Abstract
Approximately 5% of hemophilia A patients have normal amounts of a dysfunctional factor VIII (FVIII) protein and are termed cross-reacting material (CRM)-positive. FVIII is a heterodimer (domain structure A1-A2-B/A3-C1-C2) that requires thrombin cleavage to elicit procoagulant activity. Thrombin-activated FVIII is a heterotrimer with the A2 subunit (amino acid residues 373 to 740) in a weak ionic interaction with the A1 and A3-C1-C2 subunits. Dissociation of the A2 subunit correlates with inactivation of FVIII. Recently, a phenotype of CRM-positive hemophilia A patients has been characterized whose plasma displays a discrepancy between their FVIII activities, where the one-stage clotting assay displays greater activity than the two-stage clotting assay. One example is a missense mutation whereARG531 has been substituted by HIS531. An FVIII cDNA construct was prepared containing theARG531HIS mutation and the protein was expressed in COS-1 monkey cells by transient DNA transfection. Metabolic labeling with [35S]-methionine demonstrated that ARG531HIS was synthesized at an equal rate compared with FVIII wild-type (WT) but had slightly reduced antigen in the conditioned medium, suggesting a modest secretion defect. A time course of structural cleavage of ARG531HISdemonstrated identical thrombin cleavage sites and rates of proteolysis as FVIII WT. Similar to the patient phenotypes,ARG531HIS had discrepant activity as measured by a one-stage activated partial thromboplastin time (aPTT) clotting assay (36% ± 9.6% of FVIII WT) and a variation of the two-stage assay using a chromogenic substrate (COAMATIC; 19% ± 6.9% of FVIII WT). Partially purified FVIII WT and ARG531HISproteins were subjected to functional activation by incubation with thrombin. ARG531HIS demonstrated significantly reduced peak activity and was completely inactivated after 30 seconds, whereas FVIII WT retained activity until 2.5 minutes after activation. Because the ARG531HIS missense mutation predicts a charge change to the A2 subunit, we hypothesized that theARG531HIS A2 subunit could be subject to more rapid dissociation from the heterotrimer. The rate of A2 dissociation, using an optical biosensor, was determined to be fourfold faster forARG531HIS compared with FVIII WT. Because the two-stage assay involves a preincubation phase before assay measurement, an increased rate of A2 dissociation would result in an increased rate of inactivation and reduced specific activity.
PLASMA COAGULATION factor VIII (FVIII) functions within the blood coagulation cascade as a cofactor for factor IXa in the proteolytic activation of factor X to factor Xa. A quantitative or qualitative deficiency of FVIII leads to the phenotype of the bleeding disorder hemophilia A. FVIII is synthesized as a single-chain polypeptide of approximately 280 kD with the domain structure A1-A2-B-A3-C1-C2.1-3 The A domains share 35% to 40% amino acid identity and are homologous to the A-domains of ceruloplasmin. The C domains also display 35% to 40% amino acid identity to each other and are homologous to phospholipid-binding proteins, suggesting a role in phospholipid interactions.1,2,4,5 The B domain shares no significant homology with any known protein. Intracellular proteolytic processing within the B domain after residues ARG1313 orARG1648 forms a heterogeneous FVIII heterodimer consisting of approximately 90-to 220-kD heavy chain fragments (A1-A2-B) associated with an 80-kD light chain fragment (A3-C1-C2) through a monovalent copper ion-dependent linkage between the A1 and A3 domains.6
Thrombin activates FVIII through proteolytic cleavage afterARG740, ARG1689, and ARG372, generating an activated FVIII (FVIIIa) heterotrimer consisting of the A1 subunit (50 kD) in a copper ion-dependent association with the thrombin-cleaved light chain (73 kD) and a free A2 subunit associated with the A1 domain through a weak ionic interaction.7-13 An acidic amino acid-rich region at the carboxy-terminus of the A1 subunit most likely interacts with positively charged residues within the A2 subunit to retain the heterotrimeric FVIIIa configuration.14-16
FVIII activity is measured by either a one-stage or two-stage procedure.17,18 The one-stage assay is based on the ability of FVIII-containing samples to correct the prolonged activated partial thromboplastin time (aPTT) of FVIII-deficient plasma.19 The two-stage assay uses the same principle as the one-stage method, yet is split into two distinct phases.20 In the first phase, dilutions of FVIII are incubated with factor IXa, factor X, Ca2+, and phospholipid. Activation of FVIII during the first phase allows it to exhibit its procoagulant activity as a cofactor for factor IXa, leading to the generation of factor Xa. The incubation mixture is then subsampled into a second tube containing Ca2+ and a source of prothrombin and fibrinogen, and the time to fibrin formation is recorded. A modification of the two-stage assay has also been used in which the second reaction is incubated with a factor Xa-sensitive chromogenic substrate where the rate or degree of color change is proportional to the amount of FVIII added in the first stage of the assay.21
Hemophilia A is a heterogeneous disorder, with severe phenotypes associated with major disruptions of the FVIII gene. Patients with severe phenotypes usually have FVIII antigen levels that are undetectable and are termed cross-reacting material (CRM)-negative. Other genetic mutations are associated with inefficient secretion, with patient plasmas having concomitantly reduced FVIII antigen and activity levels, and are termed CRM-reduced. Approximately 5% of hemophilia A patients have considerable FVIII antigen levels (at least 30% of normal), but FVIII activity levels are significantly reduced, suggesting a protein dysfunction.22 Interestingly, approximately 40% of the CRM-positive and CRM-reduced hemophilia A patients contain missense mutations within the A2 domain.23The A2 domain, representing only approximately 10% of the entire amino acid sequence of FVIII, therefore contains a significant clustering of missense mutations resulting in hemophilia A highlighting its functional importance for FVIII procoagulant activity.
