Abstract
The small GTPase Rap1 is highly expressed in human neutrophils, but its function is largely unknown. Using the Rap1-binding domain of RalGDS (RalGDS-RBD) as an activation-specific probe for Rap1, we have investigated the regulation of Rap1 activity in primary human neutrophils. We found that a variety of stimuli involved in neutrophil activation, including fMet-Leu-Phe (fMLP), platelet-activating factor (PAF), granulocyte-macrophage colony-stimulating factor (GM-CSF), and IgG-coated particles, induce a rapid and transient Rap1 activation. In addition, we found that Rap1 is normally activated in neutrophils from chronic granulomatous disease patients that lack cytochrome b558 or p47phox and have a defective NADPH oxidase system. From these results we conclude that in neutrophils Rap1 is activated independently of respiratory burst induction. Finally, we found that Rap1 is activated by both the Ca2+ ionophore ionomycin and the phorbol ester 12-O-tetradecanoylphorbol 13-acetate (TPA), indicating that phospholipase C (PLC) activation leading to elevated levels of intracellular free Ca2+ and diacylglycerol (DAG) can mediate Rap1 activation. However, inhibition of PLC and Ca2+ depletion only marginally affected fMLP-induced Rap1 activation, suggesting that additional pathways may control Rap1 activation.
© 1998 by The American Society of Hematology.
NEUTROPHILS PLAY AN important role in the host defense to microbial pathogens. Stimulation of these cells induces multiple responses, including cell adhesion, migration, secretion, phagocytosis, and the generation of reactive oxygen species. Deregulated activation of neutrophils is implicated in the pathogenesis of a variety of inflammatory diseases leading to tissue damage. Therefore, neutrophil function is under tight control.1-5 A diverse array of receptors are expressed on the surface of neutrophils, allowing regulation by a wide range of agonists. Tyrosine kinase-linked receptors (eg, granulocyte-macrophage colony-stimulating factor [GM-CSF] receptors), serpentine receptors (eg, N-formylmethionyl-leucyl-phenylalanine [fMLP] and platelet-activating factor [PAF] receptors), and receptors that are stimulated by immune complexes such as the Fc receptors, activate distinct and overlapping signaling pathways regulating neutrophil responses6 7 (and references therein).
Several small GTPases have been implicated in controlling neutrophil function. In particular, Rac1 was shown to play an important role in the formation of the NADPH oxidase complex, thereby controlling the respiratory burst.2 In addition, receptor-dependent activation of Ras was recently demonstrated.6 Another small GTPase suggested to play a role in neutrophil function is the Ras-like small GTPase Rap1. Rap1 has an effector domain virtually identical to Ras, and it has been shown that ectopic expression of Rap1, under certain conditions, antagonizes Ras signaling. Of the two known isozymes of Rap1, Rap1A and Rap1B, Rap1A is highly expressed in neutrophils and has been found in a large complex with cytochrome b588.8 However, the physiological relevance of this association is still unknown, because Rap1 is not necessary to reconstruct a functional NADPH-oxidase complex in vitro.9However, it has been suggested Rap1 may mediate signaling events controlling the respiratory burst.10 11
We have recently developed a novel assay to measure activation of Rap1, ie, the accumulation of Rap1 in its GTP bound form.12 This assay is based on the high affinity of the Rap1-binding domain of RalGDS (RalGDS-RBD) for Rap1GTP, but not for Rap1GDP. In platelets, we observed that Rap1 is very rapidly activated by α-thrombin and various other platelet agonists. For this rapid activation, elevated levels of intracellular calcium were necessary and sufficient.12 To investigate the possible activation and function of Rap1 in human neutrophils, we have analyzed which stimuli might lead to an increase in GTP-bound Rap1. We found that Rap1 is activated by a variety of stimuli, each with distinct kinetics, including fMLP, PAF, GM-CSF, the phorbol ester 12-O-tetradecanoylphorbol 13-acetate (TPA), and IgG-coated particles. Furthermore, we show that multiple signaling pathways direct the activation of Rap1. Finally, this activation is independent of both a functional NADPH oxidase complex and the presence of cytochrome b588.
MATERIALS AND METHODS
Isolation of human neutrophils.
Blood was obtained from healthy volunteers from the Red Cross Blood Bank (Utrecht, The Netherlands). Mixed granulocytes were isolated from the buffy-coat of 500 mL 0.4% (wt/vol) tri-sodium citrate (pH 7.4) -treated blood as previously described.13 Mononuclear cells were removed by centrifugation over isotonic Percoll (1.078 g/mL) from Pharmacia (Uppsala, Sweden). After lysis of the erythrocytes in isotonic NH4Cl solution, neutrophils were washed and resuspended in incubation buffer (20 mmol/L HEPES, 132 mmol/L NaCl, 6 mmol/L KCl, 1 mmol/L MgSO4, 1.2 mmol/L KH2PO4, 5 mmol/L glucose, 1 mmol/L CaCl2) containing 0.5% human serum albumin (HSA; Central Laboratory of The Netherlands Red Cross Blood Transfusion Service, Amsterdam, The Netherlands). Neutrophils were incubated for 30 minutes at 37°C before stimulation. Neutrophils isolated in this manner were in the resting state. Neutrophils for the experiments described in Fig 4 were isolated as described.14 In all experiments, a concentration of 107 cells/mL was used for stimulation.
Neutrophil stimulation.
One milliliter of neutrophil suspension was stimulated with one of the following stimuli: fMLP (1 μmol/L), PAF (1 μmol/L), TPA (100 ng/mL), thapsigargin (100 nmol/L) (all from Sigma, St Louis, MO), GM-CSF (0.1 nmol/L; Genzyme, Boston, MA), and ionomycin (100 nmol/L; Calbiochem, La Jolla, CA). The concentrations used are known to prime neutrophils or activate the respiratory burst.15-17 In some experiments, cells were preincubated as described in the legends of the figures with one of the following inhibitors: IBMX, prostaglandin E2 (PGE2), wortmannin, staurosporine (all from Sigma), GF109203X, U73122, or LY294002 (all from Biomol, Plymouth, PA). At different time points, 0.5 mL 3× RIPA (1× RIPA: 150 mmol/L NaCl, 10 mmol/L Tris-HCl [pH 7.4], 1% NP-40, 0.5% deoxycholic acid, 0.1% sodium dodecyl sulfate [SDS], 2 mmol/L phenylmethyl sulfonyl fluoride [PMSF], 2 mmol/L benzamidine, 4 μmol/L aprotinin, 4 μmol/L leupeptin, and 4 μg/mL trypsin inhibitor) was added.
For stimulation with IgG-coated particles,14 500 μL of 0.8-μm latex beads (Difco, Augsburg, Germany) was washed with phosphate-buffered saline (PBS) and suspended in PBS (pH 8.5). One hundred microliters of human IgG (150 mg/mL; Lister Institute, Hertfordshire, UK) was added and incubated for 30 minutes at 37°C. Beads were washed two times with incubation buffer, resuspended in 500 μL incubation buffer, and added to 108 cells in 500 μL incubation buffer. Samples were stirred continuously and 200-μL aliquots were lysed at indicated time points by adding 200 μL 2× RIPA.
Rap1 activation assay.
Rap1 activation was determined essentially as described.12Cell lysates were put on ice for 8 minutes and clarified by centrifugation at 14,000 rpm in an Eppendorf centrifuge for 8 minutes at 4°C. Per sample, 14 μg His-Ral GDS RBD or glutathione S-transferase (GST)-RalGDS-RBD was precoupled for 1 hour to 15 μL of 50% Ni-NTA (nickel-nitrilotriacetic acid agarose; Qiagen, Hilden, Germany) (His-RalGDS-RBD) or 40 μL of 10% glutathione beads (GST-RalGDS-RBD). After coupling, beads were washed 4 times with RIPA, added to the cell lysate, and incubated for 30 minutes at 4°C. Samples were washed 3 times in RIPA and bound proteins were eluted in 15 μL of Laemmli sample buffer. The samples were put on SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride membranes (PVDF; NEN, Boston, MA). Rap1 was detected with a monoclonal antibody (Transduction Laboratories, Lexington, KY) and horseradish peroxidase-coupled goat antimouse (Bio-Rad, Hercules, CA) using enhanced chemiluminescence (Amersham, Buckinghamshire, UK).
Depletion of intracellular free Ca2+.
Intracellular free Ca2+ was depleted as described.18 Neutrophils were suspended in Ca2+free incubation buffer supplemented with 1 mmol/L EGTA. Indo-1/AM (Molecular Probes, Eugene, OR) was added to 1 mL aliquots of suspended cells (107 cells/mL) at a final concentration of 1.5 μmol/L. Cells were incubated for 40 minutes at 37°C. Thapsigargin (100 nmol/L) was added 10 minutes before washing to deplete internal stores. Cells were washed once with Ca2+ free incubation buffer containing EGTA. Determination Rap1 activation and Ca2+ measurements were performed with the same batch of cells. Calcium concentration was measured by a dual excitation at a wavelength of 340 nm and detected at 390 nm using Hitachi F4500 fluorescence spectrophotometer (Hitachi Ltd, Tokyo, Japan).
Respiratory burst measurements.
Neutrophils were preincubated in the absence or presence of GM-CSF (0.1 nmol/L for 20 minutes). fMLP (1 μmol/L) was added to the cells and oxygen consumption was measured using a Clark oxygen electrode.19
RESULTS
Rap1 is activated by a variety of stimuli.
Stimulation of human neutrophils with 1 nmol/L fMLP stimulation results in cell adhesion and chemotaxis. Antipathogenic responses such as degranulation and the generation of oxygen radicals occur only after stimulation with more than 1 μmol/L fMLP and require preactivation with agents such as PAF or GM-CSF. The biochemical mechanism of this preactivation or priming is not fully understood. Furthermore, the priming agents PAF and GM-CSF differ in that GM-CSF stimulation results only in chemokinesis (undirectional cell motility), whereas PAF stimulation, similarly to fMLP stimulation results in chemotaxis (directional cell movement)1,2,6,15-17 20 (and references therein).
To investigate whether fMLP, PAF, or GM-CSF can activate Rap1, we stimulated resting neutrophils isolated from human peripheral blood with different ligands for various periods of time. Rap1 was isolated with the 97 amino acid RalGDS-RBD, which specifically binds the active, GTP-bound form of Rap1, followed by detection with Western blotting.12 Stimulation with 1 μmol/L fMLP-induced a rapid increase in Rap1 activity with biphasic kinetics (Fig 1). An initial activation peak was observed by 10 seconds, which decreased around 30 seconds. Activity peaked again at 5 minutes, followed by a slow decline toward basal levels observed in resting neutrophils. The extent of activation varied somewhat between different donors (compare Figs 1, 3, and 4B for variation in Rap1 activation after fMLP-stimulation), but the kinetics of Rap1 activation remained essentially constant in all experiments. Approximately 1% of Rap1 present in the total lysate of stimulated cells was found to be bound to RalGDS-RBD (Fig 1). This was concluded after comparing the amount of Rap1 present in total lysate and that of Rap1 in the GTP bound state on Western blot. Stimulation with 1 μmol/L PAF also resulted in a rapid activation of Rap1, which peaked around 10 seconds, followed by a slow decline towards basal level. In contrast to fMLP, no second activation peak was observed.
Rap1 activation in neutrophils. Neutrophils were stimulated with either 1 μmol/L fMLP, 1 μmol/L PAF, or 0.1 nmol/L GM-CSF for indicated time points. Cells were lysed and Rap1 GTP was isolated using His-Ral-GDS RBD. Rap1 was detected by Western blot analysis using a monoclonal antibody against Rap1. The amount of Rap1 GTP isolated after 10 seconds of fMLP stimulation and unstimulated neutrophils was compared with the amount of total Rap1 (Rap1GDP and GTP) present in various amounts (%) of total lysate. The experiments were repeated at least three times with similar results.
Rap1 activation in neutrophils. Neutrophils were stimulated with either 1 μmol/L fMLP, 1 μmol/L PAF, or 0.1 nmol/L GM-CSF for indicated time points. Cells were lysed and Rap1 GTP was isolated using His-Ral-GDS RBD. Rap1 was detected by Western blot analysis using a monoclonal antibody against Rap1. The amount of Rap1 GTP isolated after 10 seconds of fMLP stimulation and unstimulated neutrophils was compared with the amount of total Rap1 (Rap1GDP and GTP) present in various amounts (%) of total lysate. The experiments were repeated at least three times with similar results.
fMLP and PAF both act via serpentine receptors. To investigate whether Rap1 could be activated via receptor-associated tyrosine kinases, we stimulated neutrophils with GM-CSF (0.1 nmol/L). This stimulation resulted in a delayed and weaker activation of Rap1, compared with fMLP- or PAF-induced Rap1 activation, reaching its maximum around 5 to 10 minutes.
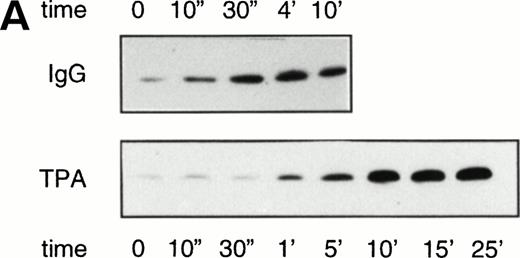
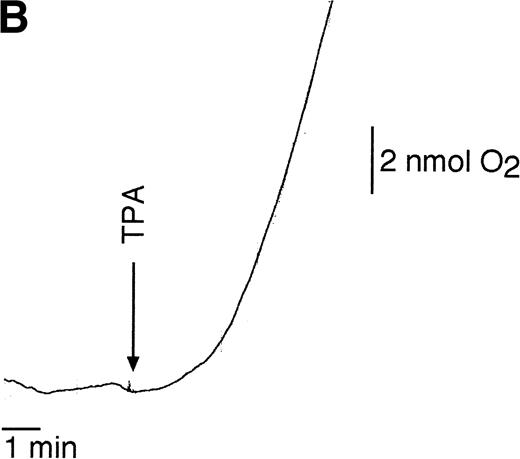
Various studies have implicated Rap1 functioning in the production of oxygen radicals. Therefore, we analyzed Rap1 activation by stimuli known to induce a respiratory burst in resting neutrophils.14 Incubation with IgG-coated particles resulted in a slow but steady increase of Rap1 activation detectable after 30 seconds, which reached its maximal activity after 4 minutes (Fig 2). A slow increase of Rap1 activation was also observed after treating resting neutrophils with TPA (100 ng/mL). In this case, Rap1 activation was detectable after 1 minute and reached a maximum after 5 minutes of TPA treatment.
Rap1 activation by stimulators of the respiratory burst in resting neutrophils. (A) Neutrophils were stimulated with 100 ng/mL TPA or IgG-coated latex beads. Samples were taken at indicated time points after stimulation. Rap1 activity was determined as described in the legend to Fig 1. (B) Respiratory burst induced in resting neutrophils by TPA (100 ng/mL) measured using a Clark oxygen electrode.
Rap1 activation by stimulators of the respiratory burst in resting neutrophils. (A) Neutrophils were stimulated with 100 ng/mL TPA or IgG-coated latex beads. Samples were taken at indicated time points after stimulation. Rap1 activity was determined as described in the legend to Fig 1. (B) Respiratory burst induced in resting neutrophils by TPA (100 ng/mL) measured using a Clark oxygen electrode.
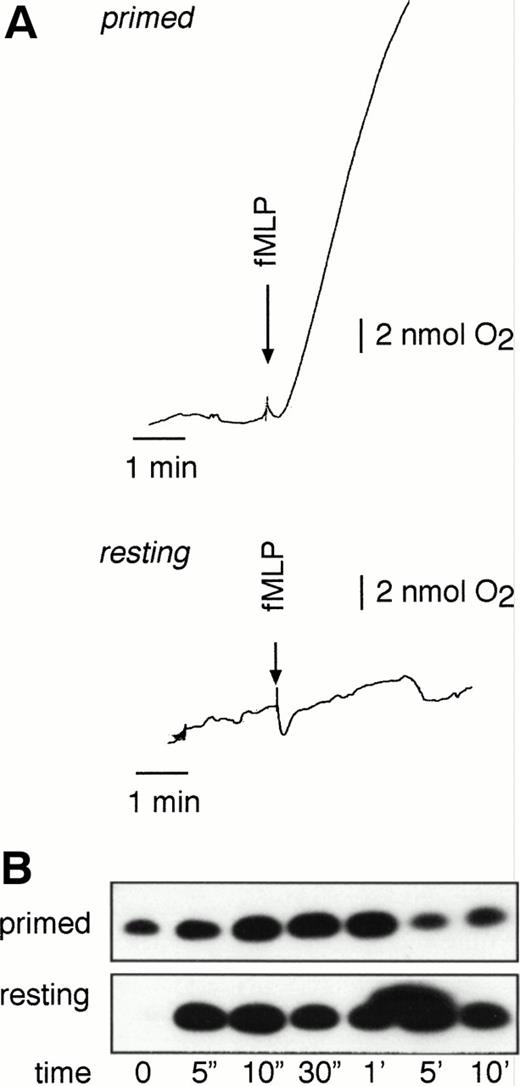
Activation of primed neutrophils by fMLP also induces the respiratory burst (Fig 3A). To investigate whether Rap1 activation is modified under these conditions, we primed neutrophils with 0.1 nmol/L GM-CSF followed by stimulation with 1 μmol/L fMLP. As shown in Fig 3B, fMLP induced Rap1 activation to a similar extent both in resting and in primed neutrophils. In general, the kinetics of activation were slightly different, with the first activation peak delayed and the absence of a second peak in the case of primed cells. As a consequence, usually only a single activation peak was observed around 30 seconds, which decreased after 1 minute. Similar results were obtained after priming neutrophils with PAF (data not shown).
Rap1 activation in resting and primed neutrophils. Rap1 activity is measured in primed and resting neutrophils. Neutrophils were primed by 20 minutes of 0.1 nmol/L GM-CSF stimulation. Subsequently, resting and primed neutrophils were stimulated with 1 μmol/L fMLP for the indicated time points. (A) Oxygen consumption was measured with a Clark oxygen electrode. (B) Rap1 activation was measured in neutrophils from the same donor as (A). Representative examples of at least three independent experiments are shown.
Rap1 activation in resting and primed neutrophils. Rap1 activity is measured in primed and resting neutrophils. Neutrophils were primed by 20 minutes of 0.1 nmol/L GM-CSF stimulation. Subsequently, resting and primed neutrophils were stimulated with 1 μmol/L fMLP for the indicated time points. (A) Oxygen consumption was measured with a Clark oxygen electrode. (B) Rap1 activation was measured in neutrophils from the same donor as (A). Representative examples of at least three independent experiments are shown.
Multiple signaling pathways direct fMLP-induced Rap1 activation.
Although Rap1 was activated by all agents used, the kinetics of Rap1 activation differed, suggesting that multiple signaling pathways might regulate Rap1 activation. To determine the mechanisms by which Rap1 GTPase is regulated, we investigated fMLP-induced Rap1 activation, because some of the signaling pathways used by fMLP in resting neutrophils are defined. fMLP activates a serpentine receptor that is coupled to various heterotrimeric G-proteins (Bokoch7 and references therein). After stimulation, phospholipase C β (PLCβ) is activated, resulting in diacylglycerol (DAG)-mediated activation of protein kinase C (PKC) and inositol 3 4 5 triphosphate (IP3)-mediated Ca2+mobilization.7,21 In addition, phosphatidylinositol-3-kinase (PI-3K) is activated, which may be responsible for the activation of protein kinase B (PKB) and of Rac GTPases.21-23
A possible signaling pathway which may mediate Rap1 activation involves changes in intracellular free Ca 2+ concentration ([Ca2+]i). We first investigated whether Ca2+ was able to induce Rap1 activation, because in human platelets α-thrombin induced Rap1 activation is Ca2+-dependent.12 Resting neutrophils were incubated with ionomycin and thapsigargin to mimic Ca2+influx and Ca2+ release from internal stores. As shown in Fig 4A, ionomycin induced a rapid activation of Rap1 to a level similar to fMLP, whereas induction of Rap1 by thapsigargin, although detectable, was clearly lower than that induced by fMLP. Because these experiments indicated that Ca2+ influx may be sufficient to induce Rap1 activation in neutrophils, we next investigated whether an increase in [Ca2+]i was essential for Rap1 activation. We therefore depleted neutrophils of Ca2+ by pretreatment with 1 mmol/L EGTA, 1.5 μmol/L indo-1/AM, or 100 nmol/L thapsigargin. Under these conditions, [Ca2+]i decreased to less than 10 nmol/L and no increase in [Ca2+]i levels was observed after fMLP stimulation (Fig 4B). Ca2+ depletion did not affect the rapid fMLP-induced Rap1 activation, but we did observe a reduction in Rap1 activation at the second activation peak as compared with the control fMLP treatment. Similar results were obtained after inhibition of Ca2+ influx by blocking Ca2+ channels with La3+ (data not shown). From these results we concluded that elevated [Ca2+]i is sufficient but not essential to induce Rap1 activation in human neutrophils.
Ca2+ dependency of Rap1 activation. (A) Neutrophils were incubated with ionomycin (100 nmol/L) or thapsigargin (100 nmol/L) for the indicated time points. Rap1 GTP was detected as in the legend to Fig 1. (B) Ca2+-depleted neutrophils were stimulated with 1 μmol/L fMLP. As a control, non–Ca2+-depleted cells were stimulated with 1 μmol/L fMLP. Ca2+-depleted and untreated neutrophils of the same donor were taken to measure [Ca2+]i after 1 μmol/L fMLP stimulation. Representative examples of at least three independent experiments are shown.
Ca2+ dependency of Rap1 activation. (A) Neutrophils were incubated with ionomycin (100 nmol/L) or thapsigargin (100 nmol/L) for the indicated time points. Rap1 GTP was detected as in the legend to Fig 1. (B) Ca2+-depleted neutrophils were stimulated with 1 μmol/L fMLP. As a control, non–Ca2+-depleted cells were stimulated with 1 μmol/L fMLP. Ca2+-depleted and untreated neutrophils of the same donor were taken to measure [Ca2+]i after 1 μmol/L fMLP stimulation. Representative examples of at least three independent experiments are shown.
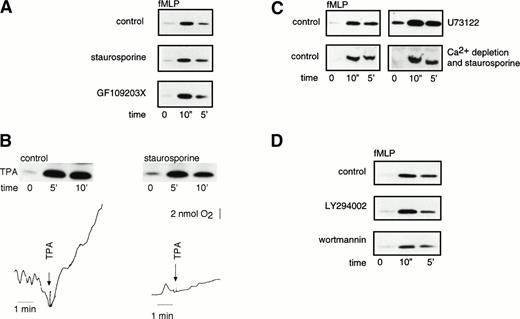
A role for PKC in Rap1 activation was suggested, because TPA induced Rap1 activation in neutrophils (see Fig 2). Therefore, we examined whether inhibitors of PKC could abolish fMLP-induced Rap1 activation. However, the broad-specificity PKC inhibitors staurosporine and GF109203X (bisindolylmaleimide) did not inhibit fMLP-induced Rap1 activation, indicating that the fMLP-induced Rap1 activation is independent of PKC (Fig 5A). Furthermore, most of the TPA-induced Rap1 activation was insensitive to staurosporine, whereas, at the concentrations used, staurosporine effectively abolished TPA-induced respiratory burst (Fig5B). Thus, Rap1 can be activated directly by TPA and does not require PKC.
fMLP- and TPA-induced Rap1 activation is independent of PKC. (A) fMLP-induced Rap1 activation is not inhibited by PKC inhibitors. Neutrophils were preincubated for 5 minutes with 200 nmol/L staurosporine or 10 minutes with 5 μmol/L GF109203X. Neutrophils were then stimulated with 1 μmol/L fMLP for 10 seconds and 5 minutes. As a control, untreated neutrophils of the same donor were used. (B) TPA-induced Rap1 activity is not inhibited by a PKC inhibitor. Neutrophils were stimulated with 100 ng/mL TPA for 5 and 10 minutes after preincubation for 5 minutes with 200 nmol/L staurosporine or buffer. Oxygen consumption was measured with a Clark oxygen electrode to measure the functionality of staurosporine treatment. Representative examples of at least three independent experiments are shown. (C) Inhibition of PLCβ does not influence Rap1 activity. Neutrophils were preincubated with 1 μmol/L U73122 (PLCβ inhibitor) for 3 minutes and subsequently stimulated with 1 μmol/L fMLP. (D) Rap1 activity is not inhibited by PI-3 kinase inhibitors. Neutrophils were preincubated with the PI-3 kinase inhibitors LY294002 (10 μmol/L) or wortmannin (100 nmol/L) for 5 minutes. Samples were taken after preincubation and 10 seconds and 5 minutes after 1 μmol/L fMLP stimulation. As a control, neutrophils of the same donor without preincubation were used. Representative examples of at least three independent experiments are shown.
fMLP- and TPA-induced Rap1 activation is independent of PKC. (A) fMLP-induced Rap1 activation is not inhibited by PKC inhibitors. Neutrophils were preincubated for 5 minutes with 200 nmol/L staurosporine or 10 minutes with 5 μmol/L GF109203X. Neutrophils were then stimulated with 1 μmol/L fMLP for 10 seconds and 5 minutes. As a control, untreated neutrophils of the same donor were used. (B) TPA-induced Rap1 activity is not inhibited by a PKC inhibitor. Neutrophils were stimulated with 100 ng/mL TPA for 5 and 10 minutes after preincubation for 5 minutes with 200 nmol/L staurosporine or buffer. Oxygen consumption was measured with a Clark oxygen electrode to measure the functionality of staurosporine treatment. Representative examples of at least three independent experiments are shown. (C) Inhibition of PLCβ does not influence Rap1 activity. Neutrophils were preincubated with 1 μmol/L U73122 (PLCβ inhibitor) for 3 minutes and subsequently stimulated with 1 μmol/L fMLP. (D) Rap1 activity is not inhibited by PI-3 kinase inhibitors. Neutrophils were preincubated with the PI-3 kinase inhibitors LY294002 (10 μmol/L) or wortmannin (100 nmol/L) for 5 minutes. Samples were taken after preincubation and 10 seconds and 5 minutes after 1 μmol/L fMLP stimulation. As a control, neutrophils of the same donor without preincubation were used. Representative examples of at least three independent experiments are shown.
Because both elevated levels of [Ca2+]i and TPA-treatment (which mimics the formation of DAG) activated Rap1, PLCβ may mediate fMLP-induced Rap1 activation. We investigated whether the PLCβ-inhibitor U7312224 could inhibit fMLP-induced Rap1. However, no inhibition of fMLP-induced Rap1 activation was observed (Fig 5C). From these results we concluded that another, distinct signaling pathway regulating Rap1 activation is activated by fMLP or that a U73122-insensitive PLC isoform is responsible for the fMLP-induced Rap1 activation. As expected, both inhibition of PKC and Ca2+ depletion did not abolish fMLP-induced Rap1 activation either (Fig 5C).
Rap1 activation is not inhibited by PGE2 and is independent of oxidase assembly and function.
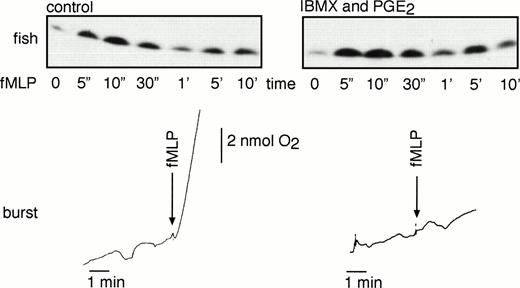
In platelets, Rap1 activation by α-thrombin is completely inhibited by prostacylin, a platelet antagonist that elevates the levels of cAMP. PGE2 is an antagonist of neutrophils and also elevates the levels of cAMP. In addition, PGE2 was reported to induce Rap1 phosphorylation via cAMP-dependent protein kinase A (PKA), resulting in the dissociation of Rap1 from cytochrome b558in vitro.25 We therefore examined if PGE2 could antagonize fMLP-dependent Rap1 activation in neutrophils. However, cotreatment of neutrophils with PGE2 and the phosphodiesterase inhibitor IBMX (which further elevates cAMP levels) did not affect fMLP-induced Rap1 activation (Fig 6). The observation that a similar treatment did inhibit fMLP-induced respiratory burst in GM-CSF–primed neutrophils indicated that cAMP does not interfere with the activation of Rap1 GTPase.
Rap1 activation is not inhibited by PGE2treatments. Neutrophils of a healthy donor were preincubated for 10 minutes with 100 μmol/L IBMX followed by 30 seconds of incubation with 30 μmol/L PGE2. Cells were stimulated with 1 μmol/L fMLP, and Rap1 activity was measured after the indicated time points. As a control, untreated neutrophils were stimulated with the same amount of fMLP. Respiratory burst was measured to control for the functionality of the cAMP treatment. Representative examples of at least three independent experiments are shown.
Rap1 activation is not inhibited by PGE2treatments. Neutrophils of a healthy donor were preincubated for 10 minutes with 100 μmol/L IBMX followed by 30 seconds of incubation with 30 μmol/L PGE2. Cells were stimulated with 1 μmol/L fMLP, and Rap1 activity was measured after the indicated time points. As a control, untreated neutrophils were stimulated with the same amount of fMLP. Respiratory burst was measured to control for the functionality of the cAMP treatment. Representative examples of at least three independent experiments are shown.
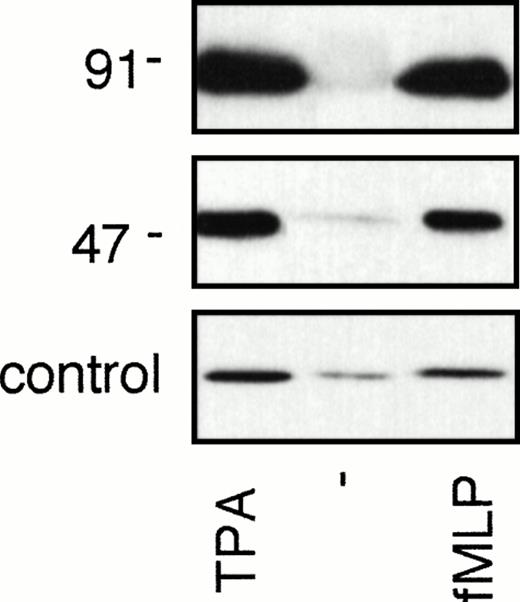
That Rap1 activation was not dependent on Rap1 association with cytochrome b558 or the formation of the oxidase complex was further confirmed by analysis of Rap1 activation in patients with chronic granulomatous disease (CGD), which lack a functional oxidase complex.26 As shown in Fig 7, Rap1 is still activated by both fMLP and TPA in neutrophils isolated from CGD patients, lacking either cytochrome b588 (p91phox) or p47phox.
Rap1 activation is independent of the presence of a functional NADPH oxidase complex. (A) Neutrophils of a p91phox-deficient or a p47-deficient patient were stimulated for 5 minutes with 100 ng/mL TPA or 1 μmol/L fMLP. Rap1 GTP was isolated using GST-Ral-GDS RBD as described in the legend to Fig 1.
Rap1 activation is independent of the presence of a functional NADPH oxidase complex. (A) Neutrophils of a p91phox-deficient or a p47-deficient patient were stimulated for 5 minutes with 100 ng/mL TPA or 1 μmol/L fMLP. Rap1 GTP was isolated using GST-Ral-GDS RBD as described in the legend to Fig 1.
DISCUSSION
In this report we have investigated the signaling events in human neutrophils that resulted in the activation of Rap1, ie, an increase in levels of Rap1GTP. We have used an assay that uses the Rap1 binding domain of a putative Rap1 effector (RalGDS-RBD) to specifically precipitate Rap1GTP. We observed that a variety of stimuli can induce Rap1 activation, but the extent and kinetics of activation varied. A very rapid activation of Rap1 was observed after fMLP and PAF stimulation, whereas slower activation was observed after stimulation with TPA, incubation with IgG-coated particles, or GM-CSF.
Our finding that several stimuli can activate Rap1 may indicate that multiple pathways are involved in the activation of Rap1. Thus far, a study of the activation of Rap1 has only been performed in human platelets and T lymphocytes. In these cells, Rap1 activation after α-thrombin stimulation is mediated by elevated [Ca2+]i, which is necessary and sufficient.12 27 In neutrophils, it appears that, in addition to elevated [Ca2+]i, other pathways lead to Rap1 activation. Indeed, both ionomycin and, to a lesser extent, thapsigargin induce Rap1 activation, but Ca2+depletion did not abolish fMLP-induced Rap1 activation as well.
Because TPA efficiently induced Rap1 activation, DAG is a strong candidate to mediate Rap1 activation. PKC, a well-known target for DAG, is not involved in Rap1 activation, because both the fMLP-induced Rap1 activation and the TPA-induced Rap1 activation were not abolished after treatment with the broad PKC inhibitor staurosporine. This implies that a different DAG target is involved in the regulation of Rap1.
Suprisingly, affecting the elevation of [Ca2+]i and DAG formation by inhibition of PLCβ did not abolish fMLP-induced Rap1 activation. This could mean that U73122 is not sufficient to block an increase in [Ca2+]i and/or the formation of DAG. Indeed, PLC-independent pathways for Ca2+ influx and DAG formation have been described.7,28 29 Alternatively, a third, still-unknown pathway is involved in the regulation of fMLP-induced Rap1 activation.
It is unlikely that fMLP-induced Rap1 activation is mediated by PI-3K,24 30 because the PI-3K inhibitors wortmannin and LY294002 also failed to abolish Rap1 activation either.
It was shown previously that Rap1 is present in a complex with cytochrome b558 of the NADPH oxidase and cotranslocates with cytochrome b558 from the specific granules to the plasma or phagosome membrane after stimulation.10,11 Our results show that association of Rap1 with cytochrome b558or an assembled oxidase complex is not necessary for Rap1 activation. This conclusion was based on our observation that Rap1 is normally activated in neutrophils from CGD patients that lack either p91phox or p47phox. Furthermore, PGE2, which was reported to induce Rap1 phosphorylation and dissociation from cytochrome b558,25 did not abolish Rap1 activation.
Our finding that Rap1 is activated after stimulation of both receptor associated-tyrosine kinases (GM-CSF) and serpentine receptors (fMLP and PAF) indicates that Rap1 functions in a pathway common to both receptor types. Interestingly, only GM-CSF but not fMLP induces phosphorylation of Clb.31 This protein has been implicated in the activation of Rap1 through the recruitment of C3G, an exchange factor for Rap1, to Cbl via the adaptor protein Crk or Crk-L.32 33However, if indeed Cbl mediates Rap1 activation, pathways inducing Rap1 activation after fMLP stimulation would be independent of Cbl phosphorylation.
Although the function of Rap1 is unknown, it has been suggested that Rap1 activity is required for respiratory burst.10,11 Our results show that Rap1 activation in vivo is not sufficient to induce a respiratory burst. Perhaps Rap1 activation is only involved in one of the events regulating the multistep process of generating a respiratory burst. Rap1 activity has also been found in human platelets.12 Both neutrophils and platelets are highly specialized, and Rap1 is possibly involved in a specific function common to both cell types. The reported association of Rap1 to cytochrome b588, in human neutrophils may indicate that Rap1 is involved in the regulation of complex formation. In platelets, a close relative of Rap1, Rap2, has been found in a complex with the major platelet integrin αIIbβ3,34 suggesting a similar role for Rap2 in platelets. Furthermore, in both cell types, Rap1 is at least partly localized on vesicular structures and is translocated to the plasma membrane upon activation.35 Interestingly, the Rap homologue in the budding yeast Saccharomyces cerevisiae, Rsr1 (Bud1) is involved in the selection of the site where a new bud will form.36 Perhaps Rap1 regulates the formation and/or translocation of protein complexes by specifying the fusion of different cellular compartments, such as the fusion of secretory vesicles with the plasma membrane. It is suggested that Rap1 is involved in regulated insulin secretion.37
To decipher the function of Rap1 it will be essential to know which effector protein binds to Rap1GTP. Interestingly, the effector domain of Rap1 is virtually identical to the effector domain of Ras,38-40 and it has been shown that Rap1 also binds to Ras effector proteins, including Ral guanine nucleotide exchange factors (RalGEFs) and Raf kinases.41-44 In neutrophils and platelets, Rap1 may activate one of the Raf1 kinase signaling cascades,43,44 or Rap1 may activate one of the RalGEFs resulting in the activation of the small GTPase Ral.41,45This protein is involved in the activation of phospholipase D,46 which, together with members of the Arf family of small GTPases, may control translocation and fusion of granules.47
ACKNOWLEDGMENT
The authors thank Drs B. Burgering and K. Reedquist for support, discussions, and critically reading the manuscript.
Supported by grants from the Netherlands Heart Association (B.F.), the Dutch Cancer Society (F.Z.), and Glaxo Wellcome (P.C.).
Address reprint requests to Johannes L. Bos, PhD, Laboratory for Physiological Chemistry, Utrecht University, Universiteitsweg 100, 3584 CG Utrecht, The Netherlands; e-mail: j.l.bos@med.ruu.nl.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


![Fig. 4. Ca2+ dependency of Rap1 activation. (A) Neutrophils were incubated with ionomycin (100 nmol/L) or thapsigargin (100 nmol/L) for the indicated time points. Rap1 GTP was detected as in the legend to Fig 1. (B) Ca2+-depleted neutrophils were stimulated with 1 μmol/L fMLP. As a control, non–Ca2+-depleted cells were stimulated with 1 μmol/L fMLP. Ca2+-depleted and untreated neutrophils of the same donor were taken to measure [Ca2+]i after 1 μmol/L fMLP stimulation. Representative examples of at least three independent experiments are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/6/10.1182_blood.v92.6.2133/4/m_blod41819004w.jpeg?Expires=1769126002&Signature=XKt77vRc7L-shKynhtl-s4U0~7uAifDUnFepKJeM783MxAfaJPJmNnUXAxcaYLnk2gl1K1m4scfyCMyY7n9Q11XEYVnKI2IDS-2x9bkJWOBtzWXdm8huO9tcv8hiq-fI-8nT0VO3Jh3M7ey8Y4dBqFX~Uv~IMkraV-J2BVAWYTMMDytcn1mzPrMxf7KIEzmtjEJRp7dNrkGuUp3BQ2ENtJeqTM1dTPwKrQweACTouvm13YE5UatzuBbr1GN0WEdG8dnmKPL1j3pwgf3Li72sJn9TMiG4zKvgq1tTxyM~kT2AT5sXtv6ZtwTRZ8gw2IceZ5kvvkDO7JMeDg3N6x7~Uw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal