Abstract
Hemoglobin E (HbE; 2β226glu-lys), globally the commonest hemoglobin variant, is synthesized at a slightly reduced rate and has a homozygous phenotype similar to heterozygous β thalassemia. Yet, when it is inherited together with a β thalassemia allele, the resulting condition, HbE/β thalassemia, is sometimes characterized by a severe, transfusion-dependent thalassemia major. The severity of this interaction has not been explained. We have explored the possibility that it may reflect the instability of HbE consequent upon globin chain imbalance imposed by the β thalassemia allele. Time-course and pulse-chase globin chain synthesis studies at 37°C on peripheral blood and bone marrow suggest that hemoglobin instability is not significant in steady-state HbE/β thalassemia; this is confirmed by density-gradient centrifugation studies that show no decrease in HbE levels relative to HbA as HbE/β+ thalassemia red blood cells age. Globin binding to membranes was assessed and only globin chains were found, in contrast to other unstable hemoglobins in which both and β chains were present. However, in experiments performed on blood from HbE/β thalassemics in the temperature range 39°C to 41°C, there was evidence of instability of HbE, a finding that was also observed in homozygous HbE. These findings suggest that the phenotype of HbE/β thalassemia is primarily the result of the interaction of two β thalassemia alleles; however, hemoglobin instability may be important during febrile episodes, contributing to worsening anemia.
© 1998 by The American Society of Hematology.
HEMOGLOBIN E (HbE; α2β226glu-lys) is the commonest hemoglobin variant, most prevalent in the rapidly expanding populations of Southeast Asia1,2 but being encountered with increasing frequency in the immigrant peoples of Europe, North America, and Australia. Although HbE was first identified in 1954,3there are still uncertainties about many aspects of its pathophysiology. Both heterozygotes and homozygotes are asymptomatic, minimally anemic, and have microcytic and hypochromic red blood cells. The latter is explained by the thalassemic nature of the βE allele,4 which reflects the activation of a cryptic donor splice site by the GAG to AAG mutation in exon 1 of the β globin gene.5 However, when the βE allele interacts with a β thalassemia mutation in the compound heterozygous state, a variable and often severe anemia is produced, with hemoglobin levels ranging from 3 to 11 g/dL.6 The severity of this interaction with β thalassemia seems disproportionate to the very mild defect in β globin chain synthesis associated with HbE; HbE homozygotes have the hemoglobin and globin chain synthesis characteristics of β thalassemia trait and have no elevation of HbF.
A clue to a possible mechanism involved in the interaction of HbE and β thalassemia lies in the observation that HbE is oxidatively unstable in vitro7,8 and that drug-induced oxidation of HbE is faster than that of hemoglobins S, A, and F.9 It is thought that this increased instability results from interference with the α1β1 interface,9 10 resulting in an increased rate of dissociation of the HbE molecule into unstable dimers. The significance of this instability in vivo is unclear, but we have recently described a family in which HbE interacts with a red blood cell enzymopathy, pyrimidine 5′ nucleotidase deficiency, to produce an unexpectedly severe anemia11; the basis for this interaction was shown to be increased HbE instability, presumably resulting from the intracellular oxidative stress associated with the enzymopathy. Using similar methods, we have explored the possible role that hemoglobin instability plays in the interaction between HbE and β thalassemia.
MATERIALS AND METHODS
Subjects.
Blood was obtained from individuals resident in the UK who were known to have HbE/β thalassemia; none of them had received blood transfusions in the preceding 6 months. Control samples were collected from relatives of the HbE/β thalassemics, β thalassemia intermedia patients (not involving HbE), and patients with known hemoglobin instability. Informed consent was obtained in each case.
Hematologic analysis.
Blood was collected into EDTA. Full blood counts were performed using an automated counter. Blood films, reticulocyte stains, and hemoglobin isoelectric focusing were performed using standard techniques.12 These methods were used to confirm the diagnosis, assess whether β° or β+ thalassemia was present, and confirm the untransfused status.
DNA analysis.
DNA was isolated from peripheral blood leukocytes.13 The nature of the β thalassemia mutation was determined by allele-specific polymerase chain reaction (PCR) amplification. α Thalassemia was screened for by digesting DNA with Bgl II, Southern blotting, and hybridization with a ζ globin-specific probe. Triplicated α globin genes were screened for with BamHI digestion and α globin-specific probe hybridization.14
Globin-chain biosynthesis.
