Abstract
Rituximab, a chimeric monoclonal antibody that binds specifically to the CD20 antigen, induced objective responses in 50% of patients with low-grade or follicular B-cell lymphoma. Because most nonfollicular B-cell lymphomas also express the CD20 antigen, we conducted a phase II study to evaluate the efficacy and tolerability of this new agent in patients with more aggressive types of lymphoma. Patients with diffuse large B-cell lymphoma (DLCL), mantle cell lymphoma (MCL), or other intermediate- or high-grade B-cell lymphomas according to the Working Formulation were included in this prospective randomized phase II study if they were in first or second relapse, if they were refractory to initial therapy, if they progressed after a partial response to initial therapy, or if they were elderly (age >60 years) and not previously treated. The patients received 8 weekly infusions of rituximab at the dose of 375 mg/m2 in arm A or one infusion of 375 mg/m2 followed by 7 weekly infusions of 500 mg/m2 in arm B. Patients were evaluated 2 months after the last rituximab infusion. Fifty-four patients were randomized from 9 centers in Europe and Australia (28 in arm A and 26 in arm B). A total of 5 complete responses (CR) and 12 partial responses (PR) were observed among the 54 enrolled patients, with no difference between the two doses. In an intent-to-treat analysis, the CR rate was 9% (CI95%, 3% to 20%) and the PR rate was 22% (CI95%, 12% to 36%), for an overall response rate of 31% (CI95%, 20% to 46%). An analysis of prognostic factors showed that response rates were lower in patients with refractory disease, patients with lymphoma not classified as DLCL, and patients with a tumor larger than 5 cm in diameter. DLCL and MCL patients had response rates of 37% and 33%, respectively. The median time to progression exceeded 246 days for the 17 responding patients. The most frequently reported adverse events were related to an infusion syndrome and were mild: 19% of the patients had a grade 3 related adverse event, slightly more in arm B, and only 1 patient had a grade 4 related adverse event in arm A. Two patients (3.7%) withdrew from treatment because of severe adverse events, one patient in each arm. In this first trial of rituximab in DLCL and MCL, patients experienced a significant clinical activity with a low toxicity. Rituximab has significant activity in DLCL and MCL patients and should be tested in combination with chemotherapy in such patients.
© 1998 by The American Society of Hematology.
RITUXIMAB IS A chimeric anti-CD20 monoclonal antibody containing human IgG1 and κ constant regions with murine variable regions.1 The antilymphoma effects of rituximab are probably due to complement and antibody-dependent cell-mediated cytoxicity,2 inhibition of cell proliferation, and induction of apoptosis.3 In phase I studies, rituximab induced a rapid depletion of CD20+normal B cells and lymphoma cells.1 Phase I trials of single doses up to 500 mg/m2 and of four weekly doses of 375 mg/m2 demonstrated clinical responses with no dose-limiting toxicity in low-grade or follicular lymphoma patients.4 In a phase II trial, four weekly infusions of 375 mg/m2 induced responses in 17 (50%) of 34 evaluable low-grade or follicular lymphoma patients, with a median time to progression of 10.2 months.5 Side effects were associated with the first rituximab infusion and usually were mild to moderate. In a recently reported large pivotal phase II study in 166 patients with low-grade or follicular lymphoma, objective response was reported for 76 (50%) of 151 evaluable patients and side effects were identical to those previously described.6 Previous experience with rituximab in patients with large B-cell lymphoma is very limited, with fewer than 12 patients having been included in the early phase I-II studies. However, nonfollicular B-cell lymphoma cells resemble follicular or small lymphocytic lymphoma cells in their expression of the target CD20 antigen and therefore may also respond to rituximab therapy.
We designed this phase II trial in patients with aggressive B-cell lymphomas, ie, intermediate- and high-grade lymphomas according to the Working Formulation, to evaluate the clinical efficacy and toxicity of rituximab monotherapy in these patients.
MATERIALS AND METHODS
Patients.