The study of missense mutations has contributed significantly to our understanding of FVIII structure-function relationships and the pathophysiology contributing to the hemophilia A disease phenotype. Missense mutations have been identified at thrombin cleavage sites critical for functional activation and at residues important in interaction with von Willebrand factor (vWF).24-28 However, to date, only two FVIII A2 domain missense mutations have been characterized as to their mechanism leading to protein dysfunction.27 29 In both cases, the reduced specific activity could be attributed to reduced interaction with factor IXa.
Recent reports have identified a CRM-positive hemophilia A phenotype in which the patient plasmas exhibit a familial discrepancy in which the FVIII procoagulant activity is higher when measured in a one-stage assay compared with a two-stage assay.30-36 This discrepancy is unusual, because one-stage and two-stage assays have been used interchangeably for some time as a standard determination of FVIII activity and the majority of patients have similar results as measured by either method.18 In this report, we have studied the mechanistic basis for one of these patient phenotypes in which an A2 domain missense mutation results in substitution of a histidine for arginine at residue 531.31 Using site-directed mutagenesis, the ARG531HISmutation was generated within the FVIII cDNA and the protein was functionally characterized after expression in transiently transfected COS-1 cells. ARG531HIS demonstrated only a modest secretion defect, had reduced specific activity, and had discrepant FVIII activity as measured by either the one-stage or two-stage methods. Upon thrombin activation, theARG531HIS A2 subunit exhibited a fourfold increased rate of dissociation from the A1/A3-C1-C2 heterodimer. The increased instability of the ARG531HISheterotrimer would reduce its specific activity in both one-stage and two-stage assays but would result in an increased disadvantage in the two-step procedure due to the incubation phase in the first step. This hemophilia A phenotype therefore supports previous in vitro studies that have suggested that nonproteolytic regulation of FVIIIa activity, via spontaneous A2 subunit dissociation, is important in vivo.
MATERIALS AND METHODS
Materials.
Anti-heavy chain FVIII monoclonal antibody (MoAb; F-8) conjugated to CL-4B Sepharose was a gift from Debra Pittman (Genetics Institute Inc, Cambridge, MA). FVIII-deficient and normal pooled human plasma were obtained from George King Biomedical, Inc (Overland Park, KS). Activated partial thromboplastin (automated aPTT reagent) and CaCl2 were purchased from General Diagnostics Organon Teknika Corp (Durham, NC). Anti-light chain FVIII MoAbs, ESH-4 and ESH-8, were purchased from American Diagnostica, Inc (Greenwich, CT). Human thrombin and aprotinin were purchased from Boehringer, Mannheim GmbH (Mannheim, Germany). [35S]-methionine (>1,000 μCi/mmol) was purchased from Amersham Corp (Arlington Heights, IL). En3Hance was purchased from Dupont (Boston, MA). Dulbecco’s modified Eagle’s medium (DMEM), methionine-free DMEM, fetal bovine serum, biotin N-hydroxy succinimide ester, and streptavidin-horseradish peroxidase conjugate were purchased from GIBCO BRL (Gaithersburg, MD). COAMATIC was purchased from DiaPharma (West Chester, OH).
Plasmid mutagenesis.
Mutagenesis was performed within the mammalian expression vector pMT237 containing the FVIII cDNA (pMT2VIII). Oligonucleotide-directed mutagenesis was used to create a Spe I-Kpn I polymerase chain reaction fragment in which codon 531 was mutated from CGC to CAC, predicting an amino acid substitution of histidine for arginine, and was ligated intoSpe I-Kpn I–digested pMT2VIII. The resulting mutant plasmid was designatedARG531HIS. The plasmid containing the wild-type FVIII cDNA sequence was designated FVIII WT. All plasmids were purified by centrifugation through cesium chloride and characterized by restriction endonuclease digestion and DNA sequence analysis.
DNA transfection and analysis.
Plasmid DNA was transfected into COS-1 cells by the diethylaminoethyl (DEAE)-dextran method as previously described.38 Conditioned medium was harvested at 64 hours posttransfection in the presence of 10% fetal bovine serum. Protein synthesis and secretion were analyzed by metabolically labeling cells at 64 hours posttransfection for 30 minutes with [35S]-methionine (300 μCi/mL in methionine-free medium), followed by a chase for 4 hours in medium containing 100-fold excess unlabeled methionine and 0.02% aprotinin. Cell extracts and conditioned medium were harvested and immunoprecipitations were performed and analyzed as described previously.38
Protein purification.
Partially purified ARG531HIS protein was obtained from 200 mL of conditioned medium from transiently transfected COS-1 cells by immunoaffinity chromatography,39 yielding 750 to 1,500 ng per purification. FVIII WT protein was purified in parallel from stably transfected Chinese hamster ovary cells. The proteins eluted into the ethylene glycol-containing buffer were dialyzed and concentrated against a polyethylene glycol (molecular weight, ∼15,000 to 20,000) -containing buffer14 and stored at −70°C.
FVIII activity and antigen assay.
