Abstract
Autologous stem-cell transplantation has become a widely used therapy in Hodgkin’s disease (HD). To appreciate the early and late risks associated with this procedure, its lethal toxicity and effects on the incidence of secondary cancers were studied. Data related to 467 French patients grafted from 1982 to 1995 for primary sensitive disease (PSD, 22%), primary refractory disease (PRD, 18%), first relapse (R1, 45%), or subsequent relapses (R2, 15%) were analyzed. Grafted patients (PSD, PRD, and R1; n = 393) were matched (3 controls for 1 case) on age, gender, clinical stage, B symptoms, and time at risk with 1179 conventionally treated patients issued from international databases. The proportional hazards (Cox) model was used to assess relative risks (RR). Among grafted patients, 8% died of toxicity related to the procedure, and 18 secondary cancers occurred leading to a 5-year cumulative incidence rate of 8.9%. In this series, risk factors for second cancer were age ≥40 years (RR = 3.73, P= .007) and the use of peripheral blood stem cells as source of graft (RR = 3.10, P = .03). Among grafted and matched ungrafted patients, risk factors for the development of secondary cancer were age ≥40 years (RR = 2.90, P < .001), relapse versus no relapse (RR = 5.22, P = .006), PRD versus other patients (RR = 3.86, P = .033), and grafted versus ungrafted patients (RR = 2.04, P = .024). Solid tumors were more frequent in grafted than in ungrafted patients (RR = 5.19, P= .001) although the incidence of myelodysplasia and acute myeloid leukemia was similar in the two groups. We conclude that high-dose chemotherapy administered as first-line treatment or after relapse is associated with an acceptable toxic death rate. The risk of secondary myelodysplasia or acute myeloid leukemia is not significantly increased after autologous stem-cell transplantation for HD, whereas an increased risk of solid tumors exists. The peripheral blood stem-cell–associated risk of secondary cancer among grafted patients needs further investigations.
© 1998 by The American Society of Hematology.
HODGKIN’S DISEASE (HD) is highly curable by both radiotherapy and/or chemotherapy, but refractory disease or early relapses are rarely cured by conventional salvage therapy.1 Since the last decade, high-dose chemotherapy (HDCT) and autologous stem-cell transplantation (ASCT) have been used to treat such patients.2,3,4 Although a single randomized study has been published showing a better event-free survival after HDCT than after conventional salvage therapy with no survival improvement,5 HDCT has become more widely used since the availability of growth factors and the emergence of peripheral blood stem cells (PBSC). ASCT has also been proposed to patients with poor initial prognostic factors,6,7 such as age ≥40 years, bulky mediastinal mass, extended stage, extranodal sites, serum lactate dehydrogenase above normal value, bone marrow involvement, and anemia. The clinical outcome of patients with three or more of these factors is very poor, with a 5-year probability of survival after conventional therapy not higher than 20.5%.8
Cohorts of patients with long survival after HDCT are now available and secondary cancers have been reported after ASCT for non-Hodgkin’s lymphoma (NHL) and HD.9 10 At University of Nebraska, the 5-year cumulative incidence of secondary myelodysplasia and acute myeloid leukemia (MDS/AML) was 4% in a series of 511 HD and NHL, whereas at the Dana Farber Cancer Institute in Boston it was 18.6% in a series of 206 NHLs.
In HD, the occurrence of MDS/AML and solid tumors after many years of conventional treatment has been also well described.11MDS/AML were generally related to age, the use and cumulative dose of alkylating agents, and splenectomy, and the risk was limited to 10 to 15 years after treatment.12 Solid tumors were mostly ascribable to radiation therapy, and showed an incidence still increasing after 20 years.13 The incidence of secondary cancers after ASCT for HD has not yet been assessed, and it is poorly understood whether such secondary cancers are due solely to prior conventional chemotherapy and radiotherapy, or whether HDCT also contributes to their development. Therefore, a series of 467 grafted HD patients was studied to evaluate the HDCT-related toxicity and risk factors associated with the development of secondary cancer. Patients grafted either for primary sensitive disease (PSD), primary refractory disease (PRD), or at first relapse (R1) were matched to 1179 patients treated with conventional therapy to assess the contribution of HDCT and ASCT to the risk of secondary cancer.
PATIENTS AND METHODS
Grafted patients.
Using the database of the Société Française de Greffe de Moelle, French centers were identified in which more than 10 ASCT for lymphomas were regularly performed per year. Fourteen of them agreed to participate in the study and represented 481 (54%) of the 889 reported HD patients who underwent HDCT in France between 1982 and 1995. Files were reviewed and, by means of a standardized form, data on initial patient characteristics, treatment type, the duration of each treatment episode, response to initial and subsequent treatments, conditioning regimen, source of stem cells, events after transplant, patient status, and cause of death were collected.
Chemotherapy data included the type of regimen: MOPP (mechlorethamine, vincristine, procarbazine, prednisone), Adriamycin containing mostly ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine), MOP/ABV hybrid, MOPP-ABVD alternating, or others and the number of cycles given. For the analysis of survival and risk of second cancer, two cycles of alternating MOPP-ABVD or hybrid MOP/ABV were considered to be equivalent to one cycle of MOPP and, on this basis, the cumulative chemotherapy was divided into four groups: ≥3 MOPP, <3 MOPP, an Adriamycin-containing regimen, and others. All patients receiving alternating- or hybrid-regimen chemotherapy are thus in the ≥3 or <3 MOPP groups. The MOPP-treated patients were divided into two groups because of the dose-dependent relationship between alkylating agents and the occurrence of secondary MDS/AML.14 15
Data relating to irradiation included the field (mantle, paraaortic, spleen, pelvis, involved field, or others) and the dose delivered to each field. Irradiation administered at different times during the treatment (including total body irradiation [TBI], post-ASCT irradiation, and irradiation for relapse) was pooled and classified as follows. Mantle-field, inverted-Y, and involved-field irradiation were considered as limited-field radiotherapy; other combinations were considered as extended-field radiotherapy. All patients receiving TBI as part of their high-dose therapy regimen were included in the extended field group for the analysis.
After each treatment episode of chemotherapy and/or radiotherapy, the disease status was evaluated as complete response (CR; disappearance of all measurable lesions), partial response (regression of at least 50% of the lesions), stable disease (regression of fewer than 50% of the lesions), or progression (progression of any lesion or new localization during treatment).
For the purposes of analysis, the whole population was divided into four groups according to the indication for HDCT and ASCT. The PRD group consisted of 86 patients who either progressed during initial therapy (78 patients), or alternatively achieved an unstable partial response lasting a median duration of 80 days (8 patients) before new progression and then received HDCT as salvage therapy. Eighty of these 86 patients received a conventional-dose salvage chemotherapy before undergoing HDCT. The PSD group consisted of 102 patients who responded to initial therapy and then received HDCT and ASCT as consolidation treatment. These patients presented with poor initial prognostic features,7 achieved a partial (66%) or CR (34%), and did not receive any additional treatment before undergoing ASCT. The other two groups consisted of patients who underwent ASCT after R1 (210 patients) or subsequent (R2) relapse (69 patients). All these patients achieved at least one CR and were treated with R1 or multiple R2 chemotherapy and/or irradiation before the stem-cell collection and HDCT. In the R2 group, 56 patients received two second-line treatments, whereas 10 received three and 5 received four. Fourteen patients who received a second stem-cell transplantation were excluded from the analysis.
After transplant, the 5-year overall survival rates of the 467 grafted patients studied were 82% (95% confidence interval [CI], 72% to 89%) and 35% (95% CI, 23% to 49%), respectively, in the PSD and PRD groups, and 44% (95% CI, 34% to 55%) and 52% (95% CI, 39% to 66%), respectively, in the R1 and R2 groups, with a median follow up of 54 months from diagnosis and 23 months from autograft. The median follow up from diagnosis of HD were 29, 47, 58, and 102 months for PRD, PSD, R1, and R2 groups, respectively. These survival rates are similar to those reported in the literature.5-8 After ASCT, patients were monitored at each center in which the diagnosis of secondary cancer was made. MDS/AML diagnosis was made after evaluation of peripheral blood, bone marrow aspirate, and core biopsy, and was based on the French-American-British (FAB) criteria. In five of eight cases, cytogenetic analysis was performed using conventional methods after short-term culture.
Ungrafted patients.
Ungrafted patients were identified through a search from the computerized HD databases from the International Database of Hodgkin’s Disease (IDHD), European Organisation for Research and Treatment of Cancer (EORTC), and Groupe d’Etude des Lymphomes de l’Adulte (GELA). The 69 grafted patients from the R2 group were not included in this analysis; they could not be matched because the date of the second or subsequent relapse is not available in the IDHD, EORTC, and GELA databases.
Three ungrafted patients were randomly matched with one grafted patient on category (PSD, PRD, R1), age at HD diagnosis, gender, clinical stage (I-II or III-IV), presence or absence of B symptoms, and follow up at least as long as the interval between the end of first therapy and the date of graft of the reference case. For few grafted patients, it was difficult to find three controls. The difference accepted between grafted patients and their controls was 3 years for age. If no ungrafted patient satisfied this requirement, the patient who was the closest was accepted as control. Age, gender, clinical stage, and B symptoms were chosen because they were generally used to select patients eligible for a given treatment. The year of HD diagnosis was not considered for matching because most ungrafted patients were diagnosed during the 1977 to 1990 period. Among grafted patients, five could not be used for matching because one of the above matching criteria was not available; these five patients belong to the PRD (three patients) and the PSD (two patients) groups. Overall, 1179 ungrafted patients were matched with the 393 grafted cases, 258 (13 from the EORTC, 245 from the IDHD) in the PRD group, 300 (57 from the EORTC, 44 from the GELA, 199 from the IDHD) in the PSD group and 621 (64 from the EORTC, 11 from the GELA, 546 from the IDHD) in the R1 group.
Statistical analysis.
Follow up began with the date of end of first HD treatment or the date of ASCT (as appropriate) and ended at the date of death, date of second cancer, or last examination, whichever came first. The cancers observed were classified by site and histologic type in accordance with the oncology section of the International Classification of Diseases.16 Two approaches were used to analyze the risk of secondary cancer after HDCT and ASCT. In the first approach, the cumulative incidence rate of secondary cancer since the end of last treatment (initial treatment or that given for salvage) or graft was calculated using Kaplan-Meier estimates.17 The Rothman method was used to calculate the CI of the rates.
In the second method, the relationship between the occurrence of secondary cancer and concomitant variables was calculated by means of the Cox proportional-hazards model,18 using a backward, stepwise regression. This model was used twice, once among the grafted population (internal comparison) and a second time when comparing grafted patients to those treated with conventional therapy (external comparison). Among the grafted patients, the variables tested were gender, age at diagnosis (20-39, ≥40 v <20), clinical stage (III-IV v I-II), B symptoms, splenectomy, type of initial treatment (chemotherapy, combined modality therapy virradiation), type of initial chemotherapy (≥3 MOPP, <3 MOPP, Adriamycin-containing, others v no chemotherapy), type of radiotherapy (limited field, extended field v no irradiation), response to initial treatment (CR v no CR), age at ASCT (20-39, ≥40 v <20), type of all chemotherapy received before graft chemotherapy (≥3 MOPP, <3 MOPP, Adriamycin-containing, othersv no chemotherapy), disease status before graft (CR vno CR), interval between diagnosis and ASCT, conditioning regimen (TBI-containing, BEAM [carmustine 300 mg/m2, etoposide 1,200 mg/m2, cytosine arabinoside 1,200 mg/m2, melphalan 140 mg/m2], CBV [cyclophosphamide 6,000 mg/m2, carmustine 300 mg/m2, etoposide 1,200 mg/m2] v others), source of stem cells (bone marrow or PBSC), platelet recovery (> 105/μL before or after 100-days postgraft), and indication for ASCT (PRD, PSD, R1, or R2). Variables significantly (P < .05 with logrank test) linked with second cancer risk were included in the Cox model.
Among the overall series of grafted and ungrafted patients, the variables tested were gender, age at last treatment or graft (≥40v <40), clinical stage (III-IV v I-II), B symptoms, splenectomy, type of overall chemotherapy administered (≥3 MOPP, <3 MOPP, Adriamycin-containing, others v no chemotherapy), type of overall irradiation given (limited field, extended field v no irradiation), and patient subgroup (PRD, PSD, or R1). Variables significantly (P < .05 with logrank test) linked with second cancer risk were included in the Cox model.
RESULTS
Grafted patient characteristics.
The characteristics of patients and ASCT are listed in Table 1. B symptoms were more often present in the PRD and PSD groups (78% and 85%, respectively) than in the R1 and R2 groups (59% and 58%, respectively). Fifty-one percent of stage-II patients exhibited B symptoms, compared with 69% of stage-III patients and 92% of stage-IV patients. First-line chemotherapy consisted in ≥3 MOPP (21%), <3 MOPP (51%), Adriamycin-containing (23%), and others (5%). Forty-two percent had no initial radiotherapy, 33% had limited-field radiotherapy, and 25% had extended-field radiotherapy. Three percent received irradiation alone and 55% combined-modality therapy. The median duration of initial treatment was 7 (1 to 16), 6 (2 to 14), 8 (1 to 20), and 8 (1 to 17) months in PRD, PSD, R1, and R2 groups, respectively. After this first-line treatment, the patients of the PRD, R1, and R2 groups received additional rescue treatments; cumulative treatments (first-line and salvage) received by all patients before ASCT are reported in Table 1. The median duration between the end of the first-line treatment and ASCT was 13 months: 6.8 (0 to 34), 2.6 (0 to 30), 31 (4 to 146), and 76 (14 to 241) months for the PRD, PSD, R1, and R2 groups, respectively.
Five percent of the patients received bone marrow and PBSC as the source of stem cells, and were pooled for the analysis with patients receiving PBSC alone. Indications for HDCT were equally distributed in these two groups (Bone marrow: PSD 23%, PRD 19%, R1 41%, and R2 17%; PBSC: PSD 24%, PRD 17%, R1 49%, and R2 10%). Hematopoietic growth factors (HGF) were commercially available in France since January 1992; therefore, PBSC-containing grafts represented 16% of the transplant procedure before 1992 and 68% after. In the PBSC-containing group, 25% of the patients were grafted before 1992 and did not receive HGF.
ASCT-related toxicity and causes of death.
Overall, 158 (34%) patients have died. HD was the main cause of death in all patient groups, followed by toxicity related to the HDCT (Table 2). Thirty-seven patients died of complications related directly to the procedure, accounting for 23% of all causes of death. Thirty-two of these patients died within the first 3 months after transplant and 5 (4 sepsis and 1 pneumopathy) died more than 3 months after transplant. Infections (especially pulmonary infections) and shock were the main causes of toxic death. The etiology of the shock was seldom available, but was presumably septic in the most cases. One patient died of a renal tubular acidosis, whereas 2 committed suicide just after the ASCT and were regarded as toxic deaths related to the procedure. Toxic deaths were less frequent in the PSD patients compared with other patients (3% v 9%,P = .037). However, most PSD patients were grafted in 1989 or later at a time in which hematological resuscitation has become efficient.
One patient in the PSD group died of second cancer (myelodysplasia), compared with five (myelodysplasia 1, acute nonlymphoblastic leukemia 1, NHL 1, solid tumor 2: lung and central nervous system) in the R1 group and one (acute nonlymphoblastic leukemia) in the R2 group.
Secondary cancers among grafted patients.
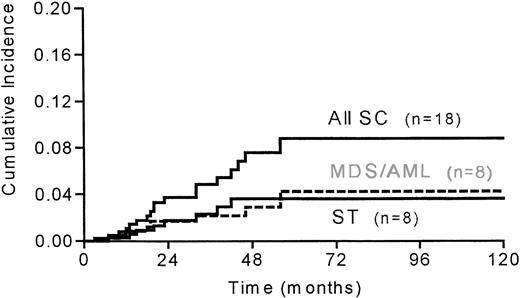
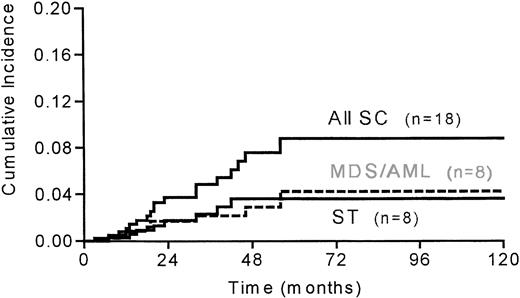
In the 467 patients, 18 secondary cancers occurred: 8 MDS/AML, 2 NHL, and 8 solid tumors 51 months (10 to 134) since diagnosis and 21 months (3 to 43) since graft. There were 4 refractory anemia with an excess of blasts, 1 refractory anemia, 1 case of AML (M2 according to FAB classification), 2 cases of unclassifiable AML, 3 lung cancers, 1 pleural carcinoma, 1 breast cancer, 1 pharyngeal cancer, 1 central nervous system (CNS) carcinoma, and 1 vulva epithelioma. No second cancer developed in the PRD group, 5 (3 MDS and 2 solid tumors) developed in the PSD group, 10 (1 MDS, 2 AML, 1 NHL, and 6 solid tumors) developed in the R1 group and 3 (1 MDS, 1 AML, and 1 NHL) in the R2 group. Cytogenetic analysis performed in 5 of the 8 AML/MDS was noninformative (ie, no mitosis) in 2 patients, and showed partial or complete deletion of chromosome 7 in 3 patients; 11q23 rearrangement was never observed. Two solid tumors (one pleural and one lung carcinoma) developed within the radiation field, two (one breast and one pharyngeal carcinoma) were borderline and four were outside the field. The overall 5-year cumulative incidence of secondary cancer was 8.9% (95% CI, 5.4% to 14.3%). The 5-year cumulative incidence was 4.3% (95% CI, 1.9% to 9.3%) for MDS/AML and 3.7% (95% CI, 1.8% to 7.3%) for solid tumors (Fig 1).
Cumulative incidence of all second cancers (SC), myelodysplasia/acute myeloid leukemia (MDS/AML) and solid tumors (ST) after ASCT in 467 HD patients (PSD, PRD, R1, and R2 groups) with a median follow up of 21 months since ASCT.
Cumulative incidence of all second cancers (SC), myelodysplasia/acute myeloid leukemia (MDS/AML) and solid tumors (ST) after ASCT in 467 HD patients (PSD, PRD, R1, and R2 groups) with a median follow up of 21 months since ASCT.
Six of the seven patients who were grafted in 1992 or after (among 190 patients) and who developed a second cancer (1 MDS, 1 NHL, and 4 solid tumors) have received hematopoietic growth factors at any time before second cancer occurred.
Risk factors for secondary cancer in the grafted population.
The following variables were included in the Cox model: age at graft, gender, B symptoms, stage (I-II v III-IV), splenectomy, radiotherapy, type of chemotherapy, high-dose chemotherapy, platelet recovery, source of graft, and time between diagnosis and graft. Taking all the secondary cancers into account, two risk factors were identified by multivariate analysis: age ≥ 40 years (RR = 3.73 with 95% CI, 1.43-9.67; P = .007) and the use of PBSC-containing graft (RR = 3.1 with 95% CI, 1.11-8.62; P = .03). There were only 4% of the 467 grafted patients who received bone marrow and PBSC as a source of stem cells; for the purpose of this analysis, these patients were pooled with those who received PBSC only. The increased risk associated with PBSC was unchanged when the analysis was restricted to patients who received bone marrow or PBSC only. The relative risk associated with splenectomy was 2.56 (95% CI, 0.89-7.36;P = .08). An analysis restricted to MDS/AML showed that splenectomy (RR = 3.86 with 95% CI, 0.91-16; P = .067) and PBSC-containing graft (RR = 3.73 with 95% CI, 0.87-16;P = .077) were at the limit of significance. No risk factor was identified for solid tumors, and the analysis was not performed for NHL because of the low number of cases.
Comparison with conventionally-treated HD patients.
The characteristics of the matched grafted and ungrafted patients are described in Table 3. Most of the grafted patients were diagnosed in the nineties (65%) whereas conventionally-treated patients were mostly diagnosed in the seventies (48%) and eighties (43%). This explains the differences between the two groups for splenectomy (10% v 32%), type of chemotherapy (Adriamycin containing: 18% v 1%), duration of follow up (21 months v 31 months), and increased death rate (33% v44%; P < .001 for each). HDCT led to an increase in toxic death (22% v 5%; P< .001) and deaths due to intercurrent disease were more frequent in the conventionally-treated group because of the longer follow up. Frequent irradiation after HDCT, either for consolidation or at relapse, was responsible for the more extensive irradiation of the grafted patients (extended field: 42% v 27%).
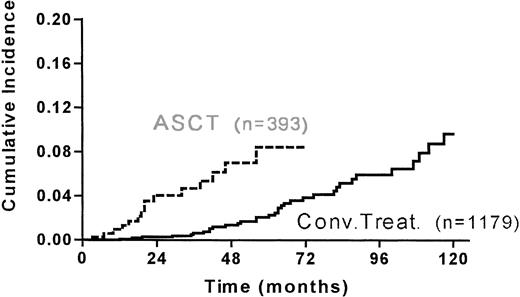
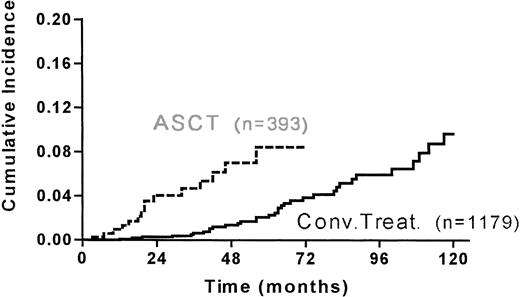
Six MDS/AML and 8 solid tumors occurred in the grafted group and 18 MDS/AML and 15 solid tumors occurred in the conventionally-treated group. The median duration after last treatment for occurrence of second cancer was shorter (28 months v 50 months) in the grafted group (Table 4). The 5-year cumulative incidence of second cancer was 10.4% (95% CI, 5.8-18.5) and 5.2% (95% CI, 3.4-7.7) for the grafted and ungrafted group, respectively (Fig 2.).
Cumulative incidence of second malignancies in the matched 393 grafted and 1179 conventionally treated HD patients (PSD, PRD, R1) since ASCT or last treatment.
Cumulative incidence of second malignancies in the matched 393 grafted and 1179 conventionally treated HD patients (PSD, PRD, R1) since ASCT or last treatment.
In multivariate analysis, four factors significantly correlated with an increased risk of second cancer in this whole population of 393 grafted and 1179 ungrafted patients (external analysis): age ≥ 40 (P< .001), relapse (P = .006), PRD (P = .033), HDCT (P = .024) (Table 5).
DISCUSSION
Since the initial suggestion by Crosby11 in 1969 that acute leukemia developing after the cure of HD could be related to the therapy, MDS/AML and solid tumors secondary to conventional chemotherapy and irradiation have been recognized as a complication of HD treatment. Alkylating agents,21 radiation therapy,22 combined modality therapy,23 and splenectomy24 have all been implicated in the development of these poor-prognosis tumors. The risk of MDS/AML is limited to a finite period of time and is at its maximum in the 5- to 9-year period of follow up. For solid tumors, the period of risk is more prolonged, even if it does seem to be a decrease in the relative risk in males after 15 years.12 25
We reported in the IDHD study that after conventional treatment, age, treatment, and gender were risk factors for all second cancer; for MDS/AML, age and combination chemotherapy with or without radiotherapy were risk factors; for NHL, age, gender, and relapse treatment were risk factors; for solid tumors, age and extended radiotherapy were risk factors.13
Myeloablative therapy with ASCT is increasingly used as part of the treatment of HD in various clinical situations, eg, for induction failure patients, after a first or subsequent relapse and also as part of the initial treatment for high-risk patients.2,3,4,8 Two recent series reported the occurrence of MDS/AML after ASCT for lymphoid malignancies9 10 but, up to the present time, there has been little data available on the incidence, timing, and risk factors for the development of MDS/AML and solid tumors after ASCT for HD alone. Whether HDCT with stem-cell support is by itself responsible for an increase in the incidence of secondary cancer after HD is unknown.
Although the 467 grafted patients included in the study were drawn from major French centers with expertise in HDCT, their characteristics and survival are not different from those of patients from other groups.5,26,27 The rather high proportion of B symptoms in our series results from the large number of PSD (mostly stage IIIB-IVB) and PRD patients (78% of B symptoms at diagnosis). The 4.3% 5-year cumulative incidence rate of secondary MDS/AML in our series is similar to the 4% reported by Darrington,9 but is somewhat lower than the 18% ± 9% 6-year actuarial risk reported by Stone et al.10 This latter study reported on NHL patients who were all administered TBI, and the definition used for myelodysplasia differed from that of the FAB classification used by Darrington9 and our group. In most series, however, the follow up of grafted patients has still only run for a short time and it is likely that the peak incidence of MDS/AML (5-9 years) or solid tumors (up to 15 years) has not been reached, suggesting that the incidence could still increase. The presence of the groups of PRD and PSD patients gave us an opportunity to analyze patients with short treatment duration before ASCT and brief intervals between HD diagnosis and ASCT, and to compare them with patients grafted after relapse.
The incidence of MDS/AML and solid tumors in the briefly treated group of PSD patients is, so far, not different from that of the R1 patients group. The reduction of the amount of mutagenic agents using up-front transplant may not have been sufficient to significantly improve the risk of secondary cancers.
No secondary cancer was observed in the PRD group, mainly a consequence of the poor outcome of these patients (35% 5-year survival) and their short follow up (29 months). Consequently, among the 467 grafted patients (internal analysis), the disease status was not a significant risk factor. However, when the matched 393 grafted and 1179 ungrafted patients were analyzed (external analysis), the disease status (PRD) with 3 second cancers in the ungrafted group emerged as a weak risk factor (P = .033).
Age and splenectomy were found to be risk factors for the development of secondary cancer after HDCT, after conventional chemotherapy, and irradiation.28,29 The risk of secondary MDS/AML is related to the number of cycles of MOPP administered14 in conventionally treated patients. This risk factor was not found to be significant in our grafted group. The number of secondary MDS/AML is small and may reflect our small median follow up that is far above the 10-year period of risk for secondary MDS/AML. Another hypothesis is that the cumulative dose of mutagenic agents received by all patients is high and may obliterate the role of MOPP exposure.
A major surprise was a higher incidence of secondary cancers observed in patients receiving PBSC-containing graft. Bhatia et al30recently reported a higher cumulative probability of MDS/AML in pooled NHL and HD patients grafted with PBSC compared with bone marrow. Traweek et al31 have also reported a trend towards a decreased risk of cytogenetic abnormalities after HDCT with the use of bone marrow alone or in combination with PBSC, compared with PBSC alone. The reasons for such observations are still unclear. First, indications for HDCT were similar in PBSC and bone marrow groups. Therefore, the higher risk in patients who received PBSC does not reflect a higher proportion of PSD patients with better survival and prolonged exposure to the risk of developing a secondary cancer. Second, HGF are commercially available in France since January 1992 and their administration correlates with the increased use of PBSC. In our series, 16% of the patients grafted before January 1992 received PBSC compared with 68% after this date. It can be speculated that widespread use of HGF, rather than PBSC, could be the risk factor associated with a higher incidence of second cancer. However, only 6 of the 18 patients who developed a second cancer have received HGF at any time between the diagnosis of HD and the diagnosis of their second cancer and among those 6, 4 developed a solid tumor that is unlikely to be related to the use of HGF. Third, prolonged immune suppression is associated with an increased risk of malignancy, because it is observable after organ transplantation or in HIV-infected patients. The difference between bone marrow and PBSC could therefore reflect a difference in immune depression after HDCT. However, PBSC graft contains more natural killer (NK) and T cells and leads to a faster hematopoietic recovery than does bone marrow; the immune reconstitution when studied is at least comparable.32
The small number of events and the, as yet, brief follow-up period mean that we can assign only limited significance to this risk factor. The evidence certainly does not entitle us to advocate discontinuation of the use of PBSC as a source of stem cells in this situation. Recent studies33,34 35 comparing PBSC and bone marrow in lymphoid malignancies have shown numerous advantages for PBSC but did not report an increase in second cancer. Further randomized trials comparing primed and unprimed bone marrow may help to clarify this question but a large number of patients will be necessary because of the low rate of events and the long necessary follow up.
Most of the previous work on secondary malignancies after ASCT has focused on MDS/AML, and the available evidence suggests that the pretransplant therapy, rather than myeloablation, is the culprit. Time from the initial therapy to the MDS/AML development, prolonged exposure to chemotherapy (ie, alkylating agents), and the fact that MDS is only rarely observed after allogenic BMT all support this hypothesis. The results from this study comparing grafted to conventionally-treated patients also suggest that chemotherapy-induced myeloablation does not, by itself, increase the risk of MDS/AML. However, the more rapid onset of solid tumors after HDCT does lead to an increased risk of second cancer for the grafted population. Solid tumors, a complication known to be associated with radiotherapy, are not due to more extensive irradiation in the grafted group because this treatment modality is not a significant risk factor in our multivariate analysis. Whether this increased risk reflects differences in the immune status of grafted and ungrafted patients or is merely a reflection of the smaller number of events is unknown and merits further investigation through a careful and prolonged follow up of these patients. However, a recent retrospective study36 showed an early increased RR of solid tumors after conventional chemotherapy for HD (1.4, 95% CI = 1.1 − 1.8). Chemotherapy was associated with an early increased risk of cancers of the bones, joints, articular cartilage, and soft tissues (RR = 6.0, 95% CI = 1.7 − 20), and cancers of the female genital system (RR = 1.8, 95% CI = 1.1 − 3.2). A statistically significant increase in the risk of respiratory and intrathoracic tumor was also observed in the first 5 years after chemotherapy (RR=2.2; 95% CI = 1.1-4.3).
Even in the absence of randomized trials, ASCT remains required for some HD patients, and, because the procedure is safe and has minor effects on the incidence of secondary malignancies, more data on the incidence and etiology of these complications is required before it would be reasonable to alter our current therapeutic approaches. However, the occurrence of secondary malignancies after HDCT is now well established and should be discussed with the patient as a potential late complication when obtaining informed consent before transplant.
ACKNOWLEDGMENT
We are indebted to Dr P. Biron, Dr H. Tilly, Dr A. Stamatoulas, Dr J.P. Jouet, J. Beaune, T. Gargi, S. Cournier, H. Desmorat, M. Bataille, and A-S. Woronoff for facilitating our access to the necessary data. We thank Dr G. Socié for fruitful discussion and encouragement during this work.
M.A. was supported by the Collège de Médecine des Hôpitaux de Paris and by the Centre Hospitalier Notre-Dame et Reine Fabiola in Charleroi, Belgium. This study was also supported by a grant from the Fondation Contre la Leucémie.
Address correspondence to Prof Christian Gisselbrecht, Hematology Institute, Hôpital Saint-Louis, 1 Avenue C, Vellefaux, 75010 Paris, France.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.