Abstract
Because cobalamin deficiency is routinely treated with parenteral cobalamin, we investigated the efficacy of oral therapy. We randomly assigned 38 newly diagnosed cobalamin deficient patients to receive cyanocobalamin as either 1 mg intramuscularly on days 1, 3, 7, 10, 14, 21, 30, 60, and 90 or 2 mg orally on a daily basis for 120 days. Therapeutic effectiveness was evaluated by measuring hematologic and neurologic improvement and changes in serum levels of cobalamin (normal, 200 to 900 pg/mL) methylmalonic acid (normal, 73 to 271 nmol/L), and homocysteine (normal, 5.1 to 13.9 μmol/L). Five patients were subsequently found to have folate deficiency, which left 18 evaluable patients in the oral group and 15 in the parenteral group. Correction of hematologic and neurologic abnormalities was prompt and indistinguishable between the 2 groups. The mean pretreatment values for serum cobalamin, methylmalonic acid, and homocysteine were, respectively, 93 pg/mL, 3,850 nmol/L, and 37.2 μmol/L in the oral group and 95 pg/mL, 3,630 nmol/L, and 40.0 μmol/L in the parenteral therapy group. After 4 months of therapy, the respective mean values were 1,005 pg/mL, 169 nmol/L, and 10.6 μmol/L in the oral group and 325 pg/mL, 265 nmol/L, and 12.2 μmol/L in the parenteral group. The higher serum cobalamin and lower serum methylmalonic acid levels at 4 months posttreatment in the oral group versus the parenteral group were significant, with P < .0005 and P < .05, respectively. In cobalamin deficiency, 2 mg of cyanocobalamin administered orally on a daily basis was as effective as 1 mg administered intramuscularly on a monthly basis and may be superior.
© 1998 by The American Society of Hematology.
THE DAILY REQUIREMENT for cobalamin (vitamin B12) is 1 to 2 μg. Such tiny doses of the vitamin are quite efficiently (∼60%) absorbed bound to intrinsic factor via a specific ileal transport system.1 Patients with pernicious anemia and other intestinal disorders develop cobalamin deficiency, because they cannot absorb the small amounts of cobalamin in food via this mechanism. It is stated in textbooks2-4and widely believed by physicians that such patients cannot reliably absorb oral cobalamin and require frequent, usually monthly, intramuscular injections for life. Indeed, in a recent survey of 245 Minnesota internists, more than 90% believed that such patients cannot absorb sufficient quantities of vitamin B12 when it is administered orally and 94% were unaware that any effective oral cobalamin preparations were available.5
However, in the 1950s and 1960s, several investigators6-11provided evidence for an additional pathway for cobalamin absorption that does not require intrinsic factor or the presence of an intact ileum.8,11-19 If very large doses of cyanocobalamin, in the range of 100 to 100,000 μg, are administered to patients with pernicious anemia, approximately 1% of the dose is absorbed, so that enough can be administered by mouth to easily exceed the daily requirement. Excellent hematologic responses were also documented in 91 of 91 patients with pernicious anemia who had received 300 to 2,000 μg or more of oral cyanocobalamin daily.8,11-19 Severe neurological involvement was also reported to respond to large oral doses.16,17,19 Finally, when patients with pernicious anemia were placed on daily oral maintenance doses of 1,000 μg, no hematologic or neurologic relapses occurred and serum cobalamin concentrations remained normal.10-12 20
However, oral treatment with the vitamin has never been the subject of a controlled study. Therefore, we conducted a randomized, controlled trial of oral versus parenteral cyanocobalamin therapy in patients with cobalamin deficiency. In addition to assessing hematologic and neurologic responses and changes in serum cobalamin levels, we measured changes in serum methylmalonic acid and total homocysteine levels, two metabolic indicators of cobalamin deficiency that are more sensitive than serum vitamin concentrations.21-23
MATERIALS AND METHODS
Selection of patients.