FVIII activity was measured by (1) one-stage aPTT clotting assay on an MLA Electra 750 fibrinometer (Medical Laboratory Automation, Inc, Pleasantville, NY) by reconstitution of human FVIII-deficient plasma or (2) by modified two-stage assay using the COAMATIC chromogenic assay according to the manufacturer’s instructions. For thrombin activation, protein samples were diluted into 50 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, 2.5 mmol/L CaCl2, and 5% glycerol and incubated at room temperature with 1 U/mL thrombin. After incubation for increasing periods of time, aliquots were diluted and assayed for FVIII activity. One unit of FVIII activity is the amount measured in 1 mL of normal human pooled plasma. FVIII antigen was quantified using a sandwich enzyme-linked immunosorbent assay (ELISA) method using anti-light chain antibodies ESH-4 and ESH-8.40 Recombinant FVIII protein purified in parallel was used as a standard.
Kinetic measurements using biosensor technology.
The kinetics of the A2 subunit dissociation from thrombin-activated FVIII WT and ARG531HIS was studied by surface plasmon resonance using the IASys biosensor (Fisons, Cambridge, UK), which measures protein binding and subsequent dissociation in real time.41 Binding of 1 ng of protein per square millimeter of the biosensor chip produces a resonance signal of 200 Arc seconds. MoAb ESH8 (50 μg/mL) in 10 mmol/L sodium acetate, pH 5.0, was covalently coupled to the activated carboxymethyldextran-coated biosensor cuvette via amino groups using succinimide ester chemistry.41 The carboxymethyldextran chip and reagents for its activation, N-ethyl-N′-(dimethylaminopropyl)carbodiimide hydrochloride and N-hydroxysuccinimide, and deactivation, ethanolamine, were purchased from Fisons. FVIII WT and ARG531HIS binding to ESH8, their dissociation from the antibody, and activation by thrombin were measured in 200 μL of 20 mmol/L HEPES, 0.15 mol/L NaCl, 5 mmol/L CaCl2, 0.01% Tween 20, pH 7.4. The chip was regenerated by the addition of 0.1 mol/L glycine, pH 3.0, for 3 minutes, resulting in complete dissociation of FVIII proteins from the capture ESH8. Identical signals for reference binding of the FVIII proteins to immobilized ESH8 were obtained before and after regeneration.
The values of the rate constants for dissociation (koff) of FVIII WT or ARG531HIS from immobilized ESH8 and those for dissociation of the A2 subunits upon thrombin activation of immobilized FVIII proteins were determined by fitting the dissociation kinetics data to the following equation describing a single-phase dissociation process: dR/dt = −koffR,42where R is observed surface plasmon resonance signal. The fitting procedure was performed using Sigmaplot 1.02 software (Jandel Scientific, San Raphael, CA).
RESULTS
Synthesis and secretion of ARG531HIS.
The synthesis and secretion of FVIII WT andARG531HIS was compared by transient DNA transfection into COS-1 monkey cells. At 64 hours posttransfection, the rates of synthesis were analyzed by immunoprecipitation of cell extracts from [35S]-methionine pulse-labeled cells. Intracellular FVIII WT and ARG531HIS were detected in their single chain forms and migrated at approximately 250 kD (Fig 1, lanes 3 and 5).ARG531HIS exhibited similar band intensity to the FVIII WT, suggesting that the missense mutation did not interfere with efficient protein synthesis. After a 4-hour chase period, FVIII WT was lost from the cell extract (Fig 1, lane 4) and was recovered from chase conditioned medium as a 280-kD single chain, a 200-kD heavy chain, and an 80-kD light chain (Fig 1, lane 8).ARG531HIS was also lost from the cell extract over the 4-hour chase period (Fig 1, lane 6), similar to FVIII WT, but had a reduced recovery from the chase-conditioned medium (Fig 1, lane 9), suggesting a modest secretion defect.ARG531HIS was also detected within the chase-conditioned medium in single-chain, heavy-chain, and light-chain forms of identical molecular mobility as FVIII WT, suggesting similar posttranslational processing and proper heavy and light chain association. From the relative band intensities within the chase-conditioned medium, ARG531HIS-secreted protein was determined to be 56% of FVIII WT. FVIII antigen determinations by ELISA were performed on unlabeled conditioned media collected from 24 to 64 hours posttransfection.ARG531HIS protein was detected at 46% and 76% of FVIII WT in two independent transfection experiments, consistent with the modest secretion defect indicated by the pulse-chase analysis.
Synthesis and secretion of FVIII WT andARG531HIS expressed in COS-1 cells. FVIII WT and ARG531HIS plasmids were transfected into COS-1 monkey cells. At 64 hours posttransfection, cells were pulse-labeled with [35S]-methionine for 30 minutes and cell extracts were harvested. Duplicate labeled cells were chased for 4 hours in medium containing excess unlabeled methionine and then cell extracts and conditioned medium were harvested. Equal proportionate volumes of cell extract (lanes 1 through 6) and conditioned medium (lanes 7 through 9) were immunoprecipitated with anti–FVIII-specific antibody and equal aliquots were analyzed by SDS-PAGE. Mock indicates cells that did not receive plasmid DNA. Cell extract pulse (P) and chase (C). The migration of FVIII from the cell extracts is indicated at the right by an arrow. FVIII from the conditioned medium is indicated at the far right as single-chain (SC), heavy-chain (HC), and light-chain (LC) forms. Molecular weight markers are shown on the left.