Patients were eligible for inclusion in this study if they had intermediate- or high- grade non-Hodgkin’s lymphoma (NHL) according to the Working Formulation,7 subtypes D to H. Patients were required to have progressive disease either after one or two prior chemotherapy regimens or were more than 60 years of age without any prior therapy. Confirmation that the lymphoma cells expressed CD20 antigen was required, as was the presence of measurable disease in at least one site not previously irradiated. Bone lesions, ascites, and pleural effusions were not considered measurable. Patients were required to be more than 18 years of age, ambulatory (Karnosfky score ≥70%), and must have given written informed consent. Patients were not included if they had other NHL subtypes, had a total doxorubicin dose greater than 400 mg/m2, had prior radioimmunotherapy for this lymphoma, had a history of other cancer, had major surgery 4 weeks before the study, had clinically significant cardiac disease or myocardial infarction during the 6 months before the study, had abnormal liver or renal functions not related to lymphoma, had active opportunistic infection, had human immunodeficiency virus (HIV)-positive serology, were HBsAg positive, or had HBc or HCV antibodies. The study was conducted according to the principles of the Declaration of Helsinki and had been approved by the local Institutional Review Boards or Ethics Review Committees according to local regulations.
Study design.
The study was an open-label, randomized, phase II trial to evaluate the clinical efficacy of rituximab, as defined by the response rate, and safety. Patients were randomized to receive weekly intravenous doses of rituximab at either regimen A (375 mg/m2 once weekly for 8 weeks) or regimen B (375 mg/m2 on day 1 followed on day 8 by 500 mg/m2 once weekly for 7 weeks). These two doses were chosen because the dose of 375 mg/m2 was the dose found to be effective and well tolerated in patients with indolent NHL,4 whereas the dose of 500 mg/m2 was the highest dose tested in phase I trials.1 Patients were allocated to treatment groups by a central randomization. The randomization was stratified between previously treated and previously untreated patients. Rituximab was administered intravenously in an outpatient setting through a peripheral or central intravenous line. Infusion was started at an initial rate of 50 mg/h. If no toxicity was observed during the first hour, the dose rate was escalated by increments of 50 mg/h every 30 minutes to a maximum of 300 mg/h. If the starting dose of rituximab was well tolerated, the starting flow rate for the administration of the second and subsequent infusions was fixed at 100 mg/h with similar increments at 30 minute intervals up to 400 mg/h. The infusion was interrupted if severe fever, rigors, edema or mucosa congestion, hypotension, or any other serious adverse events occurred. After the events resolved, the infusion was to be resumed at half the previous rate. The dose was not modified throughout the treatment period. It was recommended that patients at risk of tumor lysis syndrome receive appropriate hydration and allopurinol (300 mg/d). Treatment with corticosteroids or other chemotherapeutic agents was not permitted. Administration of oral premedication with acetaminophen at 1,000 mg and diphenhydramine hydrochloride at 50 to 100 mg was recommended 30 to 60 minutes before each infusion.
Evaluation of the tumor burden and involved sites was performed before treatment, at weeks 5 (before the fifth dose of rituximab), 9 (end of the treatment), 12, and 16 to evaluate the treatment efficacy and its persistence 2 months after the end of treatment. Patients with progressive disease (PD) during or after treatment were taken off the study. Patients with stable disease (SD) at the end of treatment (week 9) were also taken off the study and were permitted alternative treatment at the discretion of their local physician. Patients achieving a partial response (PR) or complete remission (CR) were followed-up until week 16 and were then allowed to go off study and to receive further treatment at the discretion of their local physician. Safety assessments were performed for all patients for a minimum of 8 weeks after the last infusion of rituximab. Adverse events were graded according to the World Health Organization score.
Endpoints.
The primary efficacy endpoint was the objective response rate, ie, the proportion of patients achieving either a CR or a PR at any time during the study. Response was assessed by the investigator, using the standard World Health Organization criteria, and confirmed by the sponsor based on reported tumor dimensions at each assessment. PR was defined as a decrease equal to or greater than 50% in the sum of the products of perpendicular diameters for each target lesion (SPD). CR was defined as the disappearance of all lesions, but the presence of residual lymph nodes measuring less than 1 × 1 cm on computer tomography (CT) scans was regarded as consistent with an assessment of CR. A PD was defined as any occurrence of a new lesion or an increase of 25% or more in the size (product of perpindicular diameters) of any target lesion. A SD was defined as no change in SPD or a change not corresponding to PR or PD. Secondary efficacy parameters were time to response and time to progression. Time to response was defined as the time from the first infusion to the date of the maximum response. Time to progression (TTP) was defined as the time from the first infusion to the date of disease progression. Patients receiving any other treatment at the end of the study were considered to be in PD at the time of this new therapy for purposes of this analysis. Patients with no report of PD or further therapy were censored from the analysis of TTP as of their off-study date or the date of last progression-free follow-up, whichever was the earliest. This study was designed with a short follow-up (8 weeks after the treatment for a total of 16 weeks) to allow all patients, regardless their response status, to receive further therapy at their physician’s discretion. Thus, the trial was only designed to evaluate tumor response, rather than time-dependent variables such as TTP or response duration. However, available data for TTP and response duration are presented here to provide an estimate of the extent of patient benefit resulting from rituximab therapy.
