IN THE PAST 30 YEARS, the treatment of acute myelocytic leukemia (AML) has considerably evolved and improved. The general evolution in the therapeutic strategy has invariably been on the direction of more aggressive treatment being administered early, as soon as a first complete remission (CR1) has been achieved. The rationale has been to provide maximum antitumor effect with so-called consolidation (or intensification) at a stage of minimal residual disease in an effort to eradicate the very last leukemic cells in the patient’s body. However, it is important to remember that total leukemia eradication is not mandatory, because indirect evidence has shown that cure of the disease is achievable if reduction of the tumor burden can reach a level low enough to be controlled by the patient’s own immune system. Such a level can today be obtained, although still unpredictably, by modern conventional chemotherapy or high-dose myeloablative regimens with or without total body irradiation (TBI) followed by stem cell transplantation. Numerous retrospective data exist from single institutions and from international registries on the use of conventional chemotherapy alone or allogeneic or autologous blood or marrow stem cell transplantation (ABMT). Recently, results of randomized studies comparing chemotherapy alone to allogeneic and autologous stem cell transplantation have become available. In parallel, prognostic factors have been identified, among which age, response to induction therapy in reaching CR1, and cytogenetics have proved major. Nonetheless, the choice of the therapeutic strategy has reached no consensus. Other important questions regarding ABMT, such as the role of purging the autograft from residual tumor cells and the potential benefit of autologous peripheral blood (PB) over marrow stem cells, are still being investigated.
BACKGROUND: FROM ALLOGENEIC BMT AND STEM CELL CRYOPRESERVATION TO MODERN ABMT IN ACUTE MYELOCYTIC LEUKEMIA
The history of Autologous stem cell transplantation probably started technically with the demonstration in 1955, by Barnes and Loutit,1 that bone marrow could be successfully cryopreserved, after initial works by Polge et al2 on preservation of bull sperm. Improvements in cryopreservation techniques continued untill the early 1980s.3-7 Several preclinical models in mice,8 rabbits,9monkeys,10,11 and dogs12-15 demonstrated the ability of frozen marrow to engraft and reconstitute hematopoiesis. In the dog model, the minimum dose to obtain engraftment was defined in particular and the kinetics of engraftment were shown to be correlated to the dose of marrow infused.14 15 Interestingly, the correspondence with the human situation was almost perfect. In 1975, the medical ground for ABMT was ready.
The introduction and the development of ABMT for AML first took advantage of lessons from allogeneic BMT. We reported our first successful ABMT in 1977,16 in a patient with AML in first relapse. Marrow collected in first remission and stored in liquid nitrogen was infused after a myeloablative regimen; the patient engrafted and entered a second remission. Several teams started cryopreserving CR1 marrow to treat patients in relapse.17-19 The demonstration that results achieved with allogeneic BMT were considerably better when delivered early in CR20 suggested that a similar approach should be followed with ABMT. The interest in ABMT grew substantially in Europe and North America in view of the major advantages of ABMT, ie, the absence of the necessity to find an HLA-identical family donor, the absence of development of a potentially lethal graft-versus-host disease, and the possibility of applying the treatment to older patients. It appeared later that the absence of graft-versus-leukemia, an initially unrecognized companion of graft-versus-host disease, could be in some circumstances an inconvenience.21 Another major impediment of ABMT, considered from the very beginning, was the risk to reinfuse to the patients with the graft leukemic clonogenic cells that would contribute to or initiate leukemia recurrence. From 1982, several techniques to purge the graft from residual tumor cells have been established.
ABMT is now almost exclusively used to consolidate patients in first or subsequent remission; some teams have systematically applied in vitro purging techniques to the graft,22-36 whereas others relying on agressive in vivo chemotherapy courses administered before marrow harvesting and referring to it as in vivo purging have not.37-50 However, arguments have accumulated in the past 10 years in favor of in vitro purging, and the demonstration has been brought forward that infused leukemic clones at least can contribute to relapse51; nonetheless, the clinical demonstration that in vitro purging improves the outcome is still lacking in the absence of prospective randomized studies, so that the field remains controversial. In 1985 and later, PB collected at time of recovery from chemotherapy induced aplasia was shown to contain a large number of hematopoietic progenitors able to reconstitue hematopoiesis more rapidly than marrow.52,53 Techniques of mobilization with chemotherapy and cytokines were developed. In the past 5 years, there has been a considerable interest in PB autografting for AML. However, the initial hope that the level of tumor contamination of PB would be less than marrow or even nil has not been substantiated.54 55 As of December 1997, the registry of the European Cooperative Group for Blood and Marrow transplantation (EBMT) contained information on a total of 65,000 transplants, of which 4,000 are ABMT for AML.
CLINICAL EXPERIENCE WITH AUTOLOGOUS BONE MARROW TRANSPLANTATION FOR AML
ABMT for Patients in Relapse
In the early days of ABMT, from 1977 to 1983, marrow was collected in patients with AML in CR1 to be reinfused at time of relapse,16-19,38 after myeloablation obtained with TBI or chemotherapy combinations. Marrow purging was not available. Most patients did not receive maintenance therapy posttransplantation. Results of these early trials indicated a high rate of CR2 (∼75%), with, however, no long-term survivors. Of interest was the observation that many patients experienced CR2 of longer duration than CR1, a phenomenon called at that time inversion. These early trials, although disappointing, did demonstrate the feasibility of ABMT in AML as well as the sensitivity of blast cells to high-dose cytotoxic therapy with the possibility of achieving a much higher remission rate than obtained at that time with conventional salvage regimens. In a recent reevaluation of this approach, the Seattle team56,57 has obtained a relapse-free survival of 41% at 2 years, with the longest survivor at 12 years. With a few exceptions,58 ABMT at relapse is no longer used.
ABMT for Adult Patients in Remission
Tables1-4 summarize the experience of several centers. Two pretransplant sets of regimens have mainly been used, the same as for allogeneic stem cell transplantation: one is cyclophosphamide (CY; 120 mg/kg) and TBI, originally developed in Seattle,20 with possible variations according to whether TBI is delivered in a single fraction (10 Gy)23, fractionated (12 Gy),47 or even hyperfractionated (doses up to 13.2 Gy)23 and according to whether CY is administered before or after TBI, with a separation of 48 hours between both. The other is the Busulfan-CY combination, with a total dose of 16 mg/kg busulfan over 4 days and either 200 mg/kg CY over the next 4 days, as originally designed by the Baltimore team,59 or 120 mg/kg over the next 2 days, as modified by the Columbus team60 in an effort to reduce toxicity. Of interest is that all of these regimens, when tested in the Brown Norway myelocytic leukemia rat model, have induced a 8- to 10-log leukemia cell kill.61High-dose etoposide (VP16) at 60 mg/kg has been more recently introduced by the Stanford team to be combined with TBI62 or Busulfan.27,28 63
A third regimen, the BAVC (800 mg/m2 carmustine [BCNU], 450 mg/m2 each of amsacrine [M-AMSA], and VP16, and 900 mg/m2 cytosine-arabinoside [ARA-C]) has been introduced by the Roma team and, because of its reduced toxicity, has been found particularly useful in older patients and/or in CR2.43 48
For adult patients autografted in CR1 with unpurged marrow (Table 1), reported leukemia-free survival range from 26% to 53%. Three studies have some peculiarities.