Synthesis and secretion of FVIII WT andARG531HIS expressed in COS-1 cells. FVIII WT and ARG531HIS plasmids were transfected into COS-1 monkey cells. At 64 hours posttransfection, cells were pulse-labeled with [35S]-methionine for 30 minutes and cell extracts were harvested. Duplicate labeled cells were chased for 4 hours in medium containing excess unlabeled methionine and then cell extracts and conditioned medium were harvested. Equal proportionate volumes of cell extract (lanes 1 through 6) and conditioned medium (lanes 7 through 9) were immunoprecipitated with anti–FVIII-specific antibody and equal aliquots were analyzed by SDS-PAGE. Mock indicates cells that did not receive plasmid DNA. Cell extract pulse (P) and chase (C). The migration of FVIII from the cell extracts is indicated at the right by an arrow. FVIII from the conditioned medium is indicated at the far right as single-chain (SC), heavy-chain (HC), and light-chain (LC) forms. Molecular weight markers are shown on the left.
Recombinant-derived ARG531HIS protein demonstrates a similar functional phenotype to patient plasmas.
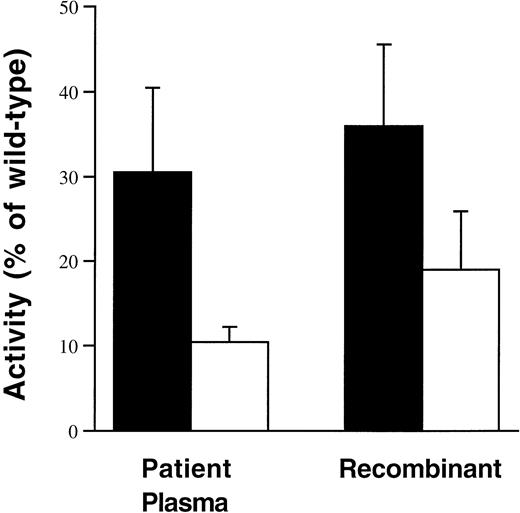
Conditioned medium was collected from COS-1 cells transiently expressing FVIII WT and ARG531HIS from 24 to 64 hours posttransfection. FVIII activity (Fig2) was measured by a one-stage aPTT clotting assay or by a modified two-stage method using the COAMATIC chromogenic assay. Similar to the patient phenotypes reported previously,ARG531HIS had discrepant activity as measured by the one-stage aPTT clotting assay (36% ± 9.6% of FVIII WT) as compared with the two-stage chromogenic assay (COAMATIC; 19% ± 6.9% of FVIII WT). The activity measurements for the patient plasmas represent all of the reported data obtained from the HAMSTeRS hemophilia A mutation database.43 The slightly higher two-stage activity results obtained with the recombinant-derivedARG531HIS are consistent with those obtained from the few reported patient plasmas in which a chromogenic assay rather than the classical two-stage assay was used.31 After immunoaffinity purification of FVIII WT andARG531HIS from the conditioned medium, similar results were obtained. Immunoaffinity purifiedARG531HIS-specific activity was 62% of FVIII WT by one-stage aPTT clotting assay as compared with 32% of FVIII WT as determined by the two-stage chromogenic assay.
Recombinant-derived ARG531HISdemonstrates a similiar phenotype to describedARG531HIS patient plasmas. Plasma activity data were derived from the HAMSTeRS hemophilia A mutation database for all reported patients identified with ARG531HIS for which both one-stage (▪) and two-stage (□) results were available. The data from recombinant-derived ARG531HISwere obtained from assaying the activity in the conditioned medium from four independent transfection experiments. Activity forARG531HIS patient plasmas and recombinant-derived ARG531HIS is presented as the percentage of wild-type (normal plasma or recombinant FVIII WT, respectively).
Recombinant-derived ARG531HISdemonstrates a similiar phenotype to describedARG531HIS patient plasmas. Plasma activity data were derived from the HAMSTeRS hemophilia A mutation database for all reported patients identified with ARG531HIS for which both one-stage (▪) and two-stage (□) results were available. The data from recombinant-derived ARG531HISwere obtained from assaying the activity in the conditioned medium from four independent transfection experiments. Activity forARG531HIS patient plasmas and recombinant-derived ARG531HIS is presented as the percentage of wild-type (normal plasma or recombinant FVIII WT, respectively).
ARG531HIS demonstrates similar thrombin proteolysis compared with FVIII WT.
[35S]-methionine–labeled FVIII WT andARG531HIS proteins were immunoprecipitated from chase conditioned medium of transiently expressing COS-1 cells labeled at 60 hours posttransfection. After Triton X-100 washes as described, the immunoprecipitated complexes were incubated with thrombin (0.1 U/mL) for increasing periods of time at 37°C before sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis. Both FVIII WT and ARG531HIS were initially detected in their 280-kD single-chain forms, and dimers of a 200-kD heavy chain in association with an 80-kD light chain (Fig 3, lanes 2 and 8). Both FVIII WT andARG531HIS were sequentially cleaved into a heterotrimer of fragments consistent with a 50-kD A1 subunit, 43-kD A2 subunit, and 73-kD thrombin-cleaved light chain, A3-C1-C2 (Fig 3, lanes 3 through 7 and 9 through 13). A 90-kD fragment appeared immediately after incubation with thrombin consistent with an A1-A2 heavy chain fragment due to cleavage after arginine 740. With higher concentrations of thrombin, this fragment is further cleaved into 50-kD A1 and 43-kD A2 subunits (data not shown). The similar pattern of electrophoretic mobility and rate of appearance of proteolytic fragments over time suggests that ARG531HIS, compared with FVIII WT, has identical sites of thrombin cleavage and sensitivity to proteolysis.