Patients were recruited from 4 ambulatory care centers that are part of the Bassett Healthcare network in central New York State. All patients who had serum cobalamin concentrations less than 160 pg/mL as measured in the Bassett Healthcare clinical laboratory between January 1993 and September 1996 were potential subjects. Of 138 such patients, 87 were excluded for one of the following reasons: location outside the immediate geographic area of Bassett Hospital; incapacity to give informed consent; refusal to participate; and associated life-threatening illness. Additional criteria for participation included confirmation of the low serum cobalamin level in another specimen and an elevation of serum methylmalonic acid, total homocysteine, or of both metabolites greater than 3 standard deviations (SD) above the mean in normal controls. As a result, another 13 patients were excluded (6 because of a normal repeat serum cobalamin and 7 in whom neither serum metabolite was elevated). The remaining 38 patients were randomized (Statistical Analysis System; SAS Institute, Cary, NC) to receive oral or parenteral therapy with cyanocobalamin. After completion of the trial, 5 were judged to have primary folate deficiency rather than of cobalamin (see Results) and were excluded from the final analysis. The remaining 33 patients, who were considered to be truly deficient in cobalamin, form the main subject of this report.
Study protocol.
Patients were interviewed and examined by one of the investigators before and after the 4-month treatment period. Informed written consent was obtained. Before and after 1, 2, and 4 months of therapy, serum specimens were obtained before the daily oral dose or before the intramuscular treatment, with the exception of 3 patients early in the study in whom serum was taken (due to error) within minutes of an injection, on a total of 7 occasions (4 at 1 month, 2 at 2 months, and 1 at 4 months). These 7 specimens were excluded from analysis of serum cobalamin in the parenteral group, but were judged valid for serum metabolite concentrations. Serum was not available at the 2-month visit from 1 patient (no. 3). In patient no. 2, folic acid (400 μg/day by mouth) was administered in addition to oral cobalamin between the second and fourth month. The study was approved by the review boards of the Mary Imogene Bassett Hospital and Columbia-Presbyterian Medical Center.
Therapeutic regimens.
Patients were randomized to receive 2,000 μg of oral cyanocobalamin (two 1,000 μg tablets; Nature’s Bounty, Bohemia, NY) administered with breakfast daily for 4 months or 1,000 μg of cyanocobalamin intramuscularly on days 1, 3, 7, 10, 14, 21, 30, 60, and 90. A research nurse administered the injections and monitored compliance.
Laboratory methods.
Serum cobalamin and folate concentrations were determined by radioassays using purified intrinsic factor and milk binder (Simultrac-S; Becton Dickinson Laboratories, Orangeburg, NY). Serum anti-intrinsic factor antibodies and unsaturated serum cobalamin-binding capacity were measured using a coated-charcoal assay.24 Serum methylmalonic acid and total homocysteine were measured using capillary gas chromatography-mass spectrometry.25 Serum pepsinogen I levels were determined by a double antibody radioimmunoassay (Sorin Biomedical Diagnostics, Incstar Corp, Steelwater, MN) as was serum gastrin (Becton Dickinson Laboratories).
Statistical analysis.
Standard methods, including the Students’ t-test and the χ2 test with Yates’ correction for continuity26 were used.
RESULTS
Characteristics of patients.
Eighteen cobalamin-deficient patients were randomized to oral cyanocobalamin (Tables 1 and2) and 15 to intramuscular treatment (Tables 3 and4). A majority in both groups were elderly women. All were white except for 1 Latina patient. All but 1 or 2 patients in each group were outpatients and most were not anemic. In 1 patient (no. 4), the serum creatinine was increased (1.7 mg/dL). In 2 anemic patients in the parenteral group (Table 3), iron deficiency appeared to contribute to the anemia. The erythrocyte mean cell volume (MCV) was elevated (>100 fL) in 7 and 8 patients, respectively, in the 2 groups. Four patients in each group had mild to moderate neurologic symptoms consistent with cobalamin deficiency.
All patients had a low serum cobalamin; 2 in each group had a decreased serum folate (Tables 2 and 4). Serum methylmalonic acid was greater than 3 SD above the mean in normal controls in all except no. 33 in the parenteral group. Serum total homocysteine was greater than 3 SD above the normal mean in 10 and 12 patients, respectively, in the oral and parenteral groups. The 2 groups did not differ significantly in mean age, pretreatment hematocrit, MCV, serum cobalamin, folate, methylmalonic acid, or total homocysteine.
Etiology of cobalamin deficiency.
Seven patients (Tables 1 and 3) had serum antibodies to intrinsic factor, establishing the diagnosis of pernicious anemia. In 4, there was a history of gastric or ileal surgery. Fourteen patients, 7 in each group, were felt to have severe chronic atrophic gastritis (which could cause either intrinsic factor deficiency or food-cobalamin malabsorption)27,28 using a combination of serum pepsinogen and gastrin concentrations29-31 (see footnote to Table 1). Three had poor dietary animal protein intake and 3 were taking agents reported to cause food cobalamin malabsorption.32 33
Hematologic and neurologic responses.
Hematologic and neurologic responses to cyanocobalamin are shown in Tables 1 and 3 and are summarized in Table5. They are similar to those observed in a larger study that used only parenteral cobalamin.34 In approximately one half of the patients in each group, a major decrease in MCV occurred. The mean decrease after 4 months of treatment was highly significant in each group (P < .005). Substantial increments in hematocrit attributable to cobalamin therapy were seen in a minority of both groups, most strikingly in patients no. 2 and 3 on oral cyanocobalamin. Four patients on each treatment experienced dramatic improvement of neurologic complaints. Improvement in mental status, gait, or vibration sense was documented in 2 and 3 patients, respectively, after oral and parenteral therapy.
Serum cobalamin concentrations.
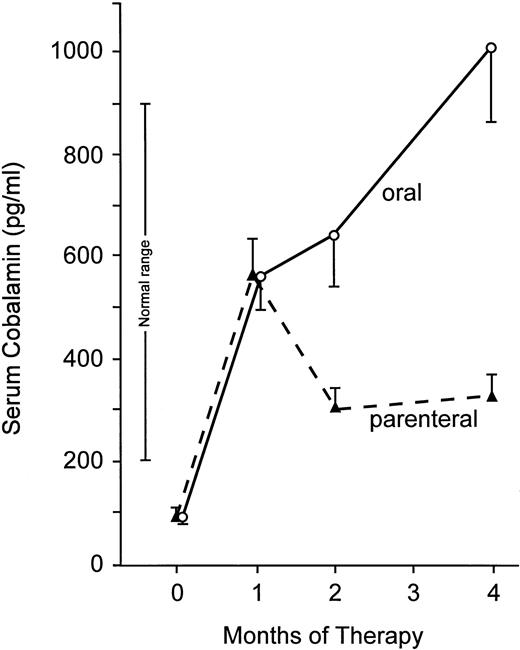
The most striking difference between the two groups was in the behavior of the serum cobalamin level (Fig 1 and Tables 2 and 4). In patients receiving oral cyanocobalamin, serum cobalamin values continued to increase throughout the 4-month treatment period. In contrast, following a substantial increase after 1 month of intramuscular therapy, the mean serum cobalamin in the parenteral group decreased at 2 months and remained essentially the same at 4 months. Serum cobalamin levels were significantly higher in the oral than the parenteral group at 2 months (643 ± 328 v 306 ± 118 pg/mL; P < .001). The difference was more than threefold at 4 months (1,005 ± 595 v 325 ± 165 pg/mL;P < .0005). The 95% confidence intervals for the 4-month serum cobalamin concentrations were 708 to 1,300 pg/mL in the oral group and 230 to 420 pg/mL in the parenteral group.
Mean serum cobalamin levels before and during 4 months of therapy with cyanocobalamin. Bars indicate ± 1 SEM. At 2 and 4 months, mean serum cobalamin concentrations were significantly higher with oral therapy (P < .001 and P < .0005, respectively).
Mean serum cobalamin levels before and during 4 months of therapy with cyanocobalamin. Bars indicate ± 1 SEM. At 2 and 4 months, mean serum cobalamin concentrations were significantly higher with oral therapy (P < .001 and P < .0005, respectively).
In patients receiving oral therapy, all serum cobalamin concentrations were obtained more than 24 hours after the last oral dose. All of the cobalamin in serum was found to be bound to protein by coated-charcoal assay.
In the parenteral group, the serum specimen at 1 month was obtained 9 days after the last of a series of six 1,000 μg injections; the subsequent 2- and 4-month samples were collected 1 month after the most recent 1,000 μg maintenance injection. The serum cobalamin concentration was normal (>200 pg/mL) in all subjects in the oral group at 1, 2, and 4 months and in all in the parenteral group at 1 month. However, the serum cobalamin was again low at 2 and 4 months in 3 and 4 patients receiving intramuscular therapy, respectively. At 4 months, the serum value was greater than 300 pg/mL in only one half of the parenteral group compared with all of those receiving oral cyanocobalamin (Tables 2, 4, and 5; P < .001).
Serum metabolite levels.
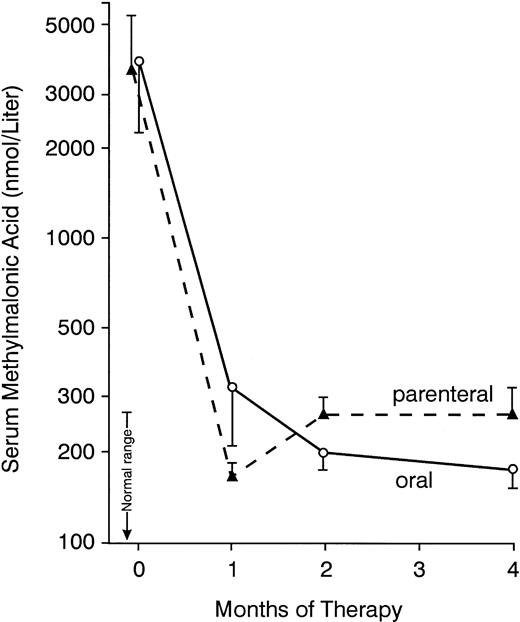
Elevated serum methylmalonic acid concentrations (Fig 2) decreased to less than 3 SD above the mean for normal controls during therapy in every patient except no. 4 (Table 2), in whom the serum creatinine was elevated. During oral treatment, the serum methylmalonic acid decreased progressively over 4 months (Fig 2). In contrast, in the parenteral group, the nadir in the serum concentration was usually reached at 1 month, with a subsequent modest rebound (Fig 2), so that the levels were significantly higher at 2 and 4 months than at 1 month in those receiving intramuscular injections (P < .001 and P < .05, respectively). The methylmalonic acid value in patients no. 20 and 25, which had been less than 3 SD above the mean for normal controls at 30 days, was elevated by 4 months (Table 4); serum cobalamin concentrations had decreased to low to borderline values in these 2 patients. Mean concentrations of the metabolite (Fig 2) did not differ significantly between the 2 treatment groups except at 4 months, when the value was higher in those receiving injections (P < .05).
Mean serum methylmalonic acid concentrations before and during 4 months of therapy with oral or parenteral cyanocobalamin. Bars indicate ± SEM. At 4 months, methylmalonic acid concentrations were signficantly lower with oral therapy (P < .05).
Mean serum methylmalonic acid concentrations before and during 4 months of therapy with oral or parenteral cyanocobalamin. Bars indicate ± SEM. At 4 months, methylmalonic acid concentrations were signficantly lower with oral therapy (P < .05).
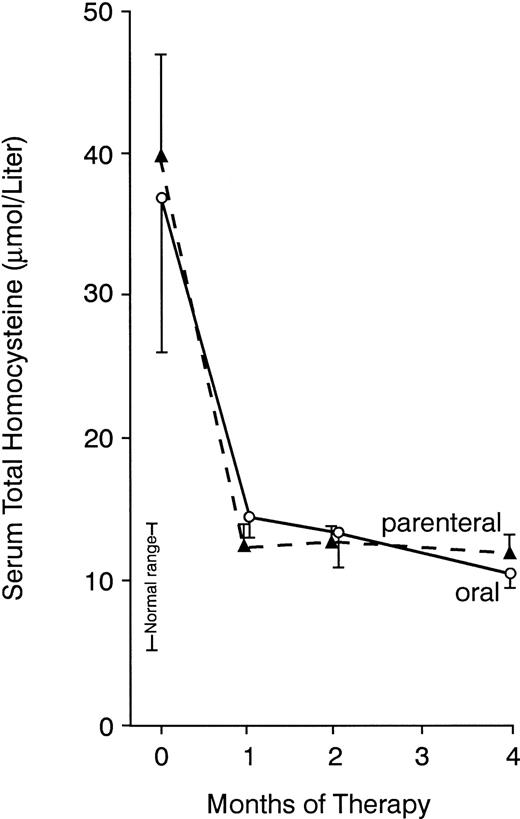
Elevated serum total homocysteine concentrations decreased to normal in most patients in both groups (Tables 2 and 4), decreasing progressively over 4 months of oral therapy, in contrast to a more pronounced decrease during the first month of injections (Fig 3). However, in a minority of patients in both groups, the response of this metabolite was not optimal. At 4 months, serum homocysteine remained greater than 3 SD above the mean for normal controls in patients no. 11 and 17 of the oral group and in patients no. 27 and 28 receiving intramuscular therapy (Tables 2 and4). All of these patients had achieved normal serum cobalamin and methylmalonic acid concentrations. Each gave a history consistent with markedly decreased dietary folate intake associated with depression, dementia, or alcoholism; in all except patient no. 11, the serum folate was low or low normal. It was not possible to undertake a subsequent trial of folic acid therapy in these patients. However, in a patient with pernicious anemia as well as severe anorexia and depression, a continued increase of serum homocysteine after 2 months of oral cobalamin therapy responded to folic acid (patient no. 2 in Table 2 as indicated in a footnote to Table 2).
Mean serum total homocysteine concentrations before and during cyanocobalamin therapy administered by mouth (—) or by injection (---). Bars indicate 1 standard error above or below mean. The number of patients at each point is the same as in Fig 2. Normal range indicates 2 SD above and below the mean in normal controls. Mean values did not differ significantly between the 2 groups at any time point.
Mean serum total homocysteine concentrations before and during cyanocobalamin therapy administered by mouth (—) or by injection (---). Bars indicate 1 standard error above or below mean. The number of patients at each point is the same as in Fig 2. Normal range indicates 2 SD above and below the mean in normal controls. Mean values did not differ significantly between the 2 groups at any time point.
Patients with primary folate deficiency.
After completion of the trial, 5 patients were considered to have primary deficiency of folate rather than cobalamin and were excluded from the analysis. At entry, each of them had low levels of both cobalamin and folate; homocysteine values (but not those of methylmalonic acid) were greater than 3 SD above the normal mean. None had evidence of underlying atrophic gastritis. Four of the five gave a history of depression, alcoholism, or malnutrition. During parenteral or oral therapy, cobalamin levels became normal in all 5 patients, but there were no hematologic responses. Homocysteine concentrations did not normalize at 4 months (and actually increased during treatment in 4). In 1 of them who was available for subsequent study, the serum homocysteine had increased from 28.1 μmol/L before therapy to 65.2 μmol/L after parenteral cyanocobalamin, at which time the serum folate remained low at 1.8 ng/mL. After a 4-week course of 1 mg of daily oral folic acid, the serum homocysteine concentration decreased to 10.0 μmol/L as the serum folate concentration increased to 27.4 ng/mL.
DISCUSSION
This randomized controlled trial of oral versus intramuscular cyanocobalamin in the treatment of well-documented cobalamin deficiency demonstrated that oral and parenteral therapy were equally effective in producing excellent hematologic and neurologic remissions, as well as robust initial metabolic responses. Furthermore, an oral regimen of 2,000 μg per day resulted in serum cobalamin concentrations that were more than 3 times greater than those obtained with a standard parenteral schedule35 of nine 1,000 μg injections administered over a 4-month period (Fig 1). Furthermore, the absorption of the oral preparation was good in every patient, so that at 4 months 18 of 18 receiving oral therapy versus only 7 of 14 receiving injections achieved serum cobalamin levels greater than 300 pg/mL, a value below which metabolic evidence of cobalamin deficiency is frequently demonstrated, especially in the elderly.23,36Indeed, the lower vitamin concentrations in the parenteral group were associated with slightly but significantly higher values for methylmalonic acid, a metabolite that is frequently abnormal before serum cobalamin levels become subnormal as deficiency of the vitamin develops.21 The clinical importance of maintaining normal serum cobalamin and metabolite levels is unclear; however, it is reasonable to use a therapeutic preparation with the greatest overall bioavailability, particularly if it enjoys high patient acceptability.
Serum homocysteine levels may be elevated in either Cbl or folate deficiency.22,24 In the absence of an increased serum methylmalonic acid, an isolated increase in serum homocysteine is usually caused by folate deficiency.22,37 It is of interest that 5 patients entered into the trial were later considered to have primary folate deficiency. Although serum cobalamin levels were low, only serum homocysteine was increased and did not respond to 4 months of parenteral or oral cyanocobalamin (and even increased further in most of the subjects). Others have reported that serum cobalamin levels may often be low in patients with folate deficiency in the absence of defects in cobalamin intake, absorption, or body stores, and have noted increases in serum cobalamin after folate therapy alone.38,39 Our findings are consistent with previous reports that only specific therapy with the vitamin in which the patient is truly deficient will normalize increased metabolite levels.40 41
In addition, among the 33 patients reported here who had clear-cut evidence of true cobalamin deficiency, despite impressive decreases in serum methylmalonic acid and the achievement of normal to increased serum cobalamin concentrations (Tables 2 and 4), 5 (3 on oral and 2 on parenteral therapy) showed only partial serum homocysteine responses. All gave a history consistent with folate deficiency, although 1 of them (patient no. 2) also had unequivocal underlying pernicious anemia. Thus, of 38 patients randomized into the study based on low serum cobalamin and increased metabolite levels, 10 (26%) were found to have folate deficiency as well, either primary or in association with lack of cobalamin.
In summary, in a randomized, controlled trial, large daily oral doses of cyanocobalamin were found equally effective in producing hematologic and neurologic responses as a standard parenteral regimen in patients with cobalamin deficiency and also resulted in clearly superior serum cobalamin levels after 4 months of treatment. Slightly better control of metabolic abnormalities was also achieved with the oral preparation. Our findings, taken together with the extensive and convincing, although uncontrolled, previous reports in the literature,8-19,42 strongly support the view that high doses of oral cyanocobalamin can replace intramuscular therapy in most situations in which the latter is currently administered. Large doses of cyanocobalamin appear to be nontoxic.43 The costs of either therapy are low, although the need for a health provider to administer monthly injections often adds considerable expense.5,44,45 It has been argued that patients are less likely to be compliant with oral treatment,41 although compliance has been reported to be very good.10,42 However, any maintenance therapy is likely to be associated with neglect, especially since today cobalamin injections are often administered outside the physician’s office by the patient, a relative, or a friend. Of a large series of patients with pernicious anemia, 11% interrupted parenteral therapy and suffered a relapse.46Oral maintenance therapy has been widely used in Sweden for more than 25 years.5,10,44,47 In a series of 64 Swedish patients maintained for several years on 1,000 μg/d, none relapsed or had a low serum cobalamin level.10 The 2,000 μg dose used in our study might therefore be considered overly generous, although it may provide an element of safety in view of the wide range of serum cobalamin concentrations found after 4 months (Table 2). Because the various preparations of oral cyanocobalamin (as well as parenteral cobalamin) are not regulated by the FDA, bioavailability may be unpredictable. Use of oral preparations other than that used in this study may not yield similar results. Compliance and effectiveness of cobalamin therapy in individual patients can and should be checked occasionally by measuring the serum cobalamin level either alone or together with metabolite levels.
Undoubtedly, parenteral treatment will continue to play a role in the treatment of cobalamin deficiency, particularly in hospitalized patients; in those with diarrhea, vomiting, or otherwise unable to take medication by mouth; in the unreliable; and in patients with severe neurologic involvement. In the initial treatment of the average outpatient with mild to moderate deficiency or in patients who require maintenance therapy, oral cyanocobalamin with a preparation of proven bioavailability should be strongly considered, if not indeed viewed as the regimen of choice.
Address correspondence to Robert H. Allen, MD, 4200 E 9th Ave, Box B-170, Denver, CO 80220.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.