The London University College study39 planned two autografts. However, a minority of patients reached second intensification. In these patients, the leukemia-free survival was 67%, but the benefit of the second intensification remains unclear because of a selection bias, with patients reaching the second intensification being more likely to belong to the good prognosis group.
A prospective study at City of Hope (Duarte, CA) evaluated a single cure of high-dose ARA-C consolidation therapy as a modality of in vivo purging before marrow collection with no subsequent in vitro purge.41 Sixty consecutive patients were included. The leukemia-free survival was 49% and the relapse incidence was 44% by intention to treat. In patients actually autografted, the leukemia-free survival and relapse incidence were 61% and 33%, respectively, with a plateau after 30 months.
In the Bologna study,42 ABMT was performed in late CR1 and comparison with historical patients treated by CT and still in CR at the same time was in favour of ABMT.
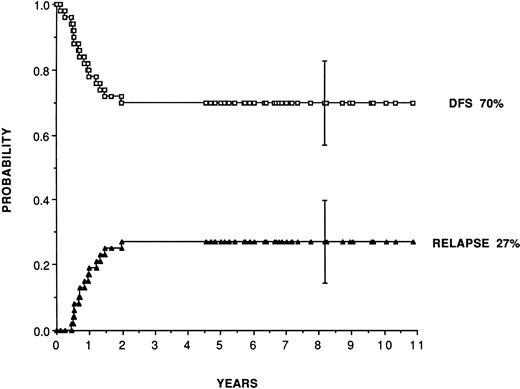
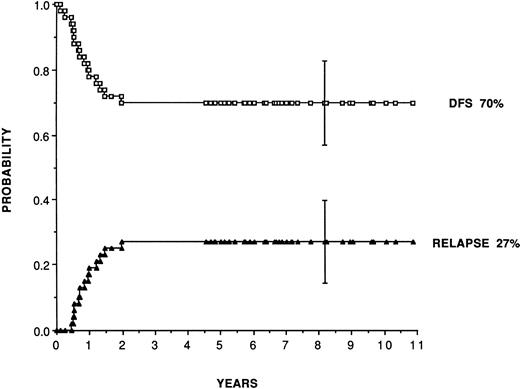
For adult patients autografted in CR1 with purged marrow (Table 2), reported leukemia-free survival range from 41% to 80% and relapse rates from 19% to 48%. Purging has consisted almost exclusively of cyclophosphamide derivatives (4 hydroperoxycyclophosphamide [4HC] or mafosfamide). Figure 1 shows, as an example, the plateau achieved for leukemia-free survival at 70% and relapse incidence at 27% in 50 consecutive patients (median follow-up, 7 years) autografted in first CR in San Francisco and Berkeley28 with marrow purged with 4HC following the original pretransplant regimen combining busulfan and etoposide introduced by the Stanford team.63 In patients in second remission, the same team reports leukemia-free survival and relapse incidence of 52% and 35%.
Leukemia-free survival and relapse rate for 50 patients transplanted in first remission with marrow purged with 4HC, following a preparative regimen combining busulfan (16 mg/kg) plus etoposide (60 mg/kg). (Reprinted with permission from Linker et al.28)
Leukemia-free survival and relapse rate for 50 patients transplanted in first remission with marrow purged with 4HC, following a preparative regimen combining busulfan (16 mg/kg) plus etoposide (60 mg/kg). (Reprinted with permission from Linker et al.28)
The Parma team25 has claimed better results when adjusting the dose of mafosfamide to the individual sensitivity of the normal colony-forming unit (CFU)-blast compartment. Only the Manchester team22 has used long-term culture conditions that they have shown to be deleterious to survival of leukemic colony-forming units-leukemic (CFU-L).
Few teams have had the simultaneous experience of autografting with purged or unpurged marrow.45,63 Within a phase 2 trial exploring the value of their preparative regimen, the Stanford team63 compared small numbers of patients autografted in CR1 receiving either unpurged marrow or marrow purged with 4HC. They initially reported a leukemia survival of 57% and a relapse incidence of 28% in the group receiving purged marrow versus a leukemia-free survival of 32% and a relapse incidence of 62% with unpurged marrow. However, with a longer follow-up, the difference never reached significance. The kinetics of engraftment were slower in the purged group.
Results of ABMT in CR2 (Table 3) indicate leukemia-free survival from 21% to 52% and relapse incidence from 30% to 63%. Purging, when used, has been performed essentially with cyclophosphamide derivatives. One notable exception has been the use of CD14+ CD15 monoclonal antibodies by the Pittsburg team.33 34 Results have been better in patients experiencing the longest duration of CR1. Some teams have claimed outcome equivalent to allogeneic BMT.
Fewer series of ABMT are available in children. Some of them in addition combine different pretransplant regimens64-66 and mix unpurged and purged autografts (Table 4). For patients autografted in CR1, the leukemia-free survival range from 41% to 87% and the retrospective analysis of the Italian registry is in favor of TBI. In CR2, reported leukemia-free survival rates are 36% and 41.5%. Results of CCSG and EBMT are presented as equivalent to allogeneic BMT.
Analyses of all these results are, of course, difficult, because they are retrospective and concern small series of patients and selection bias have likely taken place, at least in some institutions. Comparison with conventional chemotherapy is impossible, because ABMT has concerned only patients in CR. One may argue though that the clinical setting of ABMT is comparable to allogeneic BMT and propose that results achieved with ABMT indeed convey the same information (including the pitfalls) as those reported at the same periods for allogeneic BMT. Whatever the scientific significance of these results, they have demonstrated the feasibility of ABMT in CR1 and CR2. The high rate of long-term disease-free survivors observed in almost all institutions has generated hope and justified prospective controlled studies.