Thrombin proteolysis of FVIII WT compared withARG531HIS. [35S]-methionine labeled FVIII WT and ARG531HIS proteins immunoprecipitated from the chase conditioned medium of transiently expressing COS-1 cells were divided into equal aliquots and incubated with thrombin (0.1 U/mL) for increasing periods of time at 37°C. Reactions were terminated with SDS-PAGE sample buffer and protein fragments were separated by 10% SDS-PAGE. Time is in minutes, with 0 representing the absence of thrombin. Mock indicates medium from cells that did not receive plasmid DNA. FVIII protein forms are indicated at the right as follows: SC, single chain; HC, heavy chain; LC, light chain; A1+A2, A1, and A2, thrombin-cleaved heavy chain fragments; LC+IIa, thrombin-cleaved light chain; FVIIIa, predicted thrombin-activated FVIII heterotrimer. Molecular weight markers (m) are indicated on the left.
Thrombin proteolysis of FVIII WT compared withARG531HIS. [35S]-methionine labeled FVIII WT and ARG531HIS proteins immunoprecipitated from the chase conditioned medium of transiently expressing COS-1 cells were divided into equal aliquots and incubated with thrombin (0.1 U/mL) for increasing periods of time at 37°C. Reactions were terminated with SDS-PAGE sample buffer and protein fragments were separated by 10% SDS-PAGE. Time is in minutes, with 0 representing the absence of thrombin. Mock indicates medium from cells that did not receive plasmid DNA. FVIII protein forms are indicated at the right as follows: SC, single chain; HC, heavy chain; LC, light chain; A1+A2, A1, and A2, thrombin-cleaved heavy chain fragments; LC+IIa, thrombin-cleaved light chain; FVIIIa, predicted thrombin-activated FVIII heterotrimer. Molecular weight markers (m) are indicated on the left.
ARG531HIS exhibits reduced peak activity and increased rate of inactivation after functional activation by thrombin.
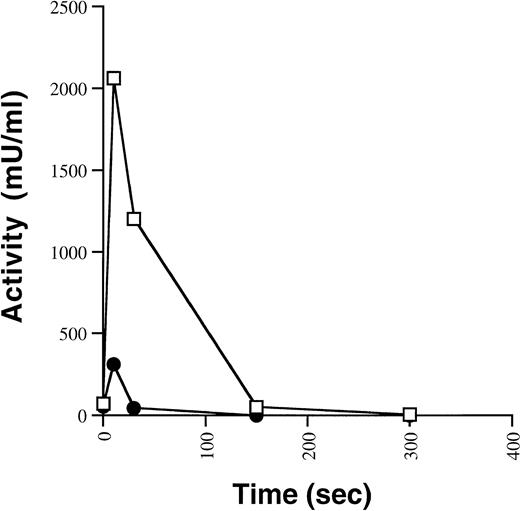
Having demonstrated a similar pattern and sensitivity ofARG531HIS to thrombin cleavage, the functional consequence of the ARG531HIS missense mutation on activation and inactivation was examined in an in vitro functional assay. Equal concentrations of immunoaffinity purified FVIII WT andARG531HIS were incubated with thrombin and assayed for FVIII activity by a one-stage aPTT clotting assay (Fig 4). Upon treatment with thrombin, FVIII WT was maximally activated within 10 seconds and then inactivated over the next 5 minutes. ARG531HIS also reached peak activity after 10 seconds of incubation with thrombin, but at approximately fivefold lower activity compared with FVIII WT. In addition, ARG531HIS was completely inactivated after 30 seconds incubation with thrombin, suggesting some increased instability of the thrombin-activated heterotrimer.
Activation and inactivation of FVIII WT andARG531HIS by thrombin. Immunoaffinity purified FVIII WT (□) and ARG531HIS (•) proteins (0.5 nmol/L) were incubated with thrombin (1 U/mL) at room temperature and assayed over time for FVIII activity by aPTT. The results are from a single thrombin activation experiment and are typical of multiple independent experiments.
Activation and inactivation of FVIII WT andARG531HIS by thrombin. Immunoaffinity purified FVIII WT (□) and ARG531HIS (•) proteins (0.5 nmol/L) were incubated with thrombin (1 U/mL) at room temperature and assayed over time for FVIII activity by aPTT. The results are from a single thrombin activation experiment and are typical of multiple independent experiments.
ARG531HIS exhibits increased rate of A2 subunit dissociation from the thrombin-activated heterotrimer.
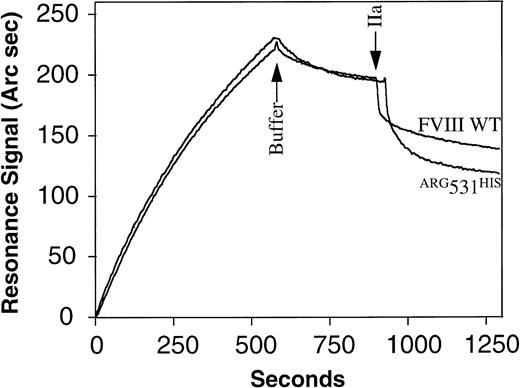
Because the A2 subunit is retained within the thrombin-activated FVIIIa heterotrimer through a weak electrostatic interaction with the acidic amino acid rich region at the carboxy terminus of the A1 subunit, we hypothesized that the charge change resulting from theARG531HIS mutation would make its A2 subunit susceptible to more rapid dissociation. The relative rates of A2 dissociation for FVIII WT and ARG531HIS were determined using an optical biosensor (Fig5). An anti-light chain antibody, ESH8, was covalently immobilized on a carboxymethyldextran-coated biosensor chip. Similar amounts (1.12 ng/mm2) of immunoaffinity-purified FVIII WT orARG531HIS protein (2.5 nmol/L) were bound to ESH8 antibody. Unbound material was removed by washing with buffer and free (nonproteolytic) dissociation from antibody was measured. The values of dissociation rate constants, koff = (8.9 ± 0.23) × 10−5 s−1, were similar for FVIII WT and ARG531HIS. Subsequently, thrombin was added to a final concentration of 1 U/mL. Because the FVIII preparations are bound to an ESH8-coated chip via their light chains, this interaction will not be disturbed by thrombin activation and the A1 subunit will remain associated with the light chain through the copper ion-dependent linkage between the A1 and A3 domains. Thus, the thrombin-induced release of the A2-subunit from the heterotrimer can be measured as a dissociation curve registered by the optical biosensor. The koff values for the FVIII WT andARG531HIS A2-subunits were (6.7 ± 0.6) × 10−3 s−1 and (2.9 ± 0.22) × 10−2 s−1, respectively. The half lives of A2-dissociation for FVIII WT andARG531HIS calculated as ln2/koffare 103 and 24 seconds, respectively. Because A2-dissociation correlates with inactivation, the ARG531HISthrombin-activated heterotrimer would be expected to inactivate fourfold faster compared with FVIII WT. Similar results were obtained via the same method, substituting an anti-A2 domain antibody (MoAb 413; Holland Red Cross Laboratory) for immobilization of the FVIII proteins (data not shown). The single exponential dissociation of the A2 subunit observed for FVIII WT in this study is comparable to the decay of FVIIIa previously reported.14 44
Determination of the kinetic parameters for nonproteolytic and thrombin-mediated dissociation of FVIII WT andARG531HIS from MoAb ESH8. MoAb ESH8 was covalently immobilized to a biosensor chip at 20 ng/mm2. FVIII WT or ARG531HIS (2.5 nmol/L) were bound to ESH8 at 1.12 ng/mm2. A resonance response of 200 Arc seconds corresponds to 1 ng of protein bound per square millimeter of the biosensor chip surface. The kinetics of FVIII WT orARG531HIS nonproteolytic dissociation from ESH8 was recorded after replacement of the ligand by dissociation buffer (at arrow). At the second arrow, thrombin (1 U/mL) was added and thrombin-mediated dissociation of the A2 subunit from immobilized dimers was followed. The koff values for nonproteolytic and thrombin-mediated dissociation were derived from dissociation kinetic curves as described under Materials and Methods.
Determination of the kinetic parameters for nonproteolytic and thrombin-mediated dissociation of FVIII WT andARG531HIS from MoAb ESH8. MoAb ESH8 was covalently immobilized to a biosensor chip at 20 ng/mm2. FVIII WT or ARG531HIS (2.5 nmol/L) were bound to ESH8 at 1.12 ng/mm2. A resonance response of 200 Arc seconds corresponds to 1 ng of protein bound per square millimeter of the biosensor chip surface. The kinetics of FVIII WT orARG531HIS nonproteolytic dissociation from ESH8 was recorded after replacement of the ligand by dissociation buffer (at arrow). At the second arrow, thrombin (1 U/mL) was added and thrombin-mediated dissociation of the A2 subunit from immobilized dimers was followed. The koff values for nonproteolytic and thrombin-mediated dissociation were derived from dissociation kinetic curves as described under Materials and Methods.
DISCUSSION
The one-stage and two-stage methods for assaying FVIII activity have been used clinically for more than 40 years. Several studies have demonstrated little differences in precision between the two methods.18 The one-stage assay, being technically simpler and easier to automate, has typically been the method of choice in recent years. Discrepancy in FVIII activity results have only been described under a few unique conditions. These have included the measurement of FVIII concentrates against plasma standards and the measurement of concentrates against concentrate standards and in the assessment of in vivo recovery of FVIII concentrate infusions.18 The discrepancies have included higher two-stage versus one-stage activities or the converse as investigated in this report. An explanation for these discrepancies has been elusive, although the Al(OH)3 adsorption step, which is used in the preparation of samples in the two-stage assay, has been suggested as a major cause of the discrepancy in assays of concentrates versus plasma. However, this discrepancy was an average of only 26% higher activity in the two-stage assay compared with the one-stage assay.45 Other suggested causes have included the choice of predilution buffer, thrombin activation of the samples, nonspecific contaminants such as lipids, and the influence of the presence of vWF.18 In the latter case, a patient with von Willebrand’s disease demonstrated a discrepancy in FVIII activity in which, even though the plasma FVIII activity measured by a one-stage assay was consistent with the antigen level, there was a 75% decrease in the plasma FVIII activity when a two-stage assay was used.35The patient was later characterized as having a von Willebrand disease Normandy defect in which there was a weaker interaction of FVIII with the mutant vWF leading to increased susceptibility of the FVIII to adsorption by Al(OH)3.36 The apparent discrepancy was corrected by adding hemophilic plasma or purified hemophilic vWF.
Several recent reports have now observed these discrepancies as part of a hemophilia A phenotype. The patients have typically been mild in phenotype and the discrepancy, a more than twofold higher activity result by the one-stage assay versus the two-stage, was observed in all affected family members.31 This phenotypic subgroup was subjected to DNA analysis to determine a responsible FVIII gene mutation.31 Several single missense mutations were identified either within the FVIII A1, A2, or A3 domains. The best characterized missense mutation was theARG531HIS mutation chosen for this study. Three independent reports collected by the hemophilia A mutation database have shown that the patients with the ARG531HISmutation have FVIII antigen levels from approximately 30% to 100% of normal, suggesting at worst a modest protein secretion defect.43 All the patients analyzed by both one-stage and two-stage assays demonstrated at least twofold higher results in the one-stage assay. Where a chromogenic based assay was also used, there was still an approximate twofold difference in the activities, although the chromogenic two-stage results were somewhat higher than those for the classical two-stage assay.31
The ARG531HIS mutation does not lie within major identified functional FVIII epitopes, such as the terminal portion of the C2 domain containing the binding site for phospholipids,46,47 the vWF binding sites (residues 1648-1689 of the A3 domain and epitopes within the terminal C2 domain),48,49 or the proposed factor IXa interaction site within the A2 domain (residues 558-565).50,51 Therefore, this particular missense mutation may require an alternative mechanism for dysfunction not yet characterized. Insights into the differences between the one-stage and two-stage assays allow a hypothesis as to a possible mechanism. The two-stage assay, divided into two separate steps, requires a prolonged phase in the first step under conditions in which FVIII becomes activated and generates the predicted FVIIIa heterotrimeric structure to exert its cofactor function with factor IXa. Previous studies have highlighted the instability of the FVIIIa heterotrimer. The FVIIIa heterotrimer exhibits a pH-dependent dissociation of the A2 subunit from the A1/A3-C1-C2 heterodimer that correlates with loss of procoagulant activity.14 The data supporting the idea that the amino acid region 558 to 565 within the A2 subunit represents a factor IXa interaction site are consistent with this observation. Porcine FVIIIa exhibits an increased affinity for its A2 subunit compared with human FVIIIa and, accordingly, demonstrates an increased specific activity.44,52,53 A genetically engineered recombinant FVIII molecule in which the A2 subunit remains covalently linked to the heterodimer after activation by thrombin exhibited a fivefold increase in specific activity.54 These observations highlight the role of A2 dissociation in limiting FVIIIa activity. The observations are consistent with positively charged residues within the A2 subunit interacting with acidic amino acid residues at the carboxy-terminus of the A1 subunit maintaining a weak electrostatic interaction to preserve the FVIIIa heterotrimer. Loss of residues 337-372 of the A1 subunit after cleavage by activated protein C or further proteolysis by thrombin leads to rapid inactivation of FVIIIa via A2 subunit dissociation.14,15,55,56 A genetically engineered mutant of recombinant FVIII, containing ARG336ILE, was resistant to proteolytic cleavage by thrombin and activated protein C and had an increased specific activity, as determined by a chromogenic assay, attributable to increased stability of the heterotrimer.12 Many of the amino acid substitutions in the porcine compared with the human A2 subunit lead to a charge alteration and may be responsible for the increased stability of the porcine FVIIIa heterotrimer. Because the two-stage assay involves the preincubation phase before subsampling, an FVIII protein with increased heterotrimeric stability would be predicted to have an increased activity in the two-stage assay. Consistent with this finding, when porcine FVIII concentrates were assayed against human concentrates by the classical two-stage method, the activity results were two to three times higher than by one-stage assays.18
However, the phenotype characterized in relation to theARG531HIS missense mutation is one in which not only the one-stage activity is reduced from that expected by the antigen levels present, but also the two-stage assay activity results are at least twofold lower than by the one-stage assay. TheARG531HIS mutation predicts a loss of charge within the A2 subunit predicting a weakened electrostatic interaction after thrombin activation. Thus, FVIIIa dissociation of aARG531HIS A2 subunit, compared with FVIII WT, would be hypothesized to be more rapid. Because the recombinant-derivedARG531HIS protein exhibited a similar phenotype to the patient plasmas, we were able to analyze the purified protein for its rate of A2 dissociation. The data using the optical biosensor confirmed the fourfold increased rate of A2 subunit loss after thrombin activation of ARG531HIS. The reduced peak activity observed for the ARG531HIS protein compared with FVIII WT can also be attributed to the increased rate of A2 subunit dissociation. Under the conditions of the aPTT clotting assay, thrombin was added to the FVIII samples in buffer and the thrombin-activated samples were then prediluted before incubation with the clotting assay reagents. The mutant would undergo spontaneous decay more rapidly than FVIII WT before assay determination. Accordingly, extrapolating from the initial slope of the inactivation phase, if FVIII WT was incubated four times longer with thrombin before assay determination, the remaining apparent peak activity would be similar to the apparent peak activity observed for the thrombin-activatedARG531HIS protein. Therefore, there is no need to invoke an additional functional defect for this mutation.
Several other genetic mutations have been described with discrepant one-stage and two-stage activities. All reported patients have mild to moderate hemophilia A phenotype and all of the mutations occur within either the A1, A2, or A3 domains.31 Typically, they involve amino acid substitutions that alter charge (eg,ARG698TRP) or hydrophobicity (eg,ALA284GLU andSER289LEU). It can be postulated that these missense mutations, although not confined to either the acidic region of the A1 domain or to the A2 subunit itself, may still interfere with the weak electrostatic interaction at this critical interaction site, thereby leading to similar instability of the FVIIIa heterotrimer.
Because the patients with the ARG531HISmutation have a mild hemophilia A phenotype, the observed FVIII dysfunction observed in vitro in these assays is apparently also important in vivo. This is an important observation for several reasons. Firstly, it is not known in vivo whether FVIIIa procoagulant activity is limited by spontaneous A2 subunit dissociation or further proteolysis. The observations from this CRM-positive mutant and porcine FVIII would suggest that the inherent instability of FVIIIa also limits its activity in vivo and is either further compromised by mechanisms that lead to increased A2 dissociation or partially abrogated by mechanisms that lead to reduced A2 dissociation. Secondly, an increased plasma level of FVIII has now been identified as a risk factor for thrombosis.57,58 If proteolytic inactivation by activated protein C was of primary importance in regulating FVIIIa, then one could predict that a mutation leading to resistance to activated protein C would also lead to an increased risk of thrombosis. Comprehensive analysis of patients with thrombophilia has failed to identify any mutations at activated protein C cleavage sites within FVIII despite the prominent association of factor V Leiden (resistant to activated protein C) with this cohort of patients.59Finally, this study predicts that even minor modifications of the A2 subunit can have major functional impacts both in vitro and in vivo. This provides further insight into research efforts directed at producing a new generation of recombinant FVIII molecules with increased specific activity. Based on the conclusions from this study and the others summarized here, we propose that the most significant mechanism of FVIIIa inactivation in vivo is dissociation of the A2 subunit.
Supported by National Institutes of Health (NIH) Grant No. HL52173 and by National Institute of Child Health and Human Development (NICHD) HD28820.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to R.J. Kaufman, PhD, Howard Hughes Medical Institute, University of Michigan Medical Center, 4570 MSRB II, 1150 W Medical Center Dr, Ann Arbor, MI 48109-0650; e-mail:kaufmanr@umich.edu.

![Fig. 1. Synthesis and secretion of FVIII WT andARG531HIS expressed in COS-1 cells. FVIII WT and ARG531HIS plasmids were transfected into COS-1 monkey cells. At 64 hours posttransfection, cells were pulse-labeled with [35S]-methionine for 30 minutes and cell extracts were harvested. Duplicate labeled cells were chased for 4 hours in medium containing excess unlabeled methionine and then cell extracts and conditioned medium were harvested. Equal proportionate volumes of cell extract (lanes 1 through 6) and conditioned medium (lanes 7 through 9) were immunoprecipitated with anti–FVIII-specific antibody and equal aliquots were analyzed by SDS-PAGE. Mock indicates cells that did not receive plasmid DNA. Cell extract pulse (P) and chase (C). The migration of FVIII from the cell extracts is indicated at the right by an arrow. FVIII from the conditioned medium is indicated at the far right as single-chain (SC), heavy-chain (HC), and light-chain (LC) forms. Molecular weight markers are shown on the left.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/1/10.1182_blood.v93.1.176/5/m_blod40107001w.jpeg?Expires=1763826025&Signature=GkuH6P~wilf25Viam~0hjkJiKG33610D0oLdc10HGT48ybVNj0gmcuPxc7mj~LDlnMZzhOkLUSlaZp-GcLFmUL64~xJ81Ha1IF0-z0P9yTTcm~s7nkbQDtsTDXoK44XErZfkby2KgdnCzzEbElYHeQ6BoizE1lxY6~fp3v4FGV9ktlYvzNCEhI8zBhFHQFdCK4RuVJhzKrx1wYRek1jN98Flg6mHixvQNtmc6S8TdY9MB~UlbOR5hSRo7IUEbLwo25p7i-c5odu9Z-Bw~VIEna6Lf5~ww7epZz6rGOto4eDx19QG4qoHllIEAp-kVQPz-Vmxf~rr4xAczjZQmkKIpg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Thrombin proteolysis of FVIII WT compared withARG531HIS. [35S]-methionine labeled FVIII WT and ARG531HIS proteins immunoprecipitated from the chase conditioned medium of transiently expressing COS-1 cells were divided into equal aliquots and incubated with thrombin (0.1 U/mL) for increasing periods of time at 37°C. Reactions were terminated with SDS-PAGE sample buffer and protein fragments were separated by 10% SDS-PAGE. Time is in minutes, with 0 representing the absence of thrombin. Mock indicates medium from cells that did not receive plasmid DNA. FVIII protein forms are indicated at the right as follows: SC, single chain; HC, heavy chain; LC, light chain; A1+A2, A1, and A2, thrombin-cleaved heavy chain fragments; LC+IIa, thrombin-cleaved light chain; FVIIIa, predicted thrombin-activated FVIII heterotrimer. Molecular weight markers (m) are indicated on the left.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/1/10.1182_blood.v93.1.176/5/m_blod40107003w.jpeg?Expires=1763826025&Signature=qGoHiGdd4BDpiVUaF9YGx760wCyIbPdi~HT3Sf~hbY-gQwXc2Qx31as0-S-9RTWASx3IGB-AQsnzGMjwvALOCPxfaPo5zNqt7Wg9E1eROLTpuuwjNfJ9rqwp0vjCckhLelsl5gpc8gFDjso46Do~kcsy3ETo4QRkTBFMibgBjNO1tRimGKEgVM4kFZ5hIWSdLZufUYwaGTMq7oV62TEwSjIfTga9rkwLnIUOviIxbGgkc1m5Bxok2msPEQUKvKASbMA71ZXEMMrTeztX~S4XeRx5QW7l6vOazr3JvUHh0cR5hhcHtAME5p6GjmaEnHQEWiVs9h0PH14fuApCUQAy6Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)