level of c-kit protein ofMiwh/Miwh mice was comparable to that of +/+ mice. The cellular response of +/+, mi/mi,Mior/Mior, andMiwh/Miwh CMCs to rmSCF was then compared using the MTT method (Fig 4). The +/+ CMCs and Miwh/Miwh CMCs responded to rmSCF in a dose-dependent manner, butMior/Mior CMCs and mi/mi CMCs did not (Fig 4).
Expression of c-kit protein in CMCs and skin mast cells. (A) Flow cytometry of the surface expression of c-kitprotein in CMCs derived from +/+, mi/mi,Mior/Mior, andMiwh/Miwh mice. Cells were incubated with either ACK2 MoAb or negative control antibody. (B) The representative field showing the content of c-kit protein in skin mast cells of day-18 pc embryos or 20-day-old mice. Specimens were observed by the confocal laser scanning microscope after staining c-kit protein with ACK2 MoAb. At least 20 fields were examined in three tissue samples obtainied from different mice, and comparable results were obtained. The intensity of fluorescence is shown by colors (the upper is the stronger). (Original magnification × 2,500.)
Expression of c-kit protein in CMCs and skin mast cells. (A) Flow cytometry of the surface expression of c-kitprotein in CMCs derived from +/+, mi/mi,Mior/Mior, andMiwh/Miwh mice. Cells were incubated with either ACK2 MoAb or negative control antibody. (B) The representative field showing the content of c-kit protein in skin mast cells of day-18 pc embryos or 20-day-old mice. Specimens were observed by the confocal laser scanning microscope after staining c-kit protein with ACK2 MoAb. At least 20 fields were examined in three tissue samples obtainied from different mice, and comparable results were obtained. The intensity of fluorescence is shown by colors (the upper is the stronger). (Original magnification × 2,500.)
Cellular response of +/+, mi/mi,Mior/Mior andMiwh/Miwh CMCs at various concentrations of rmSCF was measured with means of MTT colorimetric assay. Three independent experiments were performed with comparable results, and the result of a representative experiment is shown. Each point represents the mean of triplicate samples.
Cellular response of +/+, mi/mi,Mior/Mior andMiwh/Miwh CMCs at various concentrations of rmSCF was measured with means of MTT colorimetric assay. Three independent experiments were performed with comparable results, and the result of a representative experiment is shown. Each point represents the mean of triplicate samples.
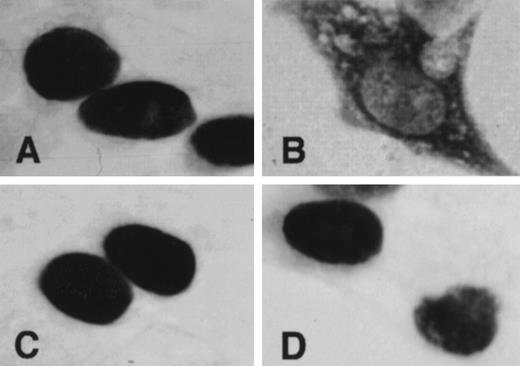
The subcellular localization of MITF was examined by immunocytochemistry. 293T cells that were transfected with expression vector containing +-MITF, mi-MITF, or-MITF, orwh-MITF cDNA were stained with the anti-MITF polyclonal antibody. Strong signals were detected in the nuclei of the cells overexpressing +-MITF, or-MITF, or wh-MITF cDNA (Fig 5). In contrast, signals were predominantly detected in the cytoplasm of the cells overexpressingmi-MITF cDNA (Fig 5).
Subcellular localization of MITF determined by immunocytochemistry. 293T cells were transfected with expression vector containing +-MITF (A), mi-MITF (B), or-MITF (C), orwh-MITF (D) cDNA. After 48 hours of transfection, cells were stained with anti-MITF antibody. (Original magnification × 4,000.)
Subcellular localization of MITF determined by immunocytochemistry. 293T cells were transfected with expression vector containing +-MITF (A), mi-MITF (B), or-MITF (C), orwh-MITF (D) cDNA. After 48 hours of transfection, cells were stained with anti-MITF antibody. (Original magnification × 4,000.)
The DNA binding ability of or-MITF and wh-MITF was examined using EGMSA. The oligonucleotide containing the CACATG motif, which represents a part of MMCP-6 promoter, was used as the probe.24 No DNA-protein complex was detected whenor-MITF or mi-MITF was incubated with the protein. The specific binding of +-MITF and wh-MITF was detected, but the amount of DNA-wh-MITF complex was smaller than the amount of DNA-+-MITF complex (Fig 6A). When the dissociation assay was performed, the dissociation rate of DNA-wh-MITF complex was higher than that of DNA-+-MITF complex (Fig 6B). In other words, the affinity of wh-MITF to DNA was lower than that of +-MITF.
DNA binding ability of +-MITF, mi-MITF,or-MITF, or wh-MITF examined by EGMSA and dissociation assay performed with +-MITF or wh-MITF. (A) EGMSA using GST-MITF fusion proteins. The labeled 5′-TGGTGGGGACACATGTTACATGGA oligonucleotide was used as a probe (hexameric motif recognized by MITF is underlined). Each lane contains 3.5 μg of GST-+-MITF, GST-mi-MITF, GST-or-MITF, or GST-wh-MITF in the absence or presence of a 200-fold molar excess of unlabeled oligonucleotide as a competitor. Coomasie brilliant blue staining for GST-+-MITF, GST-mi-MITF, GST-or-MITF, or GST-wh-MITF was shown below the result of EGMSA. The amount of protein in each lane was the same as the amount used in EGMSA. (B) Dissociation assay performed with +-MITF and wh-MITF. Reaction mixtures for DNA-+-MITF and DNA-wh-MITF complexes were prepared, and a 500-fold molar excess of unlabeled oligonucleotide was added. The reactions were sampled after further incubation time of 30, 60, 90, 120, and 150 seconds. The regions of the autoradiogram containing DNA-MITF complexes were scanned with a densitometer, and the values were plotted against the incubation period.
DNA binding ability of +-MITF, mi-MITF,or-MITF, or wh-MITF examined by EGMSA and dissociation assay performed with +-MITF or wh-MITF. (A) EGMSA using GST-MITF fusion proteins. The labeled 5′-TGGTGGGGACACATGTTACATGGA oligonucleotide was used as a probe (hexameric motif recognized by MITF is underlined). Each lane contains 3.5 μg of GST-+-MITF, GST-mi-MITF, GST-or-MITF, or GST-wh-MITF in the absence or presence of a 200-fold molar excess of unlabeled oligonucleotide as a competitor. Coomasie brilliant blue staining for GST-+-MITF, GST-mi-MITF, GST-or-MITF, or GST-wh-MITF was shown below the result of EGMSA. The amount of protein in each lane was the same as the amount used in EGMSA. (B) Dissociation assay performed with +-MITF and wh-MITF. Reaction mixtures for DNA-+-MITF and DNA-wh-MITF complexes were prepared, and a 500-fold molar excess of unlabeled oligonucleotide was added. The reactions were sampled after further incubation time of 30, 60, 90, 120, and 150 seconds. The regions of the autoradiogram containing DNA-MITF complexes were scanned with a densitometer, and the values were plotted against the incubation period.
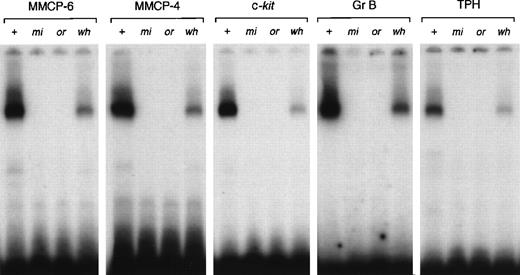
Because the affinity of transcription factors to DNA might be affected by the surrounding sequences of the core binding motif,45there is a possibility that the affinity of each mutant MITF to the CANNTG motif located in the MMCP-4, c-kit, Gr B, or TPH promoter was different from that of the CANNTG motif located in the MMCP-6 promoter. We examined the affinity of each mutant MITF to the CANNTG motif located in the MMCP-4, c-kit, Gr B, or TPH promoter. We did not examine the affinity to the CAGTTG motif located in the MMCP-5 promoter, because even +-MITF did not bind the motif.19 The result observed in the MMCP-4, c-kit, Gr B, or TPH promoter was comparable to the result observed in the MMCP-6 promoter (Fig 7).
DNA binding ability of +-MITF, mi-MITF,or-MITF, or wh-MITF to the binding motif located in MMCP-6, MMCP-4, c-kit, Gr B, or TPH promoter. Each lane contains 3.5 μg of GST-+-MITF, GST-mi-MITF, GST-or-MITF, or GST-wh-MITF.
DNA binding ability of +-MITF, mi-MITF,or-MITF, or wh-MITF to the binding motif located in MMCP-6, MMCP-4, c-kit, Gr B, or TPH promoter. Each lane contains 3.5 μg of GST-+-MITF, GST-mi-MITF, GST-or-MITF, or GST-wh-MITF.
DISCUSSION
We compared the phenotype of mast cells among +/+, mi/mi,Mior/Mior, andMiwh/Miwh mice. As reported previously,16-22 the phenotype of mast cells withmi/mi genotype was abnormal in various items. In the skin ofmi/mi mice, mast cells decreased in number and the proportions of berberine sulfate-positive, MMCP-4 mRNA+, and MMCP-6 mRNA+ mast cells decreased remarkably. The expression of c-kit protein also decreased in the skin mast cells ofmi/mi mice. Moreover, in mi/mi CMCs, the expression of MMCP-4, MMCP-5, MMCP-6, c-kit, Gr B, or TPH gene was hardly detectable, and the surface expression of c-kit receptor and the response to rmSCF were severely deficient. The serotonin content decreased significantly in mi/mi CMCs. The phenotype of mast cells of Mior/Mior genotype was abnormal in the same items as detected in mast cells of mi/migenotype. However, the magnitude of abnormality ofMior/Mior mast cells was significantly milder than that of mi/mi mast cells in the following items: the number of skin mast cells in day-18 pc embryos, the proportion of berberine sulfate-positive skin mast cells, the expression of c-kit and Gr B mRNAs, and the surface expression of c-kit protein in CMCs. The phenotypes ofMiwh/Miwh skin mast cells and CMCs were mostly comparable to those of +/+ skin mast cells and CMCs, but the decreased expression of Gr B and TPH mRNAs and the decreased serotonin content were observed in Miwh/MiwhCMCs. The abnormality of mast cells was severe in mi/mi mice, moderate in Mior/Mior mice, and slight in Miwh/Miwh mice. The degree of microphthalmia was severe in mi/mi andMior/Mior mice and moderate inMiwh/Miwh mice.4Osteopetrosis was severe in mi/mi mice, mild inMior/Mior mice, and undetectable inMiwh/Miwh mice.4 26 The rank order of abnormality in mast cells (mi/mi >Mior/Mior >Miwh/Miwh) was the same as that of the severity observed in microphthalmia and osteopetrosis.
The expression level of c-kit protein inMiwh/Miwh CMCs was comparable to that of +/+ CMCs, but that of Mior/Mior andmi/mi CMCs was significantly decreased. This was consistent with the cellular response to SCF, the ligand for c-kitreceptor tyrosine kinase. The rmSCF induced a dose-dependent response in +/+ and Miwh/Miwh CMCs but not inMior/Mior and mi/mi CMCs.
The number of mast cells in the skin appeared to parallel with the expression level of c-kit. The number of skin mast cells in day-18 pc mi/mi embryos was smaller than the number in day-18 pc Mior/Mior embryos, and the c-kit expression level of mast cells was also lower in day-18 pc mi/mi embryos than in day-18 pcMior/Mior embryos. The number of skin mast cells and the expression level of c-kit was comparable between 20-day-old mi/mi andMior/Mior mice. This is consistent with our previous result observed in Wsh/Wshmice.46 Both the number of skin mast cells and the expression level of c-kit decreased in an age-dependent manner in Wsh/Wsh mice.
The functional level of MITF necessary for the mRNA expression of each gene may be estimated by comparing +/+, mi/mi,Mior/Mior, orMiwh/Miwh mast cells. Theor-MITF was deficient in DNA binding ability but normal in nuclear localization potential. The wh-MITF had normal nuclear localization potential. The wh-MITF can bind DNA, but its affinity to DNA was lower than that of +-MITF.Miwh/Miwh CMCs normally expressed MMCP-4, MMCP-6, and c-kit genes but weakly expressed TPH and Gr B genes. The affinity of wh-MITF to the promoter of each gene may be affected by the surrounding sequences, as reported by Fisher et al45 in the case of the interaction between the Myc protein and its targets. However, no significant difference was detected among the affinity of wh-MITF to the CANNTG motif of MMCP-4, MMCP-6, c-kit, Gr B, and TPH promoters. Therefore, the deficient expression of Gr B and TPH genes may not be explained by the reduced affinity of wh-MITF to the CANNTG motifs. The relatively weak affinity of wh-MITF to the CANNTG motif may be enough for the normal expression of MMCP-4, MMCP-6, and c-kit genes but not for TPH and Gr B genes.
The DNA binding potential of wh-MITF was deficient, but it was apparently demonstratable. This was inconsistent with the finding of Hemesath et al5 that wh-MITF did not bind to the adenovirus major late promoter having the CACGTG motif as a core sequence. We produced wh-MITF as a GST fusion protein inEscherichia coli, whereas Hemesath et al5 producedwh-MITF using reticulocyte lysate. We suppose that the purity of wh-MITF used in our experiment was higher than that of Hemesath et al,5 because we purified the GST-wh-MITF fusion protein by affinity chromatography on immobilized glutathione. There is a possibility that the relatively weak affinity of wh-MITF to DNA was not detected by Hemesath et al5 due to the lower purity of the wh-MITF used in their experiment. There is another possibility that the difference between the result of Hemesath et al5 and our result may be attributable to the difference of probes.
Taken together, mast cells are suitable model for evaluating missing functions of MITF encoded by various mutant alleles.
ACKNOWLEDGMENT
The authors thank Dr S. Nomura (Osaka University) for valuable discussions, Dr Lynn Lamoreux forMior/Mior mice, Dr S. Nagata for pEF-BOS, and Kirin Brewery Co Ltd for rmSCF.
Supported by grants from the Ministry of Education, Science and Culture, the Ministry of Health and Welfare, the Mochida Memorial Foundation for Medical and Pharmaceutical Research, the Joint Research Project under the Japan-Korea Basic Scientific Promotion Program, and the Organization for Pharmaceutical Safety and Research.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Yukihiko Kitamura, MD, Department of Pathology, Osaka University Medical School, Yamada-oka 2-2, Suita 565-0871, Osaka, Japan.