Kinetics of Engraftment
The very first clinical studies had already outlined that the kinetics of engraftment after marrow autografting were particularly slow in AML, contrasting with much more rapid hematopoietic recoveries in acute lymphocytic leukemia (ALL), lymphomas, and solid tumors.67 With unpurged marrow in patients in CR1, recovery of leukocytes to 109/L or polymorphonuclears (PMN) to 0.5 × 109/L occur around days 30 through 4568and recovery of platelets to 50 × 109/L around days 70 through 100, with very wide ranges and extremes up to 1 year for leukocytes and PMN and up to 2 years for platelets. Therefore, the classical definition of engraftment failure, ie, no PMN recovery by day 28, cannot be applied in this situation, in which delayed engraftment is a more appropriate term. Although no randomized study is available to compare the kinetics of engraftment with purged and unpurged marrow, purging with CY derivatives is believed to further delay engraftment.30,36,45,69-71 In the experience of the ECOG group with 4HC purged marrow, recoveries of PMN greater than 0.5 × 109/L and platelets greater than 20 × 109/L have been observed at median days 32 and 64. In our own experience29,30 with mafosfamide purged marrow, recoveries of PMN and platelets to 50 × 109/L have been observed at days 30 and 90, and about 15% of our patient population has needed platelet support for more than 1 year. It has been shown that more rapid recoveries are obtained for younger patients, if PB counts on day of harvest are normal,71 when the transplant is performed in CR1 rather than in CR2, if in CR1 earlier after obtention of remission, and after pretransplant regimens that do not include TBI. This has been linked to the infusion of higher doses of stem cells,4,69,71-73 less damage by previous chemotherapy, and less stroma disruption, respectively. Engraftment failure accounts for approximately 10% of the cases. If other causes have been ruled out, such as defective cryopreservation and/or occurrence of complications in the posttransplant course, such as cytomegalovirus infection or liver veino occlusive disease, it has been essentially related to the infusion of poor marrow, in particular when collected in patients heavily pretreated with mitoxantrone and/or high-dose ARA-C.74 In our own experience30 with marrow purged by mafosfamide, engraftment of both neutrophils and platelets has been more rapid when purging has been adapted to the individual sensitivity of colony-forming unit–granulocyte-macrophage (CFU-GM), which has resulted in less patients receiving grafts with no detectable CFU-GM after purging; the transplant-related mortality has been significantly lower and the leukemia-free survival has been significantly higher in those in whom the dose of CFU-GM per kilogram harvested has been above the median value of 5.2 × 104/kg. Along the same line, one study in particular has correlated better kinetics of engraftment and also better outcome posttransplant to better development of progenitors from the graft in long-term culture assays.75 However, most studies evaluating the content of AML marrow collected in CR have rather found a markedly defective in vitro growth of hematopoietic progenitors and a severe functional defect of marrow stroma as compared with normal marrow or marrow of patients with other diseases, which may explain the delay to engraft observed, specifically in AML.76 The simultaneous search for the highest dose of stem cells and the lowest tumor contamination achieved by previous in vivo purging is in fact a combination of two conflicting goals.
Speeding up engraftment is, therefore, an active field of investigation. Some teams have used granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), or interleukin-1 (IL-1) after ABMT despite the theoretical concern about a potential stimulation of leukemic clones, with no major improvement of the kinetics. A possible reduction of neutropenia with IL-1 has been counterbalanced by side effects.77 The use of so-called primed marrow, ie, marrow collected after 2 to 5 days of G-CSF or GM-CSF administration, which accelerates engraftment in patients autografted for solid tumors, has not been tested in AML. IL-3 followed by GM-CSF for delayed engraftment has been of limited value.78 In fact, because the major problem in patients autografted for AML is platelet reconstitution, results of trials testing the administration posttransplant of megacaryocyte growth and development factor (MGDF) or thrombopoietin (TPO) are eagerly waited for. Inhibitors of hematopoiesis that prevent normal progenitors from entering cell cycle can be used in vitro to protect the normal stem cell compartment when purging with alkylating agents such as mafosfamide. Amifostine in particular accelerates engraftment in patients with breast cancer autografted with marrow purged with 4HC79; it is presently being evaluated in AML. The only effective measure to accelerate significantly engraftment in AML, as in other diseases, is the use of PB stem cells. Several teams80 81 have reported rapid recoveries on day 12 for PMN and day 25 for platelets; a recent pair-matched analysis from EBMT comparing PB with marrow autografts has shown recoveries of PMN and platelets to occur significantly more rapidly with PB at day 13 versus 29 for PMN and at day 42 versus 60 for platelets. However, even with PB, delays in platelet recovery do occur and extreme values have ranged up to 2 years. However, the benefit brought by the use of PB has been counterbalanced by the concern that mobilization may increase blood contamination46,54,55,80,82; reports of increased relapse incidence have generated caution and interest in adding several consolidation chemotherapy courses before leukaphereses to take advantage of in vivo purging and also interest in in vitro purging. It is presently unknown whether these manipulations performed in an effort to reduce tumor contamination will or will not slow down the kinetics.
Monitoring of the serum concentrations of hematopoietic growth factors during myeloablation and hematopoietic recovery83 has shown a sharp decrease of leukemia inhibitory factor (LIF) and an increase of IL-3 during the pretransplant regimen administration, possibly related to induction of stem cell cycling and differenciation. In the posttransplant period, IL-6, G-CSF, and IL-8 have increased from days 6 through 9 and peaked in parallel to neutrophil recovery. The peak was found higher in patients receiving PB. These observations have so far had no therapeutic implications.
Prognostic Factors Other Than Purging
Because, with a few exceptions, single-institution studies have concerned populations of patients too small to enable multivariate analyses, most identified prognostic factors have first come out of international retrospective surveys. Results from registries are criticized as relying on nonconsecutive reporting of poorly controlled data and should be interpreted with great caution. Nonetheless, the relevance of the prognostic factors listed below has later been confirmed in several prospective randomized studies.
Results of ABMT in CR1 are superior in children and in younger adults, but ABMT can still be considered in older patients.84 The outcome of 111 patients older than 50 years of age was compared with the outcome in 786 younger patients (median age, 35 years). The relapse incidence was identical in both groups, but the transplant-related mortality was higher in older patients, so that, in the end, the leukemia-free survival was significantly lower. Regimens, such as the BAVC, with a better tolerance may be favored in older patients.
Results of ABMT in CR1 are better in rapid remitters.85They are also better by cytogenetics in the standard risk group as opposed to the poor prognostic group, which includes all abnormalities of chromosomes 5 and/or 7 and hypodiploidy.86 In fact, by multivariate analyses, cytogenetics at diagnosis were found to be the strongest prognostic indicator for relapse and leukemia-free survival, not only after ABMT, but also after allogeneic BMT, as previously shown with conventional chemotherapy.
Results in CR2 are better in patients who experienced longer CR1 durations.85
Cyclophosphamide and TBI was found equivalent to the Busulfan-cyclophosphamide combinations,87 both for ABMT and allogeneic BMT, with the exception of more cases of liver veino-occlusive disease with BU-CY. A randomized comparison of CY and FTBI at 1,320 cGY versus the original BU-CY, before autografting with marrow purged with 4HC,88 has similarly shown no difference in CR1 but has suggested a trend in favor of TBI in more advanced disease.
The question of whether the dose of ARA-C administered before the transplant period to the patients had an impact on the outcome post-ABMT was considered of importance in view of the results of the CALGB randomized trial on the role of intensive postremission chemotherapy, which had shown a better survival in patients receiving the highest dose arm.89 A recent retrospective analysis on 1,629 patients autografted as well as allografted in CR1 concluded in the absence of any impact in both situations, leading to the speculation that high-dose therapy administered with ABMT would replace or erase the need for previous high-dose ARA-C.90
The timing of the transplant also is important. Results are significantly better in patients autografted late, when in CR.85 This has been mainly explained by a selection bias, with patients transplanted late having spontaneously pretransplant a higher probability of being cured. However, alternatively, one can argue that late autografts may have taken advantage of more consolidation chemotherapy administered before marrow collection, leading to a better in vivo purge of the graft.