Response rates with 95% confidence intervals (CI) are reported as an intent-to-treat analysis. TTP and corresponding 95% confidence limit estimates were computed using the standard Kaplan-Meier method of the SAS lifetest procedure. The planned sample size was adequate to determine, with 95% confidence, whether the true response rate was equal to or greater than 30%.
RESULTS
Patient characteristics.
Fifty-four patients were enrolled in the study from 9 centers in a 6-month period (September 1996 to March 1997). One patient randomized to group B received treatment A and has therefore been analyzed as part of group A, leaving 28 patients in arm A (350 mg/m2) and 26 patients in arm B (350 mg/m2, then 500 mg/m2). The characteristics of these patients at the time of their entry in the study as well as their prior lymphoma history are presented in Table 1. Thirty patients (56%) had diffuse large B-cell lymphoma (DLCL) according to the REAL Classification8 and 13 patients (24%) had a mantle cell lymphoma (MCL). One patient had a follicular large-cell lymphoma. The disease could not be precisely categorized in the REAL classification for the other 10 patients, but these patients were classified as intermediate grade in the Working Formulation (6 patients with subtype G and 4 with subtype H). Only 9 patients (17%) were previously untreated, 17 (31%) were in first relapse, 5 (9%) in second relapse, and 23 patients (43%) were in progressive disease after failure or partial response to their first chemotherapy regimen. Nine (17%) enrolled patients had been treated with intensive therapy and autologous stem cell transplantation (ABMT). Demographic and disease characteristics were well balanced between the two groups, except for a slight excess of patients in second relapse or who had received ABMT in group A and for a slight excess of MCL in group B.
Characteristics of the 54 Patients Included in the Trial at Time of Study Entry
| Variable . | Group A 375 mg/m2 (n = 28) . | Group B 500 mg/m2 (n = 26) . |
|---|---|---|
| Histology at diagnosis (REAL classification) | ||
| DLCL | 17 (61%) | 13 (50%) |
| MCL | 4 (14%) | 9 (35%) |
| FL | 1 (4%) | — |
| NOS | 6 (21%) | 4 (15%) |
| Mean time from diagnosis to study (SD) | 2.2 (2.37) | 1.6 (1.46) |
| Prior lymphoma history | ||
| First relapse | 8 (29%) | 9 (35%) |
| Second relapse | 4 (14%) | 1 (4%) |
| Primary refractory | 4 (14%) | 5 (19%) |
| PD after PR1 | 7 (25%) | 7 (27%) |
| Untreated | 5 (18%) | 4 (15%) |
| Prior ABMT | 6 (21%) | 3 (12%) |
| Median time from ABMT to study (mo) | 5 | 26 |
| Median age at study entry (range) | 62.5 (20-83) | 65 (32-86) |
| Karnofsky performance status | ||
| 90-100 | 16 (57%) | 13 (50%) |
| 70-80 | 12 (43%) | 13 (50%) |
| Median measurable tumor area in cm2 (range) | 25.4 (2-120) | 33 (5-374) |
| Variable . | Group A 375 mg/m2 (n = 28) . | Group B 500 mg/m2 (n = 26) . |
|---|---|---|
| Histology at diagnosis (REAL classification) | ||
| DLCL | 17 (61%) | 13 (50%) |
| MCL | 4 (14%) | 9 (35%) |
| FL | 1 (4%) | — |
| NOS | 6 (21%) | 4 (15%) |
| Mean time from diagnosis to study (SD) | 2.2 (2.37) | 1.6 (1.46) |
| Prior lymphoma history | ||
| First relapse | 8 (29%) | 9 (35%) |
| Second relapse | 4 (14%) | 1 (4%) |
| Primary refractory | 4 (14%) | 5 (19%) |
| PD after PR1 | 7 (25%) | 7 (27%) |
| Untreated | 5 (18%) | 4 (15%) |
| Prior ABMT | 6 (21%) | 3 (12%) |
| Median time from ABMT to study (mo) | 5 | 26 |
| Median age at study entry (range) | 62.5 (20-83) | 65 (32-86) |
| Karnofsky performance status | ||
| 90-100 | 16 (57%) | 13 (50%) |
| 70-80 | 12 (43%) | 13 (50%) |
| Median measurable tumor area in cm2 (range) | 25.4 (2-120) | 33 (5-374) |
Abbreviation: NOS, not otherwise specified.
Treatment and response rate.
Eighteen patients (33%) did not complete the 8-week treatment course (10 in group A and 8 in group B) due to PD in 14 (26%) patients (8 in arm A and 6 in arm B); adverse events in 2 (4%) patients (1 in each arm); personal reasons in 1 patient; and investigator judgement in 1 patient. Thirty-six patients (67%) completed the 8-week treatment, 14 of them being withdrawn from the study before the last scheduled visit at week 16 (7 in arm A and 7 in arm B). Eight of these 14 withdrawn patients had PD (5 in arm A and 3 in arm B); 4 had stable disease (2 in each arm); and the reason was investigator judgement in 1 patient and loss of follow-up in 1 patient. This last patient had PD later, after week 16. The median time to progression in these patients was 4 weeks (range, 4 to 56 days). Twenty-two patients (41%), 11 in arm A (39%) and 11 in arm B (42%), completed the study at the last visit of week 16.
Tumor response was assessed by the investigator and was reassessed and confirmed by the sponsor. Two patients were not evaluable for response: 1 patient was taken off study immediately after the first infusion because of an adverse event and 1 patient did not have a CT scan performed at baseline and had lung localization at the CT scan performed after week 4. This patient had a PR in all baseline sites. According to the sponsor’s confirmatory assessment, there were 5 CR (4 in arm A and 1 in arm B) and 12 PR (5 in arm A and 7 in arm B). Although there were slightly more CRs in arm A than in arm B, the overall response rate was not statistically significantly different between the two groups (32% and 31% in the intent-to-treat analysis for arms A and B, respectively). Therefore, a pooled analysis of both groups was performed. The overall response rate was 31% (CI95%, 20% to 46%) in the intent-to-treat analysis and 33% (CI95%, 20% to 47%) for the 52 evaluable patients (Table 2).
Response Rate and 95% Confidence Limits
| . | CR . | PR . | All Responses . |
|---|---|---|---|
| Responders | 5 | 12 | 17 |
| Intent-to-treat analysis (n = 54) | |||
| Response rate | 9% | 22% | 31% |
| CI95% limits | 3-20% | 12-36% | 20-46% |
| Evaluable patients (n = 52) | |||
| Response rate | 10% | 23% | 33% |
| CI95% limits | 3-21% | 13-37% | 20-47% |
| . | CR . | PR . | All Responses . |
|---|---|---|---|
| Responders | 5 | 12 | 17 |
| Intent-to-treat analysis (n = 54) | |||
| Response rate | 9% | 22% | 31% |
| CI95% limits | 3-20% | 12-36% | 20-46% |
| Evaluable patients (n = 52) | |||
| Response rate | 10% | 23% | 33% |
| CI95% limits | 3-21% | 13-37% | 20-47% |
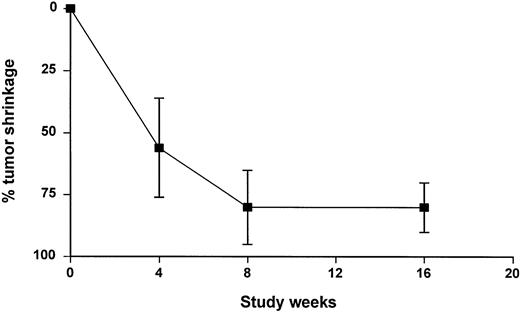
Despite a dramatic initial tumor shrinkage, some patients achieved their best response late in the course of the study (Fig 1). The median time to response was 56 days, ranging from 18 to 115 days. The median time to response was slightly shorter in arm B (41.5 days; range, 18 to 90 days) than in arm A (80 days; range, 27 to 115 days).
Parameters associated with response.
In an attempt to identify factors associated with favorable response, an analysis was performed to correlate response rates with patient characteristics at the time of the study and with patients’ prior lymphoma history (Table 3). Patients with DLCL had a 37% response rate and those with MCL had a 33% response rate. Only 1 of the 9 evaluable patients with a lymphoma not reclassified in the REAL classification responded and reached a CR. The other 8 patients had a G or H subtype (4 in each group). This difference is not statistically significant when compared with DLCL and MCL entity (P > .2). Prior lymphoma history influenced the response: patients with no prior treatment and those in first or second relapse were more likely to respond to rituximab, with response rates of 33%, 41%, and 80%, respectively. All patients who achieved a complete response were in their first or second relapse with a CR rate and an overall response rate for relapsing patients of 23% and 50%, respectively. In contrast, the response rate was 22% in patients with primary refractory disease and 8% in those who progressed after a PR to their initial treatment. However, these lower response rates were not statistically significantly different from those of relapsing patients, probably because of the low number of patients in this study. Interestingly, patients previously treated with intensive therapy and ABMT did not have a lower response rate. Tumor burden (as defined by the largest diameter of the largest lesion) at the time of rituximab treatment influenced the response: patients whose largest tumor was less than 5 cm in diameter were more likely to respond (46%) than patients whose largest tumor was greater than 5 cm (17%). No responses were observed in patients whose largest tumor was greater than 10 cm in diameter.