Approximately 20 mL of venous blood was taken into heparin and kept on ice until analyzed, a maximum of 2 hours. The cells were washed three times in cold reticulocyte saline (0.13 mol/L NaCl, 0.005 mol/L KCl, 0.007 mol/L MgCl2) before ultracentrifugation at 100,000g to concentrate the reticulocytes. White blood cells were removed from the top 0.6 mL of concentrated red blood cells using α-cellulose and microcrystalline cellulose (Sigmacell 50; Sigma Chemical Co, Poole, Dorset, UK).15 This reticulocyte-rich fraction was added to a plasma-based incubation medium and, after 10 minutes of preincubation, 200 μCi of L-[4,5-3H] leucine (Amersham International plc, Little Chalfont, Bucks, UK) was added and the incubation was continued for 2 hours.16 Incubation temperatures of 37°C, 39°C, and 41°C were used. A bone marrow aspirate sample taken for diagnostic reasons from an HbE/β thalassemic was also studied. Processing was similar apart from EDTA being used as an anticoagulant; the white blood cells were not removed.
In time-course studies, aliquots were removed from the mixture at various intervals from the start of the incubation. Each fraction was washed with cold reticulocyte saline and flash frozen before conversion to globin by acid-acetone precipitation. The globin chains were separated by cation exchange chromatography; a linear two-chambered gradient, producing 70 fractions, was used for HbA and a convex, three-chambered gradient, producing 120 fractions, was used for samples containing HbE. The radioactive incorporation into each fraction was measured by scintillation counting, and the area under the curve of each peak was calculated to measure the relative rates of synthesis of the various chains over a particular time period.16Specific activities (SA) of the globin chains were measured from each separation, after dialysis of the peak fractions against 0.5% formic acid. Hemoglobin stability was assessed by considering the rate of increase of specific activity over 2 hours. If the rate of globin chain synthesis is constant and the hemoglobin formed stable, then the SA should increase linearly with time; this was expressed as the ratio of SA increase in the first hour to that in the second hour. If the hemoglobin is stable, then this ratio is approximately 1; if the hemoglobin is unstable, newly synthesized globin chains are proteolysed, the SA increases less as the incubation progresses, and the ratio increases exponentially.
Pulse-chase studies were also performed. After incubation with L-[4,5-3H] leucine for 10 minutes, the reticulocytes were washed four times in cold reticulocyte saline to remove as much unincorporated radioactivity as possible; the reticulocytes were then added to fresh incubation medium with cold leucine replacing the radiolabeled and the incubation continued. At various time intervals, aliquots were removed, washed, frozen, and converted to globin. Globin chain separation, scintillation counting, and specific activities were performed as described earlier.
Membrane studies.
The binding of radiolabeled globin to membranes was also studied using a modification of Dodge’s method to produce Hb-free ghosts.17 18 Time-course and pulse-chase incubations were performed, the cells were washed, and the aliquots were lysed with 20 mOsm phosphate buffer, pH 7.4. The lysate was ultracentrifuged at 80,000g for 10 minutes, and the supernatant was removed. The membrane ghosts were washed four times using the phosphate lysing buffer before being resuspended with 80 mg of unlabeled carrier Hb and made into globin.
Density gradient centrifugation.
The effect of ageing on the hemoglobin composition of circulating red blood cells was analyzed by density gradient centrifugation. Washed erythrocytes from an untransfused HbE/β+ thalassemic were centrifuged at 35,000g on a 20% Percoll (Pharmacia, St. Albans, UK)/meglamine gradient for 20 minutes.19 The red blood cells were divided into three fractions: a denser older fraction, an intermediate fraction, and a less dense, younger fraction. These fractions were then washed three times in reticulocyte saline and the hemoglobin composition of each was assessed by cation exchange high-performance liquid chromatography (HPLC). Pyruvate kinase (PK) and glucose-6-phosphate dehydrogenase (G6PD) levels were also measured in each fraction to confirm that the age of the cells increased with density.20
RESULTS
Time-course globin-chain synthesis studies at 37°C.
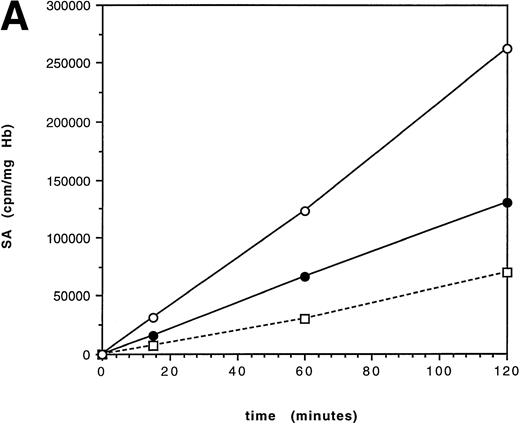
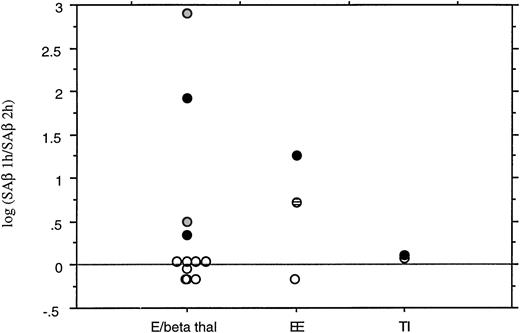
Time-course studies at 37°C were performed over 2 hours on 5 individuals with HbE/β thalassemia; DNA analysis showed the β thalassemia mutations present to be 2 cases of IVS 1-5 (G-C) (severe β+), Fr 8/9 (β°), IVS 1-130 (β°), and IVS II-654 (β°). The steady-state hemoglobin levels ranged from 6.6 to 10.4 g/dL (mean, 8.0 g/dL). Linear synthesis occurred in each case over the period of the incubation, suggesting that HbE is not significantly unstable over 2 hours (Fig1). The mean ratio of (SA increase of βE in the first hour)/(SA increase in βE the second hour) (SAβ 1 hour/SAβ 2 hours) was 0.94 (range, 0.7 to 1.1; Fig 2). A ratio of 1 results from linear synthesis with no globin instability. This result was confirmed in a sample of bone marrow from an HbE/β thalassemic [IVS 1-5(G-C)] and using whole, unwashed, blood [IVS 1-5(G-C)], with SAβ 1 hour/SAβ 2 hours ratios of 1.1 and 0.92, respectively; the latter experiment was performed to ensure that washing the cells did not remove any small molecules that might be important in exacerbating HbE instability, eg, free iron and hydrogen peroxide.21
Specific activity (SA) time-course over 2 hours in an untransfused patient with HbE/β thalassemia (IVS 1-130). The incubation temperatures are 37°C (A) and 41°C (B). Synthesis is linear at 37°C, suggesting that there is no significant hemoglobin instability. At 41°C, the curve flattens after 1 hour, consistent with hemoglobin instability. (□) γ; (•) βE; (○) .
Specific activity (SA) time-course over 2 hours in an untransfused patient with HbE/β thalassemia (IVS 1-130). The incubation temperatures are 37°C (A) and 41°C (B). Synthesis is linear at 37°C, suggesting that there is no significant hemoglobin instability. At 41°C, the curve flattens after 1 hour, consistent with hemoglobin instability. (□) γ; (•) βE; (○) .
Summary of the results of time-course globin chain synthesis studies. Results are expressed as the ratio of the increase in specific activity of the β or βE chain in the first hour to the increase in the second hour. The log10 of the ratio is plotted. If SA increases linearly, the ratio is 1 (log10 0); as the hemoglobin becomes unstable and the SA increases less in the second hour, the ratio increases exponentially. (○) Incubation at 37°C, (◍) incubation at 39°C, (•) incubation at 41°C, (⊟) sample was also deficient for pyrimidine 5’ nucleotidase. E/beta thal, E/β thalassemia (includes bone marrow sample and unwashed, whole blood); EE, homozygous HbE; TI, thalassemia intermedia. The log(SAβ 1 hour/SAβ 2 hours) is 0 in HbE/β thalassemia at 37°C, but increases markedly at higher temperatures. This effect is not seen in β thalassemia intermedia.
Summary of the results of time-course globin chain synthesis studies. Results are expressed as the ratio of the increase in specific activity of the β or βE chain in the first hour to the increase in the second hour. The log10 of the ratio is plotted. If SA increases linearly, the ratio is 1 (log10 0); as the hemoglobin becomes unstable and the SA increases less in the second hour, the ratio increases exponentially. (○) Incubation at 37°C, (◍) incubation at 39°C, (•) incubation at 41°C, (⊟) sample was also deficient for pyrimidine 5’ nucleotidase. E/beta thal, E/β thalassemia (includes bone marrow sample and unwashed, whole blood); EE, homozygous HbE; TI, thalassemia intermedia. The log(SAβ 1 hour/SAβ 2 hours) is 0 in HbE/β thalassemia at 37°C, but increases markedly at higher temperatures. This effect is not seen in β thalassemia intermedia.
Pulse-chase studies at 37°C.
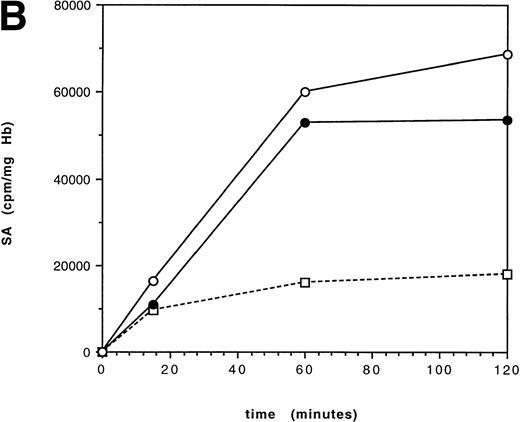
A pulse-chase incubation at 37°C was performed on an HbE/β thalassemic (IVS 1-5 G-C) over 30 minutes to detect any early hemoglobin instability (Fig 3). The specific activity increased in the first 5 minutes, as the residual radioactivity not removed by washing was incorporated; in the following 25 minutes, there was no significant change in the specific activities of any of the globin chains, suggesting that newly synthesized globin is not immediately lost.
Pulse-chase globin chain synthesis in an untransfused HbE/β thalassemic [IVS 1-5(G-C)] at 37°C. At time 0 minutes, the mixture was washed and the unused radiolabeled leucine was removed. The SA increases for the first 5 minutes as residual radiolabeled leucine is used and then plateaus, with no decrease in SA of the globin chains.(□) γ; (▪) βA; (•) βE; (○) α.
Pulse-chase globin chain synthesis in an untransfused HbE/β thalassemic [IVS 1-5(G-C)] at 37°C. At time 0 minutes, the mixture was washed and the unused radiolabeled leucine was removed. The SA increases for the first 5 minutes as residual radiolabeled leucine is used and then plateaus, with no decrease in SA of the globin chains.(□) γ; (▪) βA; (•) βE; (○) α.
Membrane studies at 37°C.
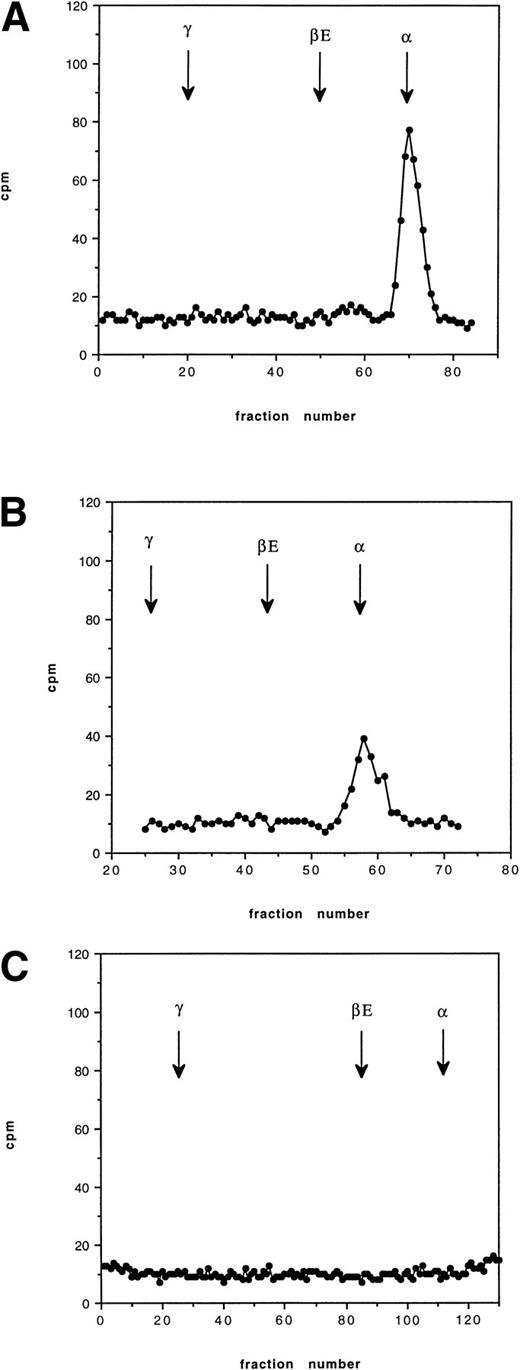
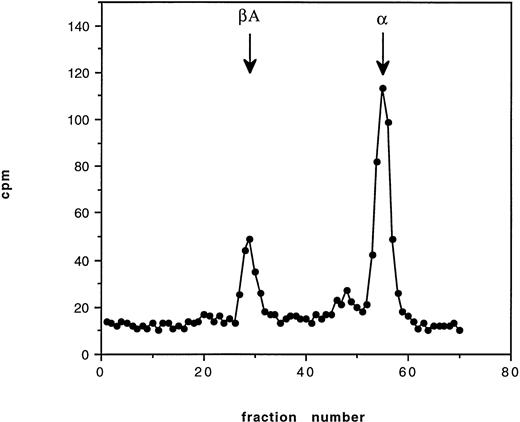
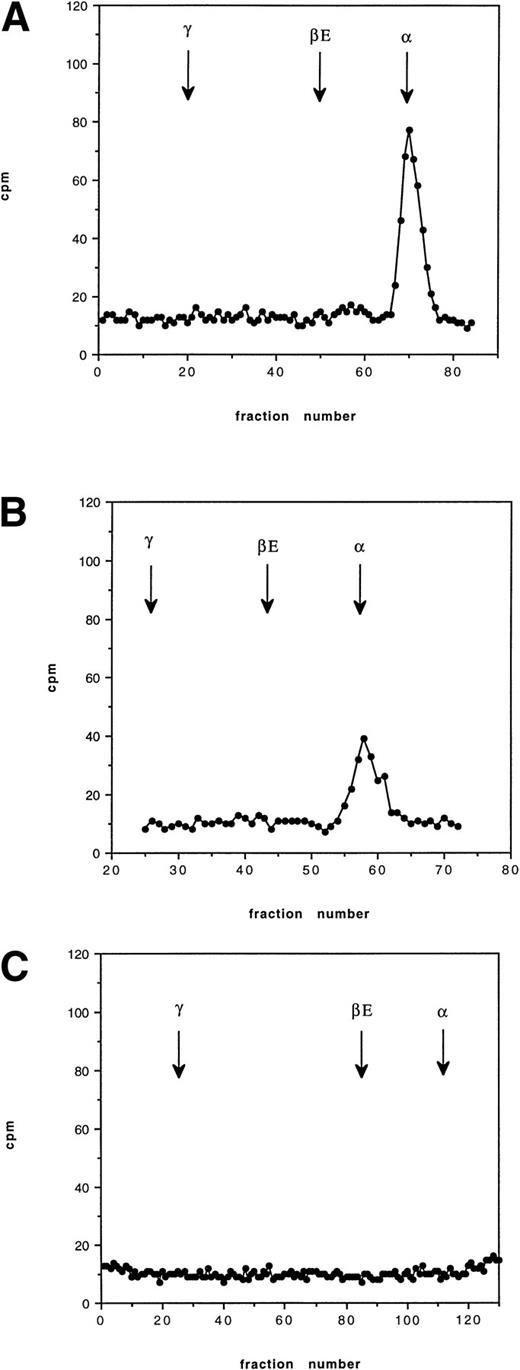
Pulse-chase incubations were performed over 12 hours using blood from an HbE/β° thalassemic (codon 16, -C). At 30 minutes, a radioactive peak corresponding to newly synthesized α globin was found to be bound to the membrane; no βE chain was observed. At 2 hours, a similar pattern was found with slightly less radioactive incorporation in the α globin peak. By 12 hours, no radioactive globin was found bound to the membrane (Fig 4). The pattern of globin binding contrasts to the findings when 60 minutes of incubation were performed on blood from a known unstable hemoglobin, Hb Bristol (β67 Val-Met→Asp), when both α and β globin chains were found on the membrane (Fig 5). This pattern of membrane-bound globin chains was also observed with Hbs Sun Prairie and Ann Arbor (data not shown).
Cation exchange chromatography of pulse-radiolabeled globin chains in an HbE/β thalassemic (Cd16) at 37°C, showing globin binding to the membrane 30 minutes, 2 hours, and 12 hours after removal of the radiolabeled leucine. The arrows show the position of each globin peak as determined by the separation of the nonradiolabeled protein. The vertical axis shows counts per minute (cpm). The initial peak of globin is lost after 12 hours.
Cation exchange chromatography of pulse-radiolabeled globin chains in an HbE/β thalassemic (Cd16) at 37°C, showing globin binding to the membrane 30 minutes, 2 hours, and 12 hours after removal of the radiolabeled leucine. The arrows show the position of each globin peak as determined by the separation of the nonradiolabeled protein. The vertical axis shows counts per minute (cpm). The initial peak of globin is lost after 12 hours.
Cation exchange chromatography showing globin binding to the membrane in Hb Bristol (β67 Val- Met→Asp) after 60 minutes of incubation with tritiated leucine. Binding of both and β globin occurs.
Cation exchange chromatography showing globin binding to the membrane in Hb Bristol (β67 Val- Met→Asp) after 60 minutes of incubation with tritiated leucine. Binding of both and β globin occurs.
Globin synthesis studies at 41°C.
The 2-hour time-course incubations were repeated at 41°C on the HbE/β thalassemics with the Fr 8/9 and IVS 1-130 mutations. At the higher temperature, there is a marked decrease in the rate of increase of specific activity, typical of hemoglobin instability (Fig 1); this pattern is similar to that seen with unstable hemoglobins at 37°C.22 Two time-course incubations of HbE/β thalassemia at 39°C also showed a similar plateau. The geometric mean of SAβ 1 hour/SAβ 2 hours at higher temperatures was 25 (range, 2.2 to 792; Fig 2), showing a marked increase compared with incubations at 37°C; this wide range reflects the fact that, as the SA increase in the second hour decreases towards zero, the ratio increases exponentially. This contrasted to a control experiment in which blood from an untransfused patient with thalassemia intermedia (Cd39/IVS 1-6) showed linear increases in specific activity with time at both 37°C and 41°C with SAβ 1 hour/SAβ 2 hours ratios of 1.2 and 1.3, respectively (Fig 2). Incubations on three controls with no hemoglobinopathies gave similar results, with SAβ 1 hour/SAβ 2 hours ratios around 1. Increased temperature also lead to a decrease in α/non-α globin chain synthesis ratios, as shown by the specific activities and total chain synthesis ratios: after 60 minutes the α/non-α ratios were 2.1 and 2.3 in the two HbE/β thalassemia samples at 37°C and 1.6 and 1.9 at 41°C. Both βEand γ increased relative to α. This temperature effect has been noted before,16 but its explanation and significance are unclear.
The pattern of hemoglobin binding to the membrane in HbE/β thalassemia at 41°C was similar to that at 37°C, with α globin but no βE chains bound.
Studies on HbE homozygotes.
Parallel experiments were performed on the red blood cells of a HbE homozygote. Time-course studies at 37°C showed linear synthesis as expected, but increasing the temperature to 41°C showed the development of a plateau, similar to that in HbE/β thalassemia. The SAβ 1 hour/SAβ 2 hours ratio increased from 0.7 at 37°C to 18 at 41°C. In contrast to HbE/β thalassemia, membrane analysis showed little globin binding at 37°C and 41°C, although at the higher temperature there was a small peak in the βEposition.
Density gradient centrifugation.
Density-gradient-separated fractions of blood from an HbE/β+ thalassemic [IVS 1-5(G-C)] were analyzed for hemoglobin composition, with the centrifugation and chromatography being performed in duplicate. The results are summarized in Fig 6. There is a relative increase in HbF, mirrored by a decrease in Hbs E and A in the older, denser fraction. The HbF/HbA ratio increased from 5.3 in the younger fraction to 9.2 in the older. On the other hand, there was no significant change in the HbE/HbA ratio across the gradient, indicating that there was no loss of the HbE relative to HbA during the lifespan of red blood cells. The pyruvate kinase activity decreased from 9.6 IU/1010 red blood cells in the top fraction to 4.15 in the bottom; G6PD levels fell similarly from 3.71 IU to 2.44.
Hb composition of three fractions of erythrocytes separated by density gradient centrifugation of blood from an untransfused HbE/β+ thalassemic [IVS 1-5(G-C)], separating red blood cells into top (least dense, youngest cells), middle, and bottom (densest, oldest cells). Each point is the average of two centrifugations from the same patient. The ratio of HbE/HbA increases slightly as the red blood cells age, suggesting that HbE is at least as stable as HbA; the ratio of HbF/HbA increases with cell age as expected. The decrease in pyruvate kinase activity with increasing cell density confirms that the denser fractions contain predominantly older red blood cells. (○) %E/%A; (•) %F/%A; (×) PK.
Hb composition of three fractions of erythrocytes separated by density gradient centrifugation of blood from an untransfused HbE/β+ thalassemic [IVS 1-5(G-C)], separating red blood cells into top (least dense, youngest cells), middle, and bottom (densest, oldest cells). Each point is the average of two centrifugations from the same patient. The ratio of HbE/HbA increases slightly as the red blood cells age, suggesting that HbE is at least as stable as HbA; the ratio of HbF/HbA increases with cell age as expected. The decrease in pyruvate kinase activity with increasing cell density confirms that the denser fractions contain predominantly older red blood cells. (○) %E/%A; (•) %F/%A; (×) PK.
DISCUSSION
This is the first study to address directly the question of HbE instability in its most important clinical context, HbE/β thalassemia. There is little previously published evidence either for or against the clinical importance of the instability of HbE in vivo. There is a single case report of dapsone causing a Heinz body hemolytic anemia in a Cambodian woman with HbE trait,23 although this has not been confirmed by subsequent observations, despite the widespread use of the drug in areas where HbE is prevalent. A second study indirectly implicated HbE instability as important in protection against malaria, by noting that HbE only offered protection if fava beans had been consumed in the preceding month.24 We have shown previously that pyrimidine 5’ nucleotidase deficiency interacts with HbE to produce hemoglobin instability and hemolysis.11However, there are a large number of reports of HbE occurring with other hemoglobin and red blood cell defects, without any suggestion of interaction: these include HbE with HbS,25HbC,26 Hb Lepore,27 Hb Hope,28 G6PD deficiency,29-31 and hereditary elliptocytosis.32,33 The interaction between HbE and HbH disease is particularly interesting: the resulting condition, HbAE-Bart’s disease, is similar in severity to HbH disease,28 suggesting that the HbE does not ameliorate the condition by reducing globin chain imbalance or exacerbate it by maximizing the hemolytic component.
The pattern of globin binding to the red blood cell membrane at 37°C is similar to that found in thalassemia major,18with only α globin present, and has been confirmed by electron microscope immunocytochemical studies of inclusions in HbE/β thalassemia.34 Time-course studies show that newly bound α globin is proteolysed within 2 and 12 hours of precipitation. This suggests that the amount of globin bound to the membrane in β thalassemia is determined by a dynamic equilibrium between the rate of precipitation and the rate of hemolysis and indicates that the majority of globin-induced damage to the membrane may occur late in development of the red blood cell, because the proteolytic capacity of the reticulocyte is very limited compared with that of the earlier nucleated red blood cell precursors.35 36
We have found no evidence that instability of HbE is important in the steady state in HbE/β thalassemia. Time- course globin chain synthesis experiments showed linear synthesis at 37°C in both marrow and peripheral blood (Fig 2). Pulse-chase studies showed that only α globin, not βE globin, binds to the red blood cell membrane, in contrast to other unstable hemoglobins such as Hb Bristol.
Density gradient centrifugation of red blood cells from a HbE/β+ thalassemic supported these findings (Fig 6). There is good evidence that in most conditions red blood cells become more dense as they age; sickle cell syndromes appear to be the exception to this.37 In our experiment, the decreasing levels of PK and G6PD activity associated with increasing density strongly suggest that the denser fractions are predominantly made up of older cells. The relative increase in HbF with cell age reflects the well-defined survival advantage of cells containing more HbF,38 but there was no significant loss of HbE relative to HbA in the older cell population. Taken together, these data suggest that HbE is not significantly unstable in vivo in the steady state.
However, the globin chain synthesis studies at higher temperatures suggest that newly synthesized hemoglobin is unstable in HbE/β thalassemia, in contrast to the effect of increasing temperature on control cells from a patient with β thalassemia intermedia. The instability involved α, βE, and γ chains, suggesting that hemoglobin tetramers containing βE chains are precipitated, rather than free globin chains, a situation that has been observed previously with unstable hemoglobins.22Instability occurred at both 39°C and 41°C, both significantly above body temperature; however, in tropical regions where malaria is endemic, febrile illnesses are common26 and the resulting exacerbation of dyserythropoiesis and hemolysis might be a significant cause of variability in hemoglobin levels in cross-sectional studies. It is known that fevers cause increased hemolysis in patients with unstable hemoglobins39 and hemoglobin H disease.40 There is an anecdotal report that fevers cause a more marked decrease in hemoglobin in HbE/β thalassemia and HbE disease,41 but this potentially important observation has not been studied subsequently.
Are there other possible interpretations of the result of the globin chain synthesis studies at higher temperatures? One possible explanation for the plateau in the rate of increase of specific activity is that the rate of mRNA translation starts to decrease over 2 hours at 41°C; the maintenance of linear synthesis in thalassemia intermedia at 41°C goes against this. Although the hemoglobin appears to be unstable at higher temperatures in HbE/β thalassemia, the pattern of globin binding to the membrane is unchanged compared with that at 37°C, with only α globin found. This suggests that precipitated βE chains are either proteolysed rapidly from the membrane or that they are unable to bind to the membrane at all. This absence of β globin binding to the membrane is similar to that in the interaction between HbE and pyrimidine 5′ nucleotidase deficiency.11 It is also interesting that the HbE homozygote showed a similar temperature effect to HbE/β thalassemics, with a plateau occurring in specific activity after 1 hour at 41°C and an SAβ 1 hour/SAβ 2 hours ratio of 18; it differed, however, in that no definite detectable peak of radioactivity was found attached to the membrane. The clinical correlate of this finding in homozygous HbE is unclear, with little information on the effects of fever on homozygotes.
This study suggests that the nature of the interaction between HbE and β thalassemia is primarily the interaction of two β thalassemia alleles and that the resulting degree of anemia is related to the amount of globin chain imbalance. Similarly, the marked variability in severity between different individuals with HbE/β thalassemia principally results from differences in globin chain imbalance, including variability in γ chain synthesis and coexistent α thalassemia. However, we have also found evidence that HbE can be significantly unstable when subjected to mild oxidative stress, such as increases in body temperature, and this may also contribute to the variability and severity. The significance of these temperature effects needs to be addressed in clinical studies.
ACKNOWLEDGMENT
The authors thank Dr J.B. Porter, Dr C. Hatton, Dr P. Darbyshire, and Dr A. Yardumian for referring patients used in this study and J. Darley for red blood cell enzyme assays.
Supported by the Medical Research Council, UK.
Address reprint requests to D.C. Rees, MRCP, MRC Molecular Haematology Unit, University of Oxford, Institute of Molecular Medicine, The John Radcliffe, Headley Way, Headington, Oxford, OX3 9DS, UK.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


![Fig. 3. Pulse-chase globin chain synthesis in an untransfused HbE/β thalassemic [IVS 1-5(G-C)] at 37°C. At time 0 minutes, the mixture was washed and the unused radiolabeled leucine was removed. The SA increases for the first 5 minutes as residual radiolabeled leucine is used and then plateaus, with no decrease in SA of the globin chains.(□) γ; (▪) βA; (•) βE; (○) α.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/6/10.1182_blood.v92.6.2141/4/m_blod41805003x.jpeg?Expires=1769099221&Signature=bQ4WTPwV-hqlEH5ItVvFPfBu8mV8FugACYJyODsQ3o8hf0b59l4gIvRCvAiAy5B8VU-ikVwmdWJgChwCwyFlJw2DhR6TR0kiOKRk60jRSeJrCl3NyQ0g5LnUQX06g782XCHjy2rqW75yvxpn6d4yXKzADHs6TqaQOhux5Kbat0PcWWLZ8WnGvyqi55bJH8LzIXs6Qo8LiJaKWvHSBcc06p2Imyj-V2iFgWiQMxhBSwjvifYGUOLOs47zCD1dCxsKNKCBm93PGMo3r64IeuFf6hIksqMpOBKILR5sI80I31pt4nXiB3YjnTpxFSWKk8L61cX8QccY9F7LPzjtPXRjYg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Hb composition of three fractions of erythrocytes separated by density gradient centrifugation of blood from an untransfused HbE/β+ thalassemic [IVS 1-5(G-C)], separating red blood cells into top (least dense, youngest cells), middle, and bottom (densest, oldest cells). Each point is the average of two centrifugations from the same patient. The ratio of HbE/HbA increases slightly as the red blood cells age, suggesting that HbE is at least as stable as HbA; the ratio of HbF/HbA increases with cell age as expected. The decrease in pyruvate kinase activity with increasing cell density confirms that the denser fractions contain predominantly older red blood cells. (○) %E/%A; (•) %F/%A; (×) PK.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/6/10.1182_blood.v92.6.2141/4/m_blod41805006x.jpeg?Expires=1769099221&Signature=2hubEC5WocAgPXEHqMYZQTPzxvBmf~zHHo6vd-yUkvREpf0AF-XHz7jxSDJQ-YRLs-3ubKTp3oq89wXI8Po0omZmhRGXVo646Z86PYF4BAN7Am1jsVRiZpHMadeVkbW2k-t6ft25JXVibrMZXvK3Zx0GVrbDDBK6rsa5qwoKzv0VuG7IeMRVQTEE0w8ee11W6GNhpP1vPSAn6j0RfLHHsXJ-Qf2-pTDpHC93a~C-T5DUK0PLRzHjLgScM67elnyPbZaA2FYfDiabnGBhoiEpSJBVPMuc0OXc8~Sbz67BJ1-LjyUgUtg1DkvoRjtY8KmAeXS8EImrxyDHBa7wAaRZng__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



![Fig. 3. Pulse-chase globin chain synthesis in an untransfused HbE/β thalassemic [IVS 1-5(G-C)] at 37°C. At time 0 minutes, the mixture was washed and the unused radiolabeled leucine was removed. The SA increases for the first 5 minutes as residual radiolabeled leucine is used and then plateaus, with no decrease in SA of the globin chains.(□) γ; (▪) βA; (•) βE; (○) α.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/6/10.1182_blood.v92.6.2141/4/m_blod41805003x.jpeg?Expires=1769099222&Signature=4pCFxSH8lz6iBRd791hL2SFIRy-JkzLPlgQ5oGaRNjWr~jy55qDCI6G7yYqFPFbfe6xJXwTzbm7JwkGDLqRotX02HWTvVlpBKiV1MC5TXEuH5OGNacnNCmQEcfA2GHiACvI8FIuOEhClvC0AaJoVi6rwr6Q5hp0L~IgLv-3RjXxGZfETKbYIc0gTYqy0~drkjZqu~L5o1d719iVFejpClQ~72iI3qXrmEYlPWaRfAAZG8kR3F0bTHWZawj6r-oCio2BbhBGtpY-wJPq~EYUmTqVjeJnzas3SbvaFmtie9cdz6JcglWdfIaQUSa3~oONN0nsBIgCKt--LKpMPjOazJw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 6. Hb composition of three fractions of erythrocytes separated by density gradient centrifugation of blood from an untransfused HbE/β+ thalassemic [IVS 1-5(G-C)], separating red blood cells into top (least dense, youngest cells), middle, and bottom (densest, oldest cells). Each point is the average of two centrifugations from the same patient. The ratio of HbE/HbA increases slightly as the red blood cells age, suggesting that HbE is at least as stable as HbA; the ratio of HbF/HbA increases with cell age as expected. The decrease in pyruvate kinase activity with increasing cell density confirms that the denser fractions contain predominantly older red blood cells. (○) %E/%A; (•) %F/%A; (×) PK.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/6/10.1182_blood.v92.6.2141/4/m_blod41805006x.jpeg?Expires=1769099222&Signature=fqMHPpFGSSzBTcFsWZybuHrsEcaSmDyU-pD7JurPzilT01H87M7xYmmy0faQOfetfE9VL~vAVgEbxcl9v2HXbLePkOnZH0RTfb4kVfOftvOzbTEpkOkwOfqjNZy8Y0i4NBfjJcBJKIsQKN4nVVa71PujYIrnObJ0MegQ9MCkD9nIr2jkIvhmGKiQtkmTtHEIOaSPyskrLHxKfiZu1Lk5LWrMyBK~uSh3AGqD5alE6Ke6YmHgbpEoox7-lT7ZRZ24UIYy88lUXa52L2tLdRHXAS55YnSfvL~wKB~qxYwYtIzvVKPUd81Ke2Dm~hWhZP9SD6XfcWniXhjMFsAKBWEJyQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)