IN VITRO PURGING
The amount of theoretical, experimental, preclinical, and clinical work published in the past 15 years on purging has been considerable. Purging in AML has mostly been performed using cyclophosphamide derivatives. Cyclophosphamide itself has no antitumor action. It is metabolized in the liver to generate the active catabolites such as 4HC. Mafosfamide is an analog that generates in vitro free 4 hydrocyclophosphamide.
Data have accumulated in favor of purging for ABMT in acute leukemia, most of all AML, at the same time when similar observations have been gathered for non-Hodgkin’s lymphomas,94 chronic myelocytic leukemia,95 and, more recently, solid tumors.54
However, it has been argued that in vivo purging obtained by agressive chemotherapy delivered before stem cell collection could bypass the need for additional in vitro treatment. Also, as seen above, because the kinetics of engraftment after ABMT for AML may be slower with purged marrow, it has been proposed that any advantage of purging in terms of a reduction in the relapse incidence could be obliterated by the toxicity of a longer aplasia duration.
Rationale
The Brown Norway rat model of human AML, extensively studied by the Johns’ Hopkins group,96-98 has provided the first rationale for purging. In this model, the dose of leukemic cells that, when infused to the animals, kills 50% of them (LD 50), is 25. With the average weight of an animal of 250 g, this was postulated to translate in the human situation into a LD 50 around 10,000 leukemic cells for an adult of 70 kg. Leukemic rats submitted to TBI, followed by infusion of syngeneic marrow purposedly contaminated with 1% leukemic cells, to mimic the human situation, survived, attesting to succesful engraftment; however, they did not develop recurrent lethal disease only if the graft had been treated in vitro with 4HC at a dose greater than 60 nmol/L, attesting to the succesful purging above this 4HC dose threshold. Later experiments using the IPC 81 subline grown in culture from the original rat leukemia predicted that a similar purging approach with human marrow would result in CFU-GM residual values around 1%.98
Numerous studies in humans using immunophenotyping,99,100serial karyotyping,101 growth of leukemic progenitors CFU-L,102 and, more recently, molecular detection of tumor cells by polymerase chain reaction (PCR)103-105 have clearly shown that positive detection of residual tumor cells in the marrow of patients with AML in CR predicts for relapse; some of these studies have inversely correlated the level of minimal residual disease to the probability of cure. The Johns’ Hopkins group in particular has been able to grow CFU-L from the majority of their patients autografted in CR2 or CR3 with marrow purged by 4HC.102 They compared the sensitivity of CFU-L and normal CFU-GM to 4HC and identified two groups of patients according to whether the sensitivity of CFU-L was higher (sensitive group) or identical to (resistant group) the sensitivity of CFU-GM. The relapse incidence was 15% in the sensitive group and 80% in the resistant group. They proposed that a better purging had been obtained in the sensitive group. However, an alternate explanation whereby CFU-L sensitivity to 4HC in fact was but a surrogate marker of the sensitivity of the disease to chemotherapy in general could not be ruled out.
The most important demonstration that leukemic cells harvested with the autologous graft at least can contribute to relapse has come from gene marking studies.51 106 The neomycin resistance gene in a retroviral vector has been used to mark autologous unpurged marrow infused to patients; in the few patients who relapsed, the resurgent blast cells contained the neomycin-resistance gene marker, clearly indicating that they originated from the graft. Similar observations were made with neuroblastomas, chronic myelocytic leukemia, and solid tumors.
One aspect of purging that has been overlooked for many years is the contribution of freezing. AML-CFU are more sensitive to cryopreservation than their normal counterparts. Freezing induces a one-log reduction in clonogenic AML-CFU.107Furthermore, cells fragilized by in vitro treatment are more sensitive to freezing and thawing.108 Also, it is unclear whether dimethylsulfoxide infused to the patients with the autograft, in case it is not washed before hand, has any antileukemic impact.109
All things considered, calculations extrapolating from the rat leukemia model to a human autograft contaminated by 0.5% leukemic cells, taking into account the fact that 1% only of leukemic cells are indeed clonogenic and freezing is the obligatory first step of purging, would indicate that a single log tumor reduction in the graft may in fact be of considerable importance. These theoretical considerations will have to be reevaluated in view of the recent identification of the AML-initiating cell (SLIC) by transplantation of human leukemic cells into SCID and NOD-SCID mice.110 Because the frequency of SLIC in PB of leukemic patients has been shown by limiting dilution assays to be around 1/250,000, ie, more than a 1,000fold lower than the AML-CFU, the necessary reduction by purging would need to be less than estimated from AML-CFU data, but the sensitivity of SLIC to Cyclophosphamide derivatives is unknown.
Although the major action of cyclophosphamide derivatives is tumor cytotoxicity, other actions also have been claimed, in particular an increase in natural killer (NK) cell population and activity111 as well as induction of apoptosis in leukemic cells that can be further enhanced by IL-3 and IL-6.112 An increase in NK cell activity has been documented in AML patients after ABMT with marrow purged by mafosfamide,113 suggesting that purging with mafosfamide may add to tumor destruction in the graft some secondary antitumor immunomodulation.
Purging With Cyclophosphamide Derivatives and Doses of Marrow Infused
In the context of purging, an intriguing observation made at several centers has been the relationship existing between the doses of marrow infused and the outcome of the transplanted patients. This observation has conveyed the message that what is done to the graft itself may have an impact posttransplant.
(1) The Baltimore team generated a clinical program for AML at high risk of relapse. Patients in CR2 and beyond were intensified by the BU-CY combination followed by infusion of autologous marrow purged in vitro by 4HC at the constant dose of 100 μg/mL.36,59,71The probability for the patients to remain in CR was correlated to the residual fraction of the normal progenitors CFU-GM in the graft after in vitro treatment.114 It was 40% versus 18% for CFU-GM residual surviving fractions in the autograft below or above the 1% median value, respectively.
Our own group designed in 1982 a program of high-dose intensification with cyclophosphamide and TBI, followed by reinfusion of autologous marrow purged by mafosfamide.29,30 115-117 By multivariate analysis, the leukemia-free survival was significantly higher in patients who received richer bone marrow, as evaluated before purging. In a more recent update on 234 patients, we also found, as in Baltimore, the relapse incidence to be correlated to the CFU-GM residual fraction after purging. It was 50% versus 31% for patients receiving autografts with CFU-GM residual fraction after purging above or below the median, respectively. The speculation was that less residual CFU-GM meant more agressive purging, which in turn resulted in less relapses.
(2) The Roma team had a different experience with unpurged marrow. The leukemia-free survival was lower in patients receiving doses of marrow evaluated in nucleated cells per kilogram above the median value. They concluded that more leukemic cells had probably been reinfused with the higher unpurged marrow doses.
These observations may be taken as indicating that better results indeed are achieved with the highest doses of stem cells provided that high levels of contamination by tumor cells should be avoided; this would, in theory, correspond to AML treated with CY derivatives. Conversely, this explanation would account for the absence of such a dose effect both in AML in the absence of purging and also in ALL despite attempts at purging, because ALL progenitors have been shown to be highly resistant. A similar observation has been reported in allografted recipients, with a better leukemia-free survival in those receiving the higher doses of marrow.118 The intervention of a stem cell competition effect has been postulated, whereby an expanded normal stem cell pool would express a growth advantage and/or a higher resistance (eg, to inhibitors of leukemic origin) when faced by a minimal residual tumor population.119
(3) Normal progenitors and, by implication, CFU-L experience individual levels of sensitivity to mafosfamide. We adapted the dose of mafosfamide for purging to the individual sensitivity of each patient normal CFU-GM in an effort to determine the highest possible dose of mafosfamide and ensure maximal tumor reduction.29,30 The dose selected was the LD95 defined as the dose sparing 5% CFU-GM, evaluated 15 days before marrow collection, on a small aspirate from which aliquots had been taken and incubated with increasing doses of mafosfamide. With this approach, the doses of mafosfamide used for marrow incubation ranged from 15 μg to 170 μg/2 × 107 buffy coat cells/mL. This individual sensitivity also was taken into account by the Parma team, who adjusted the dose of mafosfamide from 61 to 146 μg/mL to reach a 50% inhibition of the normal CFU-Blast compartment. Not only did this team report on better leukemia-free survival in their patients autografted with marrow treated at adjusted levels of mafosfamide over constant dose purging and over no purging,25 but, interestingly, patients receiving adjusted dose mafosfamide purged marrow were the only ones to respond to recombinant GM-CSF,70 a finding that was explained by dose adjustment sparing the more primitive CFU-Blast compartment.
International Registry Retrospective Analyses on Purging With Cyclophosphamide Derivatives
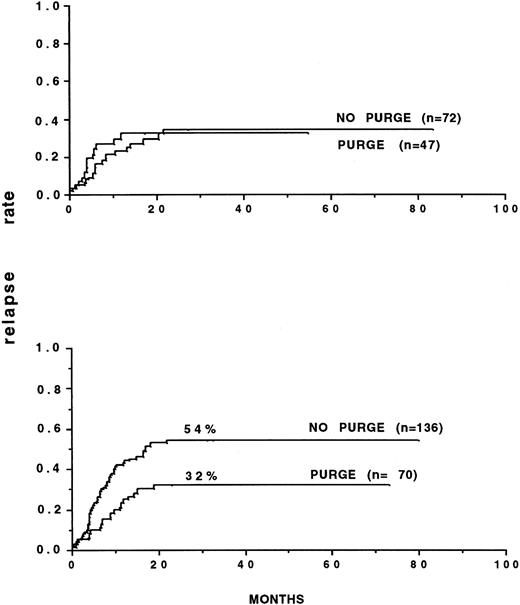
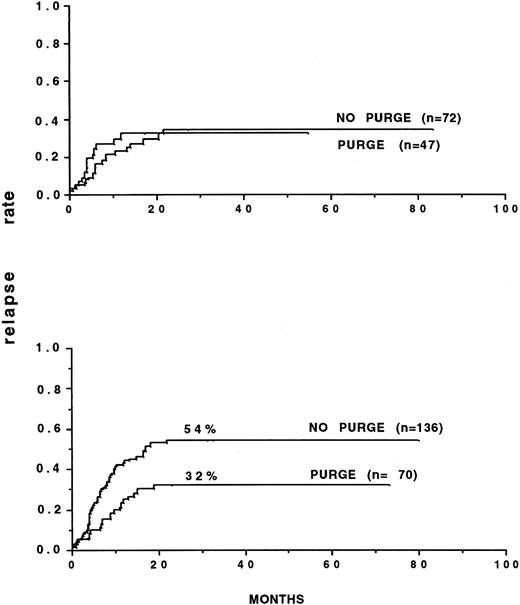
Several retrospective analyses on purging have been performed.85 92 The most important one involved 59 European teams that had reported 919 autografts for consolidation of AML up to December 31, 1989. Marrow was purged with mafosfamide in 269 patients. The outcome posttransplant was correlated to the initial response to CR induction: inital rapid responders had a better leukemia-free survival (53% v 42%) and lower relapse incidence (46% v 57%) than did slow responders. Multivariate analysis in several populations showed significantly the efficacy of marrow purging in AML in CR1. In patients autografted after TBI, the relapse incidence with purged marrow was 29% versus 50% and 16% versus 60% when considering only those autografted within 6 months of CR. In slow responders, the results were 20% versus 61%, significantly in favor of purging, whereas the relapse incidence were similar in rapid responders. The relapse patterns were different in that the plateau for persisting remission started at 23 months with purged marrow and at 32 months with unpurged marrow. In CR2, marrow purging was also associated with a better leukemia-free survival in patients receiving TBI, but the relapse incidence was not significantly different. It was concluded that purging was most likely to bring benefit to a specific category of patients, ie, those transplanted early (Fig2) and slow responders, in whom the probability that leukemic cells might still persist in the graft at time of collection was higher.
Relapse rates of patients autografted in first remission, after TBI, with either purged or nonpurged bone marrow. (A) Patients autografted within 6 months of obtaining remission. (B) Patients autografted later than 6 months after remission. (A) P = .02. (B) P = not significant. (Reprinted with permission from Gorin et al.85)
Relapse rates of patients autografted in first remission, after TBI, with either purged or nonpurged bone marrow. (A) Patients autografted within 6 months of obtaining remission. (B) Patients autografted later than 6 months after remission. (A) P = .02. (B) P = not significant. (Reprinted with permission from Gorin et al.85)
These data were later confirmed by an IBMTR-ABMTR retrospective study on patients with AML autografted in the United States. In this last study, the kinetics of engraftment were slower in those autografted with purged marrow, both for neutrophils and platelets, but the relapse incidence at 3 years was lower in CR1 (40% v 70%) and in CR2 (50% v 90%) and likewise the leukemia-free survival better in CR1 (50% v 30%) and in CR2 (40% v 15%; unpublished data).
Future Direction: Cytoprotective Agent-Mafosfamide Combination
A recent approach to prevent toxicity of purging with mafosfamide towards the normal stem cell pool has been the use of cytoprotective agents. Amifostine, a phosphorylated aminothiol prodrug is more selectively transformed into its active cytoprotective metabolite in normal tissues that have a higher concentration of alkaline phosphatase and a neutral pH environment.120
The Denver team79 has investigated the impact of amifostine on the protection against 4HC in the context of purging for autologous BMT in breast cancer. Leukocyte engraftment was achieved 10 days earlier and the average number of platelet transfusions and days of antibiotic therapy were reduced by 50%.
The ability of amifostine to selectively protect normal bone marrow progenitor cells versus leukemic progenitor cells from the cytotoxic effect of mafosfamide has been evaluated in vitro.121Amifostine pretreatment has resulted in a statistically significant protection of CFU-GM and burst-forming unit-erythroid (BFU-E) and quite unexpectedly was found to increase the cytotoxicity of mafosfamide on the fresh human leukemia progenitor cells, with, in the end, an estimated 6 log therapeutic ratio.
A prospective multicentric randomized study of the role of amifostine in the context of purging with mafosfamide is presently ongoing.
Other Purging Means
Cyclophosphamide derivatives represent more than 90% of the global experience in the field of purging for ABMT in AML. Other drugs have been occasionally proposed and used, with no demonstrated advantage: these include VP-16,31,122 alkyl lysophospholipids,123 and Simvastatin, an antithypercholesterolemic drug that inhibits HMGCoA.124
Antimyeloid monoclonal antibodies, CD14 and CD15 (PM-81 and AML 2-23) with complement, have been tested in CR2/CR3 patients and shown to allow rapid hematologic engraftment without toxicity.33,125A trial with anti-CD33 antibody, eliminating virtually all committed myeloid progenitors, on the other hand, has resulted in a significant engraftment delay.126 Enhancement of in vitro immunological antitumor activity has been obtained using several approaches: the use of bispecific monoclonal antibodies anti-CD13 and anti-CD3,127 selective targeting of lymphokine-activated killer cells by CD3 monoclonal antibody against the interferon-inducible Fcγ receptor on AML blast cells,128and the combination of monoclonal antibodies wth low-dose 4HC.129 None of these has been tested in a clinical situation.
The Manchester group developed in 1986 a technique of long-term culture that, when applied to AML marrow in relapse, appeared to selectively destroy the leukemic clones to the advantage of the normal hematopoietic progenitors. This has then been used to purge marrow collected in CR1.130 In a small series of 26 patients (Table 2), the leukemia-free survival has been as high as 80%, but a selection bias cannot be ruled out. A similar approach has been used with success by the Vancouver group in chronic myelocytic leukemia,131 in which the conditions of the long-term culture have favored the survival and development of the Phi-negative compartment.
IL-2 to generate in vitro LAK cells with antileukemic activity132 has unfortunately resulted in the destruction of immature hematopoietic progenitors, causing failure to engraft.
Finally, leukemic cells have a higher heat sensitivity than the normal hematopoietic subset.133 The therapeutic gain can be augmented by preincubation with the tetrapeptide AcSDKP (Goralatide). A hyperthermic purging protocol with incubation of the marrow at 43°C for 90 minutes has been proposed. Combination with cyclophosphamide derivatives would be a further logical step.
Immune Therapy and ABMT
After anecdotal reports of complete or partial remission obtained with IL-2 in patients with advanced AML, IL-2 has also been used post-ABMT in an effort to stimulate immune functions and NK cell activity.134,135 Some pilot studies have concerned patients relapsing post-ABMT; other studies have used IL-2 after ABMT to maintain CR in higher risk patients. Except for the Seattle study136 in which a leukemia-free survival of 71% at 4 years has been reported in a small number of patients autografted either in relapse or in CR2, the general experience has remained unconvincing.137,138 An interim analysis of a randomized study of linomide (a potent inductor of endogenous IL-2 secretion and NK cell activation) post-ABMT in CR1 has failed to show any difference in the two arms. Cyclosporine A at low doses post-ABMT has been administered in an effort to generate graft-versus-host disease/graft-versus-leukemia and indeed has induced cutaneous manifestations of graft-versus-host disease in most patients, but with no demonstration of any antileukemic effect.139
PB STEM CELL TRANSPLANTATION FOR AML
Despite the original assumption that PB would be less contaminated than marrow and even might be free of tumor, early experiences with PB transplants were disappointing and, in fact, suggested that, at least after induction, a large amount of leukemic clones were mobilized. Massive reinfusion of these cells to patients led to early relapses.55,80 82 Leukaphereses after consolidation rather than after induction were recommended.
The first retrospective EBMT studies comparing marrow to PB autografts for AML in CR1 indicated at 8 years a leukemia-free survival of 51% in 1,279 recipients of marrow and of 44% in 100 recipients of PB with no significant difference (unpublished data).
However, when discriminating between purged and unpurged marrow, results with purged marrow were significantly superior to PB, with a leukemia-free survival of 57% in 251 purged marrow autografts versus 44%; the relapse incidence was 50% versus 37% (P = .0006). These results did not differ from the original single institution retrospective comparison of purged marrow versus PB observed by the Heidelberg group in 1989.24
There was no difference whether the mobilization had been achieved using chemotherapy, growth factors, or both. However, the number of chemotherapy courses precollection shown was important, but only in rapid responders. Patients receiving leukaphereses collected after a minimum of two chemotherapy courses had a significantly lower relapse incidence (20% v 62%,P = .008) and a significantly better leukemia-free survival (69% v 35%, P = .02) than patients receiving leukaphereses collected after only one chemotherapy course. (manuscript submitted).
The conclusion of these studies was that autografting with PB may do as well as purged ABMT, provided that it takes advantage of in vivo purging represented by additional courses of chemotherapy administered to the patients before collection. Recent results from several institutions that follow this therapeutic strategy look promising, with reported leukemia-free survival as high as 71% at 5 years in CR1 and only a slight engraftment delay.81Alternatively, because it has been clearly shown that stem cell mobilization techniques also mobilize tumor cells, PB purging is being considered.140
COMPLICATIONS OF ABMT FOR AML
Complications of autografting for AML depend on several variables, among which the population age, the nature of the pretransplant regimen used, and the source of stem cells infused (which influences the duration of aplasia) are essential. As predicted from theory, graft-versus-host disease has almost never been observed, despite a few anecdotal reports. Apart from this, the nature of the complications observed after ABMT in AML has not differed from complications of allogeneic BMT; however, reported incidences have usually been lower. Early complications have included mucositis (up to 100% with high-dose melphalan and etoposide), bacterial and fungal sepsis, pulmonary aspergillosis, cytomegalovirus infection and disease (up to 20% with TBI and BU-CY), and liver veno-occlusive disease (up to 20% with BU-CY). Transplant-related mortality has varied from as low as 5% in single experimented institutions to an unexpected high of 12% in the large MRC 10 randomized trial, which included small and very small centers. In a large recent retrospective EBMT study,47 the transplant-related mortality has been 13% and 8%, respectively, in adults and children autografted in CR1 and 20% and 12% in CR2. Late complications combine sterility, cataracts, and secondary malignancies. Sterility is almost constant after TBI or BU-CY,141although successful pregnancies have been occasionally reported. However, alternate chemotherapy combinations may offer better chances, although this has to be balanced with a lower antileukemic effect.142 Cataracts are directly correlated to the use of TBI. Several studies have indicated that TBI fractionation decreases its incidence. In a recent retrospective study143 of 1,063 patients transplanted after TBI, which included 490 AML, the risk was found to be significantly lower after ABMT as compared with allogeneic BMT and also lower in younger patients, in cases with heparin administration (as administered in some centers for prevention of liver veno-occlusive disease), and in the absence of long-term steroid administration.
In 1985144 and later in 1993,101 we reported on the occurrence of multiple chromosome abnormalities in patients with acute leukemia autografted with marrow purged by mafosfamide after TBI: 30% of our patients autografted with marrow purged with mafosfamide had complex chromosome abnormalities detectable at some time, as late as 88 months posttransplant, in a minority of cells, which either persisted or were only transient, and appeared not to be related to the original leukemic clone. These observations have so far not been correlated to any unfavorable outcome. Because we did not find similar abnormalities in patients autografted with purged marrow but after non-TBI pretransplant regimens, we postulated that these rare abnormal circulating cells were endogenous progenitor cells that had survived the previous repeated aggression of chemotherapy induction and consolidation courses and the pretransplant regimen including TBI. Similar findings have been reported by others in AML145 and patients with non-Hodgkin’s lymphoma,146 as well as after allogeneic transplantation, especially with T-cell–depleted marrow, supporting the same explanation. Myelodysplastic syndromes post-ABMT have been described as a late complication, occuring with a frequency of up to 14.5% at 5 years, in patients with non-Hodgkin’s lymphoma.147 Occurence of myelodysplastic syndromes or secondary leukemia in patients autografted for AML has been mentioned in meetings but so far not published. It is possible that some have been confused with recurrence of the original disease. The recent introduction of high-dose etoposide in pretransplant regimens may major the risk.148
THE AML-3 EXCEPTION
Old data,149 collected before the introduction of all-trans retinoic acid (ATRA), had indicated in patients autografted for an acute promyelocytic leukemia (M3) defined by cytology a leukemia-free survival at 7 years of 48% in CR1 and 31% in CR2. Results achieved with allo-BMT were not superior because of a very high transplant-related mortality of 42%, as opposed to 18% for ABMT.
A more recent analysis86 on 999 patients with evaluable cytogenetics has found the t(15,17) translocation to be the strongest favorable prognostic factor for the outcome post-ABMT, with a leukemia-free survival of 56% and an overall survival of 68% at 5 years.
Meanwhile, however, the whole field of AML3 has changed since 1990150 with the advent of ATRA, its use in combination with chemotherapy for induction of remission,150-152 the detection of the PML/RARα fusion transcript by PCR and the demonstration of its prognostic relevance for detection of minimal residual disease,104,105 and the design of a better treatment strategy. In the recent AIDA trial,104 induction with ATRA and Idarubicin followed by 3 consolidation courses has resulted in 95% CR and a leukemia-free survival of about 80% at 2 years. Moreover, at the end of the consolidation period, 98% of the patients had undetectable PML/RAR transcripts. Therefore, stem cell transplantation cannot be recommended as part of the upfront therapy of M3. In contrast, in the minority of patients who still relapse, induction of a CR2 again with ATRA and chemotherapy is easily achievable and ABMT should be seriously considered. It has been proposed that patients in CR2 remaining PCR positive proceed to allo-BMT if possible, whereas those reaching PCR negativity be intensified with ABMT.105 Purging the autograft in M3 might take advantage of several peculiarities: CD34+ selection, because a majority of M3 leukemic progenitors have been reported to be CD34− (in contrast to other types), and in vitro incubation with ATRA, not taking into account more classical means such as monoclonal antibodies or cyclophosphamide derivatives. The unique possibility for monitoring minimal residual disease by PCR further makes studies and protocols along these lines appealing; unfortunately, the predicable small patients accrual has so far generated little enthusiasm.
THE PLACE OF ABMT IN THE THERAPEUTIC STRATEGY OF AML
Several retrospective studies comparing chemotherapy with allo-BMT and ABMT have been performed in the past 15 years. In a first joint EORTC-EBMT analysis, 236 patients treated according to the AML 5 and 6 EORTC chemotherapy protocols were compared with 453 allografted and 182 autografted patients registered by EBMT.153 At 6 months posttransplant, allografted recipients had a significant better leukemia-free survival than the chemotherapy group and the autografted group was in between, with no significant advantage, however, over chemotherapy. In a retrospective analysis of their own single institution experience, the Genoa team154 has compared the outcome of 159 AML patients allografted or autografted with unpurged marrow in CR1 after TBI. At 8 years, results have been identical, with leukemia-free survival rates of 52% and 49%, respectively. Of interest was the finding that the relapse incidence after ABMT (39%) was identical to the incidence in allotransplant recipients who developed no chronic graft-versus-host disease (37%), but was higher than in those with chronic graft-versus-host disease (30%). Two recent EBMT retrospective studies compared the outcome of patients with AML after ABMT; the first one to allo-BMT with an HLA-identical sibling47 and the second one to allo-BMT with an HLA-identical volunteer donor.155 Results indicated a significant higher transplant-related mortality for allografting and a significant higher relapse rate for autografting.
In terms of leukemia-free survival, results were significantly superior for patients allografted with an HLA-identical sibling marrow in CR1 but not in CR2. Results of ABMT and unrelated marrow transplants did not differ, an observation also made by the Seattle team.156
Table 5 lists the results of prospective randomized studies completed: some have consisted of two arms and compared allogeneic BMT with ABMT68,157-159 or ABMT with conventional chemotherapy.64 Others with three arms have compared the three treatment modalities.160-163 In all, except the ECOG study,162 marrow used for ABMT was unpurged. Results are given in the majority by intention to treat. As an example, in the study conducted in Boston, all patients entering CR1 were offered allogeneic BMT or auto-BMT after a consolidation course using high-dose ARAC (6 g/m2 total). The actuarial leukemia-free survival in the two groups of patients actually transplanted was identical (62%). The contribution of high-dose ARAC was felt to be important. Of all the studies, the two biggest by patient accrual and number of institutions involved have been the international AML8 EORTC-GIMEMA trial161 and the UK MRC 10 trial.163
The AML8 trial compared in 941 patients 10 to 45 years of age in CR1 allogeneic BMT (in those with an HLA-identical sibling) with either autologous BMT or a second course of high-dose cytarabine and daunorubicin in the others. The relapse incidence was the highest in the intensive chemotherapy group and the lowest in the allogeneic transplantation group; the transplant-related mortality was the highest after allogeneic transplantation and the lowest after chemotherapy. In the end, the leukemia-free survival was significantly better after both transplant modalities, over chemotherapy with values at 5 years of 55% for allogeneic BMT, 48% for ABMT, and only 30% for conventional chemotherapy. Another finding of the trial was that many patients who relapsed in the conventional arm could be rescued by ABMT, which in the end resulted in a similar overall survival in the three groups.
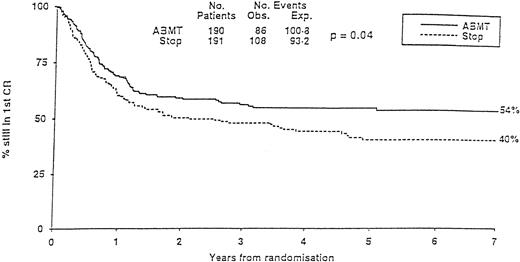
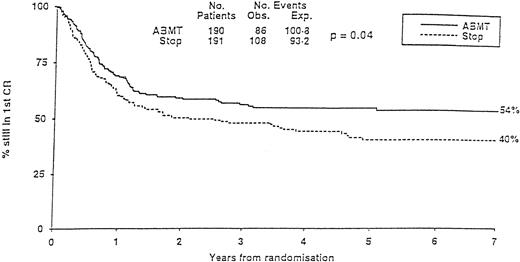
The MRC10 trial recruited 1966 patients from 163 institutions. Patients reaching CR1 received high-dose consolidation before going either to allogeneic transplantation if they had an identical family donor or being randomized to additional high-dose chemotherapy with no further treatment (STOP) or ABMT. The overall survival of the population at 7 years was 40%. Prognostic factors defined three risk categories. The good-risk group had favorable cytogenetics. The poor-risk group cumulated unfavorable cytogenetics to poor blast clearance after the first induction course. All other patients belonged to the so-called standard group. Having a donor for an allogeneic transplant was significantly better in term of leukemia-free survival in the standard group but not in the others. In all categories, ABMT was superior to the STOP arm, with, at 7 years, a significantly lower relapse incidence (37% v 58%) and a significantly higher leukemia-free survival (54% v 40%; Fig 3). Because the transplant-related mortality in the ABMT arm was high, it was felt that some improvement might be searched in this direction in the future. Two other multicentric trials, one in children64 and the other one in adults,160 failed to show any advantage of ABMT over conventional chemotherapy. In the first one (POG), a lower relapse incidence in the ABMT arm was counterbalanced by a higher transplant-related mortality. In the second one (GOELAM), results of allogeneic BMT, ABMT, or intensive chemotherapy consolidation were identical. The investigators attributed their findings to an unexpected high relapse rate in the allo BMT arm and the use of high-dose ARAC (24 g/m2) in the chemotherapy arm, which was felt to reproduce results achieved with the higher dose arm in the CALGB ARAC dose study.89 They concluded that intensive chemotherapy consolidation with high-dose ARAC was similar to ABMT.
MRC-10 Study. Leukemia-free survival of patients randomized to ABMT or STOP arm. (Reprinted with permission from Burnett et al, “Randomized comparison of autologous bone marrow transplantation to intensive chemotherapy for acute myeloid leukaemia in first remission: Results of MRC-AML 10 trial,” volume 351, page 705. © by The Lancet Ltd, 1998.163)
MRC-10 Study. Leukemia-free survival of patients randomized to ABMT or STOP arm. (Reprinted with permission from Burnett et al, “Randomized comparison of autologous bone marrow transplantation to intensive chemotherapy for acute myeloid leukaemia in first remission: Results of MRC-AML 10 trial,” volume 351, page 705. © by The Lancet Ltd, 1998.163)
In summary, with all trials considered, comparisons of allogeneic BMT to ABMT have been in favor of the first transplant modality. Comparisons of ABMT to conventional chemotherapy have shown in most instances a significantly reduced relapse incidence in the ABMT arm. Also, in most studies, ABMT has been associated with a better leukemia-free survival, except when a high transplant-related mortality has suppressed the benefit resulting from the reduced relapse incidence.64,163 No study has shown an advantage of conventional chemotherapy over ABMT. This indicates that ABMT represents the highest available antitumor consolidation regimen, which is limited, however, by its feasibility and toxicity, as recently confirmed by a metaanalysis of seven of the randomized trials conducted between 1984 and 1995.164 It is possible but yet to be proven that high-dose ARAC, as tested by the CALGB, offers similar results.
Because of the considerable increase in the numbers of stem cell transplantations performed throughout the world, cost effectiveness has become of paramount importance: several teams have financially evaluated the cost of allogeneic transplants, ABMT, and conventional chemotherapy for AML.165-167 For ABMT, depending on whether TBI has been or has not been included in the preparative regimen and whether the source of stem cells has been marrow purged or unpurged or PB, figures have ranged from $50,000 to $120,000 US dollars, which is much higher than those reported for lymphomas and solid tumors, which have been in the range of $35,000 to $50,000 US dollars. The only logical way to compare strategies is to include the results in term of disease-free survival and express the cost per year of saved life, as initially performed for allo-BMT, with an evaluation of about $10,000 US dollars per additional year of life.165 A recent Swedish study167 has evaluated and compared the cost of all three procedures: allo-BMT, auto-BMT, and conventional chemotherapy. In this study, the costs per year of saved life have been $10,000, $10,000, and $14,000 US dollars, respectively, for a patient of 20 years of age and $18,000, $18,000, and $24,000 US dollars, respectively, for a 50-year-old patient. These results indicate that ABMT for AML is as expensive as allo-BMT and that both modalities in the end are not more expensive than conventional chemotherapy.
CONCLUSION AND FUTURE DEVELOPMENT
Autologous stem cell transplantation is nowadays a recognized therapeutic option for AML therapy. Prospective randomized studies have shown its superiority over conventional chemotherapy, with the possible reservation that none has tested high-dose ARAC in a parallel arm. Arguments in favor of in vitro purging have accumulated at the same time when agressive in vivo purging used before stem cell collection may have reached the same goal, so that the two purging means may not necessarily have to be combined. There is an increasing move towards PB rather than BM as the source of stem cells to accelerate engraftment. Expansion of marrow protected by amifostine and treated with mafosfamide is an interesting alternative for research. Improvement in pretransplant regimens with radiolabeled antimyeloid monoclonal antibodies to increase tumor reduction and better control of residual tumor posttransplant with leukemia vaccines are on the verge of being tested. Some teams are even considering the combination of autografting to achieve high tumor reduction without graft-versus-host disease, with delayed allogeneic lymphocyte infusion to later take advantage of graft-versus-leukemia. As it presently stands, indications of ABMT for many teams still reside largely in impossibilities of allogeneic transplants and concern primarily good- or standard-risk AML in older patients with no family donor. An important randomized international study evaluating the source of stem cells and possibly purging with the help of the NOD-SCID mouse model for evaluation of minimal residual disease is needed. A foreseen reduction in transplant-related mortality with PB and a hopefully successful search for reduction in relapse incidence with immunomodulation or leukemia vaccines would be the keys for ABMT to become the first rather than the second therapeutic option.
ACKNOWLEDGMENT
The author expresses his gratitude to Veronique Skalli for help in preparing the manuscript and secretarial assistance.
Address reprint requests to N.C. Gorin, MD, Department of Hematology and Centre de Recherche Claude-Bernard sur la Thérapie Cellulaire, Hôpital Saint-Antoine AP-HP, 184, rue du Faubourg Saint-Antoine, 75012 Paris, France.