Tumor Response and Patient’s Characteristics
| . | All Patients (n = 52) . | Responders (n = 17) . | % . |
|---|---|---|---|
| Prior lymphoma history | |||
| First relapse | 17 | 7 | 41% |
| Second relapse | 5 | 4 | 80% |
| Primary refractory | 9 | 2 | 22% |
| PD after PR | 12 | 1 | 8% |
| Untreated | 9 | 3 | 33% |
| Histology (REAL classification) | |||
| DLCL | 30 | 11 | 37% |
| MCL | 12 | 4 | 33% |
| Follicular | 1 | 1 | — |
| NOS | 9 | 1 | 11% |
| Prior ABMT | |||
| Yes | 9 | 3 | 33% |
| No | 43 | 14 | 33% |
| Bone marrow involvement | |||
| Yes | 7 | 3 | 43% |
| No | 13 | 2 | 15% |
| Unknown | 32 | 12 | 37% |
| Largest tumor lesion | |||
| <5 cm | 28 | 13 | 46% |
| 5-10 cm | 19 | 4 | 21% |
| ≥10 cm | 5 | 0 | 0% |
| . | All Patients (n = 52) . | Responders (n = 17) . | % . |
|---|---|---|---|
| Prior lymphoma history | |||
| First relapse | 17 | 7 | 41% |
| Second relapse | 5 | 4 | 80% |
| Primary refractory | 9 | 2 | 22% |
| PD after PR | 12 | 1 | 8% |
| Untreated | 9 | 3 | 33% |
| Histology (REAL classification) | |||
| DLCL | 30 | 11 | 37% |
| MCL | 12 | 4 | 33% |
| Follicular | 1 | 1 | — |
| NOS | 9 | 1 | 11% |
| Prior ABMT | |||
| Yes | 9 | 3 | 33% |
| No | 43 | 14 | 33% |
| Bone marrow involvement | |||
| Yes | 7 | 3 | 43% |
| No | 13 | 2 | 15% |
| Unknown | 32 | 12 | 37% |
| Largest tumor lesion | |||
| <5 cm | 28 | 13 | 46% |
| 5-10 cm | 19 | 4 | 21% |
| ≥10 cm | 5 | 0 | 0% |
Abbreviation: NOS, not otherwise specified.
Duration of response.
A long-term follow-up beyond the visit scheduled at week 16 was not planned in the protocol, and patients’ management after the off-study date varied substantially between centers. Consequently, the definition of time to progression was defined conservatively by the date of either a recorded disease progression or an investigator’s decision to begin a new treatment. Only 6 of the 17 responding patients (35%) had progressed at the time of analysis with a progression occurring between day 83 and day 246 after the first rituximab infusion. Because most patients had not progressed and because progression was imputed rather than observed for patients who received further treatment, the conclusion of the TTP analysis is limited to the statement that the median TTP for patients in arm A and arm B exceeded 105 days and 121 days, respectively. When both groups were pooled, the median TTP of the 54 patients enrolled in the study exceeded 105 days (range, 0 to 336+ days). The median TTP for the 17 responding patients exceeded 246 days (range, 83 to 336+ days).
Toxicity.
All patients enrolled in this study received at least 1 infusion of rituximab. Eighteen patients in each arm received the 8 infusions planned in the protocol. The mean total infusion time for the first infusion was approximately 5 hours for patients in both arms, ranging from 0.5 to 10.1 hours. Thereafter, the infusion time decreased in both arms, being slightly longer in arm B (3.7 to 4.5 hours compared with 3.1 to 3.3 hours in arm A). The number of patients requiring an interruption, a slowing of the infusion rate, or a discontinuation was slightly higher in arm B than in arm A (6, 2, and 1, respectively, for arm A patients compared with 14, 4, and 5, respectively, for arm B patients).
