Abstract
The Fcγ receptor IIIb (FcγRIIIb) for the Fc domain of IgG is expressed exclusively on neutrophils. The FcγRIIIb bears allotypic polymorphisms referred to as NA1, NA2, and SH, which are known for their frequent involvement in alloimmune and autoimmune neutropenias as well as in transfusion reactions. The bactericidal capacity of isolated neutrophils is easily activatable, and activation results in self-desintegration, thus preventing storage of neutrophils. As a result, only freshly isolated granulocytes can be used for antibody screening, often making it impossible to use typed panel cells. To provide a readily available source of typed panel cells, we therefore established stable mammalian cells expressing recombinant NA1, NA2, and SH antigens. We isolated mRNA from typed neutrophils and then transcribed it in cDNA. The cDNA that codes for the different forms of the FcγRIIIb was amplified by polymerase chain reaction and was subsequently subcloned into the mammalian expression vector pcDNA3. Chinese hamster ovary (CHO) cells were transfected with allele-specific constructs, and stable cell lines expressing FcγRIIIb were selected by flow cytometry. Because human sera show high background fluorescence with transfectants in flow cytometry, the monoclonal antibody–specific isolation of granulocyte antigens (MAIGA) assay was performed. By MAIGA assay, we tested 14 well-characterized human alloantibodies directed against the antigens NA1, NA2, and SH; 5 FcγRIIIb-specific isoantibodies; and 12 FcγRIIIb-reactive autoantibodies. Except one NA1- and one SH-specific alloantibody, all other antibodies could be identified by the use of CHO transfectants. In contrast to neutrophils, fixed CHO cells can be stored at 4°C for at least 4 weeks or stored frozen for a longer period. This longer shelf life of the transfected CHO cells compared with isolated neutrophils will simplify the detection of the clinically most important FcγRIIIb-reactive alloantibodies and autoantibodies.
THE Fcγ RECEPTOR IIIb (FcγRIIIb = CD16) is a low-affinity receptor for the Fc region of complexed but not monomeric IgG antibodies and removes preferentially small immune complexes from the circulation.1 Immunological studies have shown that FcγRIIIb is rather polymorphic, bearing the important NA1 and NA2 antigens,2 the low-frequency antigen SH,3 and the high-frequency antigens LAN and SAR.4,5Alloantibodies directed against these antigens cause alloimmune immune neutropenia,6 immune neutropenia after bone marrow transplantation,7 and transfusion-related acute lung injury.8 In addition, neutrophil alloimmunization can result in refractoriness to granulocyte transfusions.9,10About 0.1% of the European population do not express the FcγRIIIb on their neutrophils as a result of FcγRIIIB gene deficiency.11 Because these neutrophils do not display the NA1 and NA2 antigens, this phenotype is called NA null.12Women with the NA null phenotype can form isoantibodies to the FcγRIIIb causing severe isoimmune neonatal neutropenia.13Neutrophil autoantibodies detected in the sera of patients with autoimmune neutropenia are frequently directed against the FcγRIIIb with preferential binding to the NA1 isoform.14
In contrast to red blood cells and platelets, granulocytes are not storable without activation and ensuing autolysis as a result of granule enzyme and oxygen radical release. Therefore, cumbersome and time consuming isolation of granulocytes from the donor’s blood for each test series is necessary for antibody screening. Because the FcγRIIIb is the major immunogenic glycoprotein on the neutrophil membrane, we established stable mammalian cell lines expressing the polymorphic forms of the FcγRIIIb and showed their usefulness in detecting FcγRIIIb-reactive granulocyte antibodies.
MATERIALS AND METHODS
Sera.
Human NA-specific typing sera and FγRIIIb-specific isoantibodies were obtained from immunized mothers’ infants with alloimmune or isoimmune neonatal neutropenia. Sera containing autoantibodies were from infants with primary autoimmune neutropenia.
Monoclonal antibodies (MoAbs).
MoAb 3G8 directed against a monomorphic epitope of FcγRIIIb (CD16) was purchased from Immunotech (Hamburg, Germany) and a second FcγRIIIb-reactive MoAb BW 209/2 was a generous gift of Dr Kurrle (Marburg, Germany). MoAbs CLB gran 11 and GRM1 directed against NA1 and NA2 allelic forms of FcγRIIIb were generous gifts of Dr Garrido (Granada, Spain) and Dr von dem Borne (Amsterdam, The Netherlands).
Amplification of full-length FcγRIIIB cDNA.
Full-length FcγRIIIB cDNA was synthesized by polymerase chain reaction (PCR) amplification of granulocyte mRNA from of an NA1-homozygous donor and an NA2-, SH-positive donor as previously described.3 The FcγRIII-specific primers were constructed based on the published sequence of Ravetch and Perussia.15In brief, 3 μL cDNA was mixed with 8 μL PCR buffer, 0.5 μmol/L of sense primer (5′1-TCTTTGGTGACTTGTCCA-18 3′), 0.5 μL antisense primer (5′886-AGAGGCCTGAGGATGAT-870 3′), 200 μmol/L of each dNTP, and 1.5 U of Taq GOLD polymerase and were amplified on a GenAmp 9600 DNA thermal cycle (Perkin Elmer, Weiterstadt, Germany) for 39 cycles. After heating at 95°C for 5 minutes, PCR was performed under following conditions: denaturation at 95°C for 60 seconds, annealing at 47°C for 90 seconds, and extension at 72°C for 60 seconds. An aliquot of 5 μL PCR product (dilution 1:100) was amplified again using nested primers (5′ 16-CCACTCCAGTGTGGCATC-33 3′) and (5′ 831-GCCACTGCTCTTATTACT-814 3′) for 39 cycles. Each cycle consisted of denaturation at 95°C for 45 seconds, annealing at 49°C for 50 seconds, and extension for 72°C for 60 seconds. In the final cycle, the samples were kept at 72°C for 10 minutes and then chilled to 4°C. Amplified cDNA was analyzed on 1.5% agarose gel electrophoresis and purified by Geneclean (Dianova, Hamburg, Germany).
Construction of allele-specific FcγRIIIB expression systems.
Purified cDNA was flushed using Klenow DNA polymerase (Biolabs, Bad Schwalbach, Germany) and was subcloned into the EcoRV site of the mammalian expression vector pcDNA3 (Invitrogen, Leek, The Netherlands). To determine the right insert orientation within the vector, NA1-, NA2-, and SH-constructs were digested with KpnI endonuclease (Biolabs). Plasmid from positive clones were then analyzed for NA1-, NA2-, and SH-polymorphic sites by PCR and restriction analysis. In brief, plasmid DNA were amplified by PCR using sense primer no. 5 (5′ 41-AGCTGCTCCTCCCAACTG-58 3′) antisense primer no. 6 (5′ 393-CTCCTTGAACACCCACCG-376 3′) for 35 cycles. Each cycle consisted of denaturation at 95°C for 30 seconds, annealing at 59°C for 50 seconds, and extension at 72°C for 30 seconds. Amplified DNA was analyzed by restriction digestion with TaqI endonuclease (Biolabs). To determine the allele specificity of NA1-, NA2-, and SH-constructs, PCR products were subjected to restriction analysis using TaqI and SfaNI endonucleases (Biolabs). Purified plasmids used for subsequent transfection were validated by nucleotide sequence analysis.
Stable expression of allele-specific FcγRIIIB recombinant antigens.
Chinese hamster ovary (CHO; American Type Tissue Collection, Rockville, MD) cells were grown in RPMI 1640 medium (GIBCO-BRL, Eggenstein, Germany) containing 10% fetal calf serum (Seromed, Berlin, Germany), 1% sodium pyruvate, 1% glutamine, and 1% penicillin/streptomycine (complete medium) and were transfected with NA1-, NA2-, and SH-constructs by the use of Lipofectin (GIBCO-BRL). In brief, 6 μg of each construct was mixed with 25 μL Lipofectin in 2 mL OptiMEM medium (GIBCO-BRL) and then added to subconfluent 10-cm plate of CHO cells for 12 hours. Nine milliliters complete medium was added and the incubation was continued for 48 hours. After splitting, CHO transfectants were selected with Genicitin (G418; final concentration 1 mg/mL; GIBCO-BRL) for about 2 weeks. Stable transfectants were analyzed for high expression of FcγRIIIB recombinant proteins by flow cytometry (see below). After subcloning, stable transfectants were grown in complete medium supplemented with 200 μg/mL G418.
Flow cytometry.
Stable transfectants were obtained with Trypsin-EDTA (GIBCO-BRL), washed in phosphate-buffered saline (PBS; GIBCO-BRL), and fixed with 1% paraformaldehyde (PFA). After three times washing 40 μL (5 × 103/L) cells were incubated with 10 μL MoAb 3G8 specific for FcγRIIIb for 30 minutes at room temperature. Sensitized cells were then washed twice, stained with 40 μL fluorescein-isothiocyanate–conjugated rabbit anti-mouse IgG (dilution 1:30; Dako, Hamburg, Germany) and analyzed by flow cytometry (Ortho Diagnostics, Neckargemuend, Germany).
Antigen capture assay with stable transfectants.
The assay with stable transfectants was performed using the MoAb-specific immobilization of granulocyte antigens (MAIGA) assay as previously described for granulocytes with minor modifications.16 In brief, 100 μL (2 × 103/μL) of transfectants fixed with 1% paraformaldehyde were incubated (30 minutes, 37°C) with human serum and a MoAb. The FcγRIII-specific MoAbs 3G8 and BW 209/2 were used in different reaction mixtures. The cells were washed and solubilized by adding 100 μL of lysis buffer (1% Triton-X 100, 5 mmol/L EDTA, 2 mmol/L phenylmethylsulfonylfluoride [PMSF], 0.5 μg/mL Leupeptin, 500 KIE/mL Aprotinin in 20 mmol/L Tris-buffered saline, pH 7.4) for 30 minutes at room temperature. After sonication (2 minutes) and centrifugation at 15,000g for 30 minutes, the supernatant of each reaction mixture was transferred to a separate tube coated with goat anti-mouse antibodies. Unattached antibodies were removed by washing, and goat anti-human IgG (heavy + light chain) antibodies conjugated with peroxidase were added. After washing and subsequent addition of a substrate containing luminol, hydrogen peroxide, and 4-iodophenol, the emitted light (chemiluminescence) was measured over a period of 15 minutes in a luminometer (Lumat LB 9501; Berthold, Wildbad, Germany).
Immunoprecipitation.
Immunoprecipitation was performed as recently described.3In brief, for immunoprecipitation 5 × 107 unfixed CHO cells in PBS were biotinylated (5 mmol/L NHS-LC-Biotin; Pierce, Rockford, IL) for 30 minutes on ice. After washing, 100 μL of cell suspension, 100 μL of serum, or MoAb solution (0.01 mg/mL) was added to each sample and incubated for 30 minutes at 37°C. The cells were washed and solubilized by adding lysis buffer (see MAIGA assay) for 30 minutes at room temperature. After sonication and centrifugation the supernatants were incubated with rabbit anti-human IgG or anti-mouse Ig (Dako) antibodies coupled to Protein A-Sepharose CL-4B (Pharmacia, Freiburg, Germany). The Protein A-Sepharose beads were washed and resuspended in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer, boiled, and then subjected to 10% SDS-PAGE. After electrophoresis, proteins were transferred onto nitrocellulose (Hibond C, Amersham, Braunschweig, Germany). For visualization, the nitrocellulose was first blocked with bovine albumin and then incubated with streptavidin conjugated to peroxidase. Unbound streptavidin was washed out, the nitrocellulose was incubated with a chemiluminescent substrate (ECL Western Blotting Detection System; Amersham), and then exposed to x-ray films.
NA and SH genotyping by PCR with sequence-specific primers (PCR-SSP).
For genotyping of NA antigens, a slightly modified PCR-SSP technique was used as previously described.17 Briefly, DNA isolated from CHO cells was amplified by PCR using a thermal cycler with 2 U Taq DNA polymerase. The PCR reaction consists of 30 cycles (denaturation, 98°C/30 seconds; annealing, 57°C/1 minute; extension, 71°C/30 seconds; final extension, 71°C/5 minutes). The NA-specific primers were designed as sense primers and were situated at position 208 to 227 for NA1 and at position 130 to 147 for NA2. To enhance the specificity of the NA1 primer, at position 4 from the 3′ end, the correct nucleotide A was substituted by a T. The nonspecific antisense primer is situated at position 331 to 348. As internal positive PCR control, two primers (HGH-I and HGH-II, see below) amplifying a 439-bp fragment of the human growth hormone gene (HGH) were used. After electrophoresis in 1.6% agarose gel and staining with ethidium bromide, the PCR products were visualized by ultraviolet illumination and photographed.
For genotyping of the SH antigen the recently described PCR-SSP method was used.3 Five microliters of DNA was amplified in a total volume of 50 μL using 0.5 μM SH(+) sequence-specific antisense primer no. 7 (5′-285TCTGTCGTTGACTGTGTCAT266-3′) or the antithetical SH(−) sequence-specific antisense primer no. 8 (285TCTGTCGTTGACTGTGTCAG266-3′), 0.5 μmol/L sense primer no. 9 (5′-95AAGATCTCCCAAAGGCTGTG115-3′), 200 μmol/L of each dNTP, 5 μL 10× PCR buffer, 2 U Taq polymerase on a DNA thermal cycler (Perkin Elmer) for 30 cycles. To enhance the specificity of the primers, at position 4 from the 3′ end the correct nucleotide, G was substituted by a T. Coamplification of the HGH gene using 0.125 μmol/L HGH I primer (5′-CAGTGCCTTCCCAACCATTCCCTTA-3′) and 0.125 μmol/L HGH II primer (5′-ATCCACTCACGGATTTCTGTTGTGTTTC-3′) was run as internal control. Each cycle consisted of denaturation at 95°C for 30 seconds, annealing at 60°C for 1 minute and primer extension at 71°C for 30 seconds.
RESULTS
Characterization of allele-specific constructs.
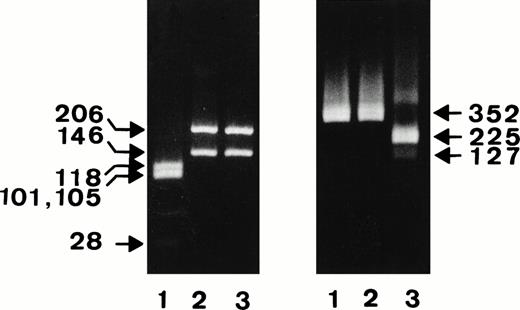
Figure 1 shows the results of the restriction analysis of the constructs digested with the NA-specific TaqI and the SH-specific SfaNI endonucleases.
Restriction analysis of the NA1-, NA2-, and SH-constructs. After PCR amplification of the NA1- (lanes 1), NA-2 (lanes 2), and SH-constructs (lanes 3), FcγRIIIb fragments encompassing nucleotides 41-393 were digested with NA-specific TaqI (left panel) or SH-specific SfaNI endonuclease (right panel) and were analyzed on 4% or 1.5% agarose gel, respectively.
Restriction analysis of the NA1-, NA2-, and SH-constructs. After PCR amplification of the NA1- (lanes 1), NA-2 (lanes 2), and SH-constructs (lanes 3), FcγRIIIb fragments encompassing nucleotides 41-393 were digested with NA-specific TaqI (left panel) or SH-specific SfaNI endonuclease (right panel) and were analyzed on 4% or 1.5% agarose gel, respectively.
Expression of allele-specific FcγRIIIb on CHO cells.
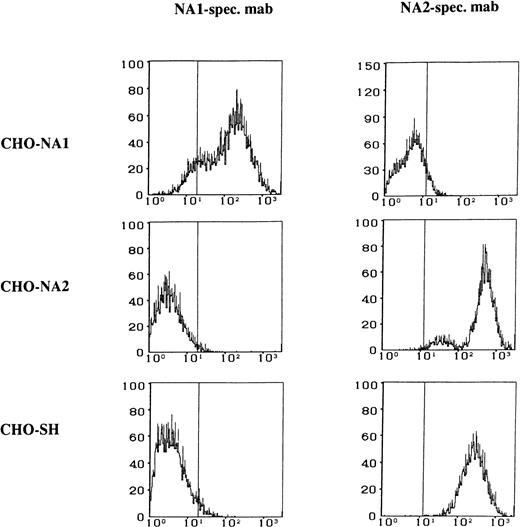
The FcγRIIIb specificity of the transfected CHO cells was tested by the use of human alloantibodies and MoAbs recognizing different isoforms of the FcγRIIIb. The NA1, NA2, and SH isoforms of the FcγRIIIb expressed by the transfected CHO cells could be clearly identified by flow cytometry (Fig 2).
Flow cytometry analysis of the stable CHO transfectants expressing FcγRIIIb NA1-, NA2-, and SH-isoforms. Paraformaldehyde-fixed CHO cells were stained either with NA1-specific MoAb CLB Gran 11 or with NA2-specific MoAb GRM 1, washed, and labeled with fluorescein-conjugated rabbit anti-mouse IgG.
Flow cytometry analysis of the stable CHO transfectants expressing FcγRIIIb NA1-, NA2-, and SH-isoforms. Paraformaldehyde-fixed CHO cells were stained either with NA1-specific MoAb CLB Gran 11 or with NA2-specific MoAb GRM 1, washed, and labeled with fluorescein-conjugated rabbit anti-mouse IgG.
Immunochemical characterization of the FcγRIIIb recombinant antigens.
Immunoprecipitation using the MoAb 3G8 showed the expected Mr of ∼58 kD for the FcγRIIIb-NA1 form and the Mr of ∼73 kD for the FcγRIIIb-NA2 and ∼63 kD for FcγRIIIb-SH forms, respectively (Fig 3). The base pair exchange responsible for the SH polymorphism influences glycoslyation of the FcγRIIIb, resulting in a slightly lower Mr of the SH isoform.
Immunoprecipitation of allele-specific FcγRIIIb recombinant isoforms from biotin surface-labeled CHO stable transfectants expressing SH (lane 1), NA2 (lane 2), and NA1 (lane 3) granulocyte antigens. After solubilization CHO lysates were immunoprecipitated with MoAb 3G8 specific for FcγRIIIb. Immunoprecipitates were separated on 7.5% SDS-PAGE under nonreducing conditions and were transferred by immunoblotting. Biotin-labeled proteins were visualized using streptavidin and chemiluminescence substrate system. Note the different electrophorectic migrations of the various FcγRIIIb isoforms.
Immunoprecipitation of allele-specific FcγRIIIb recombinant isoforms from biotin surface-labeled CHO stable transfectants expressing SH (lane 1), NA2 (lane 2), and NA1 (lane 3) granulocyte antigens. After solubilization CHO lysates were immunoprecipitated with MoAb 3G8 specific for FcγRIIIb. Immunoprecipitates were separated on 7.5% SDS-PAGE under nonreducing conditions and were transferred by immunoblotting. Biotin-labeled proteins were visualized using streptavidin and chemiluminescence substrate system. Note the different electrophorectic migrations of the various FcγRIIIb isoforms.
Application of CHO stable transfectants expressing allele-specific recombinant antigens for detection of human granulocytes FcγRIIIb-reactive alloantibodies and autoantibodies.
In immunofluorescence, human sera showed a high background staining so that interpretation of the results was often quite difficult. Therefore, we used a CHO cell modification of the antigen-specific MAIGA assay for alloantibody identification in human sera. By the MAIGA assay, false positive results due to antibodies recognizing structures other than the FcγRIIIb are excluded. Fourteen human sera containing well-characterized alloantibodies directed against the NA1, NA2, and SH antigens as well as five sera with human isoantibodies to the FcγRIIIb were tested in the MAIGA assay for their reactivity with the transfected CHO cells (Table 1) and freshly isolated granulocytes. Whereas all isoantibodies to the FcγRIIIb were detected, one NA1 and one SH alloantibody were not detectable by MAIGA assay using CHO cells as well as granulocytes.
In addition, we tested 12 sera containing autoantibodies to the FcγRIIIb as shown by MAIGA assay using human granulocytes (Table 1). The autoantibodies tested showed preferential binding to NA1 homozygous test cells in granulocyte immunofluorescence. Five of the 12 sera showed binding to both NA1- and NA2-transfected cells. However, the binding reactivity to NA2 CHO cells was weaker. Two other sera reacted additionally with the SH transfected CHO cells.
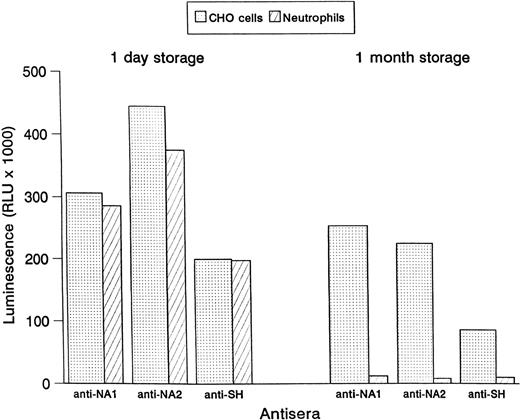
CHO cells were stored for 1 month and tested in the MAIGA assay for their reactivity with human alloantibodies as compared to stored granulocytes. In contrast to the human neutrophils, no significant loss of reactivity was observed with the CHO cells (Fig 4). Similar results were achieved when CHO cells and human neutrophils were stored frozen (data not shown).
Binding of human antisera to NA1-, NA2-, and SH-expressing CHO cells and human granulocytes. Reactivity in the MAIGA assay is shown after 1 day storage and 1 month storage at 4°C.
Binding of human antisera to NA1-, NA2-, and SH-expressing CHO cells and human granulocytes. Reactivity in the MAIGA assay is shown after 1 day storage and 1 month storage at 4°C.
Genotyping analysis of FcγRIIIB alleles by sequence (allele)-specific PCR.
Sequence (allele)-specific PCR was performed using DNA isolated from transfected CHO cells (Fig 5). The PCR products were identical to the products obtained by allele-specific PCR using DNA isolated from human leukocytes. The results show that the transfectants can be used as a source of NA1, NA2, and SH reference DNA for genotyping.
Genotyping analysis of the stable CHO transfectants expressing NA1-, NA2-, and SH-isoforms by allele-specific PCR. DNA were isolated from the transfectants and were amplified using allele-specific NA1- (lanes 2, 5, and 8), NA2-(lanes 3, 6, and 9), and SH- (lanes 4, 7, and 10) primer. PCR products were analyzed on 1.5% agarose gel using MW VI (lane 1; Boehringer Mannheim, Mannheim, Germany) as molecular weight standards.
Genotyping analysis of the stable CHO transfectants expressing NA1-, NA2-, and SH-isoforms by allele-specific PCR. DNA were isolated from the transfectants and were amplified using allele-specific NA1- (lanes 2, 5, and 8), NA2-(lanes 3, 6, and 9), and SH- (lanes 4, 7, and 10) primer. PCR products were analyzed on 1.5% agarose gel using MW VI (lane 1; Boehringer Mannheim, Mannheim, Germany) as molecular weight standards.
DISCUSSION
For granulocyte antibody screening, a combination of the granulocyte immunofluorescence and agglutination has been found to be the best means of detection.18 However, these tests require nonactivated and, for agglutination, also functionally active cells. Therefore, only granulocytes stored up to 4 hours can be used in granulocyte immunofluorescence and agglutination tests.19This need for freshly isolated granulocytes is a major problem in granulocyte serology, making granulocyte antibody screening often impossible and hampering the detection of antibodies to low-frequency antigens. These problems are avoided by the introduction of stable mammalian cell lines such as CHO cells transfected with cDNA encoding for selected human antigens. CHO transfectants expressing human antigens have been shown to be useful reagents for the detection of alloantibodies that are difficult to identify such as antibodies to Cromer antigens.20
Antibodies binding to the FcγRIIIb are of the same clinical importance in granulocyte serology as antibodies to the glycoprotein IIb/IIIa in platelet serology or Rhesus antibodies in red cell serology. Two thirds of all cases of neonatal immune neutropenia are caused by FcγRIIIb-reactive allo/isoantibodies,21 and about a third of the autoantibodies in primary autoimmune neutropenia in infancy are directed against the FcγRIIIb.14Therefore, we transfected CHO cells with the NA1, NA2, and SH isoforms of the FcγRIIIb. We could show that our NA1, NA2, and SH transfectants exhibit the same antigenic and immunochemical heterogeneity as the isoforms show on human neutrophils. CHO cells also glycosylate FcγRIIIb in a similar manner to normal human neutrophils.22
Although MoAbs to FcγRIIIb can be easily detected by flow cytometry, human sera caused such a high background in immunofluorescence that interpretation can become very difficult. Therefore, we used a modified MAIGA assay procedure for antibody identification using CHO cells instead of human granulocytes. By this procedure, we could detect most of the FcγRIIIb-reactive antibodies. In contrast to neutrophils, the CHO cells could be stored for at least 1 month at 4°C.
The alloantibodies that were not detected were also not detectable by MAIGA using human neutrophils. Usually, it is assumed that there is a steric hindrance between the human antibody and the two MoAbs used for antigen immobilization, although the MoAbs are directed against different epitopes because they do not hinder each other in their binding to the FcγRIIIb. Another explanation is based on the observation that the glycosylphosphatidylinositol-anchored FcγRIIIb cooperates with the transmembrane leukocyte adhesion molecule CD11b/CD18 for signal transduction via the cell membrane.23Possibly, the epitopes recognized by these alloantibodies are stabilized by the CD11b/CD18 complex and are disrupted during FcγRIIIb isolation.
Supported by a grant from the Deutsche Forschungsgemeinschaft DFG Bu 770/3-3.
This work is part of an academic thesis (Phd) of Karin Kissel.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Juergen Bux, MD, Institute for Clinical Immunology and Transfusion Medicine, Justus Liebig University, Langhansstrasse 7, D-35385 Giessen, Germany; e-mail:Juergen.Bux@immunologie.med.uni-giessen.de.