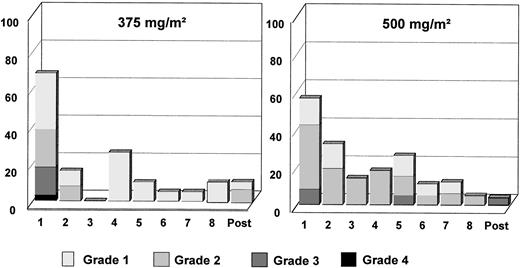
A summary of all adverse events (AE) reported during treatment probably or possibly related to rituximab or with unknown relationship is listed in Table 4. Two and 3 patients did not experience any AE in arm A and arm B, respectively. Altogether, 26 patients experienced 125 AE in arm A and 23 patients experienced 143 AE in arm B. The majority of these events (90% in arm A and 86% in arm B) were of mild to moderate severity. Nine patients (29%) experienced 13 grade 3 or 4 AE in arm A and 9 patients (35%) experienced 20 grade 3 or 4 AE in arm B. The most frequent AE in both groups were in the body as a whole body system. These AE were fever, rigors, hypothermia, and edema. Mild to moderate hypotension was reported in 6 patients (21%) and 3 patients (11%) in arms A and B, respectively. Hypotension was severe only in 1 patient in arm B. The most frequent respiratory AE were dyspnea (21% and 8% of patients in arms A and B, respectively) and coughing (11% in both arms). AE involving the skin were principally increased sweating (18% and 19% of patients in arm A and B, respectively) and pruritus (11% in both arms). In both arms, the majority of AE occurred during or after the first infusion (Fig 2). The frequency of adverse events and their severity decreased for the subsequent infusions. However, more patients in arm B than in arm A experienced AE from the second infusion onwards (Fig 2). Grade 3 and grade 4 AE were predominantly seen during or shortly after the first infusion. Two deaths during the study period were reported, both in arm B, and both were judged to be secondary to the study disease. Before death, both patients had been taken off the study because of progressive disease.
Adverse Events Reported During the Trial Period Possibly or Probably Related to Rituximab or With Unknown Relationship
| . | Arm A (375 mg/m2) . | Arm B (500 mg/m2) . | ||||||
|---|---|---|---|---|---|---|---|---|
| Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . | |
| Any adverse event | 35 | 23 | 4 | 1 | 32 | 41 | 12 | 0 |
| General disorders | 13 | 13 | 3 | 1 | 8 | 8 | 4 | 0 |
| Fever | 4 | 8 | — | — | 4 | 5 | 1 | — |
| Rigors | 3 | 4 | 3 | — | — | 4 | 1 | — |
| Hypothermia | 4 | — | — | — | 4 | — | — | — |
| Any pain | 1 | 1 | — | — | — | 1 | 1 | — |
| Oedema | — | — | — | — | — | — | — | — |
| Anaphylactic shock | — | — | — | 1 | — | — | — | — |
| Cardiovascular, mainly hypotension | 5 | 3 | — | — | 1 | 5 | 1 | — |
| Nervous disorders | 3 | 2 | — | — | 2 | 1 | 1 | — |
| Gastrointestinal disorders | 4 | 1 | — | — | 8 | 8 | — | — |
| Respiratory disorders | 6 | 3 | 1 | — | 6 | 3 | — | — |
| Skin (pruritus) | 2 | — | — | — | 3 | 5 | 1 | — |
| Urinary disorders | 1 | 1 | — | — | — | 1 | — | — |
| Thrombocytopenia | — | — | — | — | — | — | 1 | — |
| Anemia | — | — | — | — | — | 1 | 1 | — |
| Neutropenia | — | — | — | — | — | — | 1 | — |
| . | Arm A (375 mg/m2) . | Arm B (500 mg/m2) . | ||||||
|---|---|---|---|---|---|---|---|---|
| Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . | |
| Any adverse event | 35 | 23 | 4 | 1 | 32 | 41 | 12 | 0 |
| General disorders | 13 | 13 | 3 | 1 | 8 | 8 | 4 | 0 |
| Fever | 4 | 8 | — | — | 4 | 5 | 1 | — |
| Rigors | 3 | 4 | 3 | — | — | 4 | 1 | — |
| Hypothermia | 4 | — | — | — | 4 | — | — | — |
| Any pain | 1 | 1 | — | — | — | 1 | 1 | — |
| Oedema | — | — | — | — | — | — | — | — |
| Anaphylactic shock | — | — | — | 1 | — | — | — | — |
| Cardiovascular, mainly hypotension | 5 | 3 | — | — | 1 | 5 | 1 | — |
| Nervous disorders | 3 | 2 | — | — | 2 | 1 | 1 | — |
| Gastrointestinal disorders | 4 | 1 | — | — | 8 | 8 | — | — |
| Respiratory disorders | 6 | 3 | 1 | — | 6 | 3 | — | — |
| Skin (pruritus) | 2 | — | — | — | 3 | 5 | 1 | — |
| Urinary disorders | 1 | 1 | — | — | — | 1 | — | — |
| Thrombocytopenia | — | — | — | — | — | — | 1 | — |
| Anemia | — | — | — | — | — | 1 | 1 | — |
| Neutropenia | — | — | — | — | — | — | 1 | — |
Fifteen patients experienced a serious AE, 7 (25%) in arm A and 8 (31%) in arm B. When considering the events judged to be related to treatment with rituximab, 3 and 11 events were reported for 3 (11%) and 6 (23%) of patients in arms A and B, respectively. All patients recovered from these AE. Only 2 patients stopped treatment because of a drug-related AE: 1 patient in arm A presented an anaphylactic shock after the first infusion; 1 patient in arm B presented severe rash, arthralgia, fever, and thrombocytopenia after the second infusion. Both patients recovered from the AE. Severe neutropenia was observed in only 1 and 3 patients in arms A and B, respectively. Severe thrombocytopenia was observed in 3 patients in arm B but not in arm A. Severe anemia was reported in only 1 patient in arm A. The most frequent hematologic AE was lymphocytopenia, which was severe in 17 (61%) and 21 (81%) patients in arms A and B, respectively. However, it was present at baseline in the majority of these patients.
An infection was defined as any occurrence of an AE suggestive of an infection and/or any occurrence of therapeutic use of anti-infectious drugs (antibiotics, antivirals, and antifungals). Infections were classified as bacterial, viral, fungal, or unknown. An episode of infection was recorded in 39% and 31% of patients in arms A and B, respectively (Table 5). Five patients experienced a respiratory infection (bronchitis, pneumonitis, pneumonia, and febrile pleural effusion), 2 patients had pharyngitis, and 3 patients had rhinitis. Urinary tract infection occurred in 5 patients, an abscess in 2, and a flu-like syndrome in 2. Conjunctivitis, gastroenteritis, herpes simplex infection,Escherichia coli septicemia, candida mucositis, and arthritis occurred in 1 patient each. Only 10 episodes were thought to be related to rituximab, none of them being serious or related to opportunistic pathogens and the remainder being described as related to either concomitant disease or to study disease.
Summary of Clinically Documented or Presumed Infections
| . | Arm A (375 mg/m2) . | Arm B (500 mg/m2) . | ||
|---|---|---|---|---|
| All Episodes . | Related Episodes . | All Episodes . | Related Episodes . | |
| Patients with infection | 11 (39%) | 5 (18%) | 8 (31%) | 5 (19%) |
| Infection episodes | 14 | 5 | 13 | 5 |
| Bacterial | 8 | 2 | 8 | 4 |
| Viral | 2 | — | 1 | — |
| Fungal | 1 | — | — | — |
| Unknown | 3 | 3 | 4 | 1 |
| Severity of infectious episodes | ||||
| Grade 1 and 2 | 12 | 4 | 12 | 4 |
| Grade 3 and 4 | — | — | — | — |
| Unknown | 2 | 1 | 1 | 1 |
| . | Arm A (375 mg/m2) . | Arm B (500 mg/m2) . | ||
|---|---|---|---|---|
| All Episodes . | Related Episodes . | All Episodes . | Related Episodes . | |
| Patients with infection | 11 (39%) | 5 (18%) | 8 (31%) | 5 (19%) |
| Infection episodes | 14 | 5 | 13 | 5 |
| Bacterial | 8 | 2 | 8 | 4 |
| Viral | 2 | — | 1 | — |
| Fungal | 1 | — | — | — |
| Unknown | 3 | 3 | 4 | 1 |
| Severity of infectious episodes | ||||
| Grade 1 and 2 | 12 | 4 | 12 | 4 |
| Grade 3 and 4 | — | — | — | — |
| Unknown | 2 | 1 | 1 | 1 |
CD20+ B cells were rapidly depleted from circulating blood for most patients after three infusions of rituximab. CD20+cells remained below normal limits until week 16. The mean serum IgG, IgA, and IgM levels remained within normal range throughout the study.
DISCUSSION
This study is the first trial intended to evaluate the efficacy and the safety of an anti-CD20 monoclonal antibody therapy in patients with aggressive lymphoma (defined as the intermediate- and high-grade subtypes of the Working Formulation). The study was designed to evaluate the safety of two dosing regimens of rituximab and to define whether the true response rate was equal to or greater than 30%.
The patients’ baseline characteristics were well balanced between the two groups. The dominant features of this population were a relatively old age, as compared with the published literature, and a high proportion of previously treated patients. These characteristics were expected because young patients in either refractory or relapsing disease stage are usually treated with intensive therapy and ABMT. Thus, only patients who had declined or who were too old for high-dose therapy were included in this trial.
There were no major differences in the response rates between the two treatment groups. The overall response rate was 31% in the intent-to-treat population of 54 patients and 32% with a 10% CR rate in the evaluable patients (n = 52). This response rate is above the minimal desirable threshold that was defined by the protocol and is similar to what would be expected with single-agent therapy in this patient population. In contrast, it is below the response rate usually obtained with combination chemotherapy such as the DHAP,9ESHAP,10 or VIM11 regimens used in this setting. These types of regimens are often used in younger patients and have been associated with a much higher toxicity, particularly hematologic toxicity. Therefore, a direct comparison of the merits of rituximab monotherapy versus combination chemotherapy is not considered appropriate. In addition, such a comparison would be limited by the fact that elderly patients are commonly excluded or underrepresented in published trials. In our study, 50% and 62% of patients enrolled were older than 60 years of age in arms A and B, respectively. Additionally, comparison of any single agent administered for 8 weeks and intensive, high- dose chemotherapy regimens administered over several months is not appropriate.
The estimated time-to-progression exceeded 105 days and 246 days for the whole population and for the responding patients, respectively. Taking into account that patients in this study were refractory or relapsed and that these results were achieved with a biological therapy, these data should be viewed at least as encouraging. Some patients reached long-lasting progression-free intervals up to 11+ months. The results of this study suggest that the factors that may be associated with a higher likelihood of response are a relapsed or previously untreated disease, a histology of DLCL, a low tumor burden, and a good performance status. Interestingly, a reasonably high response rate was associated with MCL, an entity known to be refractory to chemotherapy.
Importantly, these results were obtained without the characteristic toxicity of combination chemotherapy regimens and over a shorter treatment period. Even if a majority of patients experienced events thought to be related to rituximab, only 2 patients (4%) were withdrawn from the study because of adverse events and no toxic deaths were observed. Most of the adverse events were mild to moderate in severity (Fig 2). As previously described in trials in patients with low-grade or follicular lymphoma, most adverse events occurred during or after the first infusion of rituximab, and the number and the severity of adverse events decreased with the subsequent infusions. The most frequent AE were events usually described under the term of infusion-related syndrome, such as fever, chills, rigors, mild to moderate hypotension, and headache. Two serious adverse events reported in this study were not previously described in the earlier trials with rituximab. First, a patient experienced serum sickness that occurred after the second infusion and recovered with corticosteroid treatment. Second, a patient suffered from a severe hypotension and bronchospasm (described as anaphylactic shock) during his first infusion, which prompted his withdrawal from the study.
The very low incidence of cytopenia is a clinically significant difference from the toxicity usually associated with combination chemotherapy regimens. Only 4% and 8% of patients experienced severe neutropenia in arms A and B, respectively. Similarly, severe thrombocytopenia was not reported for any patient in arm A and for 12% of patients in arm B. The frequency of grade 3 and grade 4 adverse events (23%) and of serious adverse events (23%) was higher in arm B treatment than in arm A (18% and 16%, respectively). Patients in arm B required more infusion alterations due to these adverse events. These results indicate that the higher dose of rituximab was slightly less well tolerated than the standard dose of 375 mg/m2. The infectious episodes were not different between the two doses (Table 5) but were slightly more frequent than in the previous reports in low-grade lymphomas.4 5 However, the episodes qualified as related to rituximab treatment by the investigators were not statistically different. It is thus difficult to incriminate the longer duration of the treatment as responsible for this slow increase in infectious rate.
In conclusion, the results of this study indicate that rituximab therapy has significant anti-lymphoma activity in DLCL and MCL patients without the toxicity commonly observed with combination chemotherapy regimens. There was no marked differences in efficacy between the two dosing regimens, but the safety profile of the higher dose (500 mg/m2) was less favorable as compared with the standard dose regimen (375 mg/m2). This regimen should be evaluated in combination with standard chemotherapy in patients with aggressive B-cell lymphoma.
Address reprint requests to B. Coiffier, MD, Service d’Hématologie, Centre Hospitalier Lyon-Sud, 69495 Pierre-Bénite Cedex, France; e-mail:coiffier@hematologie.univ-lyon1.fr.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal