Abstract
To elucidate the contributions of GATA-1 to definitive hematopoiesis in vivo, we have examined adult mice that were rendered genetically defective in GATA-1 synthesis (Takahashi et al, J Biol Chem272:12611, 1997). Because the GATA-1 gene is located on the X chromosome, which is randomly inactivated in every cell, heterozygous females can bear either an active wild-type or mutant (referred to asGATA-1.05) GATA-1 allele, consequently leading to variable anemic severity. These heterozygous mutant mice usually developed normally, but they began to die after 5 months. These affected animals displayed marked splenomegaly, anemia, and thrombocytopenia. Proerythroblasts and megakaryocytes massively accumulated in the spleens of the heterozygotes, and we showed that the neomycin resistance gene (which is the positive selection marker in ES cells) was expressed profusely in the abnormally abundant cells generated in the GATA-1.05 mutant females. We also observed hematopoiesis outside of the bone marrow in the affected mutant mice. These data suggest that a small number of GATA-1.05 mutant hematopoietic progenitor cells begin to proliferate vigorously during early adulthood, but because the cells are unable to terminally differentiate, this leads to progenitor proliferation in the spleen and consequently death. Thus, GATA-1 plays important in vivo roles for directing definitive hematopoietic progenitors to differentiate along both the erythroid and megakaryocytic pathways. The GATA-1 heterozygous mutant mouse shows a phenotype that is analogous to human myelodysplastic syndrome and thus may serve as a useful model for this disorder.
TRANSCRIPTION FACTOR GATA-1 is expressed within the hematopoietic hierarchy in erythroid, megakaryocytic, eosinophilic, and mast cell lineages1-5 as well as in Sertoli cells of the testis.6 The GATA-1 gene has been mapped to the X chromosome7 and two alternative promoters/first exons with differential tissue-specific usage have been identified. The 5′ IT promoter and the downstream IE promoter dictate GATA-1 transcription in Sertoli cells and erythroid cells, respectively.8
The functional roles of GATA-1 in hematopoietic cell development have been analyzed by gene targeting (reviewed in Yamamoto et al9). Analysis of chimeric animals generated by GATA-1–null embryonic stem (ES) cells indicated that the mutant cells do not contribute to the mature erythroid population.10 In vitro, GATA-1–null ES cells are also unable to differentiate into mature erythroid cells,11-13 thus suggesting that GATA-1 is required for the terminal differentiation of committed erythroid progenitor cells.
Because previous attempts at generating germ line GATA-1–null alleles were unsuccessful,10 we prepared an erythroid promoter-specific knock-down of the GATA-1 gene.14 Because male embryos bearing this mutation expressed GATA-1 mRNAs at approximately 5% of the level that accumulated in wild-type embryos, we therefore referred to this mutant allele as GATA-1.05. All mutant male embryos (of GATA-1.05/Y genotype) died by 12.5 days post coitus (dpc) due to impaired primitive hematopoiesis. Analysis of the male embryos (by specifically marking primitive progenitors with alacZ transgene) demonstrated that the maturation of these progenitors was blocked at the proerythroblast stage in theGATA-1.05/Y embryos, indicating that GATA-1 is necessary for terminal differentiation during primitive erythropoiesis.14This conclusion is consistent with that of another recent analysis studying GATA-1–null mutant embryonic erythropoiesis.15
However, the in vivo functional contributions of GATA-1 to definitive hematopoiesis are still unknown, because mutant male mice (GATA-1.05/Y) die before full commencement of definitive hematopoiesis due to a severe deficit in primitive hematopoiesis. To address this question, we took advantage of the fact that GATA-1 gene is X-linked,7 and because of random inactivation of the X chromosome (ie, Lyonization16) in somatic tissues, heterozygous mutant females should always be chimeric for GATA-1 gene expression. Analysis of heterozygous (GATA-1.05/X) female mice showed that GATA-1 is required for the terminal differentiation of definitive erythroid and megakaryocytic cells. These results indicate that GATA-1 is a key regulator of definitive hematopoietic cell differentiation in vivo and suggest that the loss of GATA-1 is an accelerating factor for abnormal expansion of erythroid and megakaryocytic lineage progenitor cells.
MATERIALS AND METHODS
Mice.
Mice bearing the GATA-1.05 allele15 were bred in clean rooms at the Animal Research Institute of Tohoku University and in the Animal Research Center at the University of Tsukuba (Tsukuba, Japan). Hematological examination of mice heterozygous for theGATA-1.05 allele was performed by phlebotomy from the retro-orbital plexus. After initially noting that adult heterozygous mutant female mice have abnormally shorter life spans, they were monitored carefully after becoming anemic so that necropsies could be performed within 12 hours after death. Biopsy of overtly healthyGATA-1.05 heterozygous mutant females was performed on a group of 11 young mice of 8 to 12 weeks of age.
Histological analysis of mice.
Samples of major organs of the GATA-1.05 heterozygous mice and their littermates were obtained either by necropsy or biopsy. The samples were fixed in 10% buffered formalin and embedded in paraffin. Sections were stained with hematoxylin and eosin for histological examination. Cryo-sections were processed for immunohistochemistry with N6 anti–GATA-1 antibody6 and antimouse glycoprotein IIb antibody (Komeno et al, submitted for publication). Acetylcholinesterase staining for megakaryocytes was performed as described.17
Reverse transcriptase-polymerase chain reaction (RT-PCR) analysis of GATA-1 and neomycin resistant gene expression.
RNA was isolated from spleens using the guanidine thiocyanate/cesium chloride ultracentrifugation method.18 First-strand cDNA was synthesized using Superscript reverse-transcriptase (GIBCO BRL, Gaithersburg, MD) at 37°C for 1 hour, and 1 μL of this 20 μL reaction mixture was used for the PCR reactions. Amplified products were analyzed on 2% agarose gels. Sequences of the primers used were as follows: erythroid-specific GATA-1 transcript (expected size, 483 bp), 5′-TAAGGTGGCTGAATCCTCTGCATC and 3′-CCGTCTTCAAGGTGTCCAAGAACGT; testis-specific GATA-1 transcript (expected size, 529 bp), 5′-CGTGAAGCGAGACCATCGTC and the 3′-primer was the same as the erythroid 3′-primer; and neomycin phosphotransferase (expected product, 339 bp), 5′-AAGTATCCATCATGGCTGATG and 3′-TAGCCAACGCTATGTCCTGATA. Primers for glucose-6-phosphate dehydrogenase (G6PD; expected size, 162 bp) were synthesized as described previously.19
RESULTS
Female mice of GATA-1.05/X genotype have a shortened life span.
We have previously reported that GATA-1.05/X mutant female mice suffered varying degrees of anemia in the embryonic and early neonatal stages.14 Because the wild-type siblings displayed none of these symptoms, we suspected that this heterogeneity in phenotype was probably due to variable extent of random inactivation of the wild-type X chromosome.16 We therefore selected 19 heterozygous and 8 wild-type female mice and followed their life span. TheGATA-1.05/X mice had a shortened life span in comparison to wild-type littermates. More than half of the heterozygous mutant female mice died within 7 months after birth (Fig1), whereas all 8 control female animals survived beyond the test period. Thus, the GATA-1.05 lesion leads to a dramatically foreshortened life span in mature heterozygous females.
Cumulative mortality in GATA-1.05 heterozygous (•) and wild-type (▵) female mice. Heterozygous GATA-1.05females14 were mated with wild-type and the resultant female progenies were first genotyped. The life span of 19 heterozygous mutant and 8 wild-type litter mates was then examined.
Cumulative mortality in GATA-1.05 heterozygous (•) and wild-type (▵) female mice. Heterozygous GATA-1.05females14 were mated with wild-type and the resultant female progenies were first genotyped. The life span of 19 heterozygous mutant and 8 wild-type litter mates was then examined.
Anemia and thrombocytopenia in adult GATA-1.05 female mutant mice.
To elucidate the cause of death in the heterozygous mutant mice, we dissected and analyzed the moribund animals. We found that most of them displayed marked splenomegaly (varying principally in degree) and that the appearance of the enlarged spleen was light red to pink in color, rather than the typical dark red color (Fig 2A). Furthermore, expansion of the spleen was so pronounced that other abdominal organs were compressed, as is also often seen in human myeloproliferative disorders.
Splenomegaly in the GATA-1.05 heterozygous mutant female mice. (A) Spleens were removed from wild-type (upper) andGATA-1.05 heterozygous (lower) littermates after genotyping and hematocrit screening of animals. Note that the spleen of theGATA-1.05 heterozygous mouse is markedly enlarged and is somewhat pink as compared with the dark red spleen from the wild-type mouse. (B) Sizes of the spleens from 6 wild-type (wt) and 22 heterozygous mutant (+/GATA-1.05) female mice. +/− b shows the spleen weights of the biopsied 8- to 12-week-old healthyGATA-1.05 heterozygous mice (those listed in Table 1). +/− a indicates mice that were found dead whose spleen weights were determined upon necropsy. (○) Mice no. 12 through 14 of Table 1 are indicated.
Splenomegaly in the GATA-1.05 heterozygous mutant female mice. (A) Spleens were removed from wild-type (upper) andGATA-1.05 heterozygous (lower) littermates after genotyping and hematocrit screening of animals. Note that the spleen of theGATA-1.05 heterozygous mouse is markedly enlarged and is somewhat pink as compared with the dark red spleen from the wild-type mouse. (B) Sizes of the spleens from 6 wild-type (wt) and 22 heterozygous mutant (+/GATA-1.05) female mice. +/− b shows the spleen weights of the biopsied 8- to 12-week-old healthyGATA-1.05 heterozygous mice (those listed in Table 1). +/− a indicates mice that were found dead whose spleen weights were determined upon necropsy. (○) Mice no. 12 through 14 of Table 1 are indicated.
Acetylcholinesterase and anti-glycoprotein IIb staining of megakaryocytes in the spleens of female mice. Megakaryocytes in a heterozygous mutant spleen (C and D) were positive for acetylcholinesterase staining. The appearance of the positive staining cells is morphologically similar to megakaryocytes in a wild-type spleen (A and B). Note that there are more megakaryocytes in the mutant mouse spleen. These cells were also positive for glycoprotein IIb (E and F). Original magnifications × 20 (A, C, and E) and × 80 (B, D, and F).
Acetylcholinesterase and anti-glycoprotein IIb staining of megakaryocytes in the spleens of female mice. Megakaryocytes in a heterozygous mutant spleen (C and D) were positive for acetylcholinesterase staining. The appearance of the positive staining cells is morphologically similar to megakaryocytes in a wild-type spleen (A and B). Note that there are more megakaryocytes in the mutant mouse spleen. These cells were also positive for glycoprotein IIb (E and F). Original magnifications × 20 (A, C, and E) and × 80 (B, D, and F).
Eight litters from GATA-1.05 mutant female and wild-type male mice crosses either by biopsy or autopsy soon after death were examined. We found that all of the mice that died spontaneously were of the GATA-1.05/X genotype and that most of these showed enlargement of the spleen to variable degrees (Fig 2B, +/− a). A second group of 11 8- to 12-week-old female mutant mice were killed, and their spleens were examined by biopsy. These mice were considerably younger than the previous set of mutant females examined that had died spontaneously. We again observed slight enlargement of the spleen among these overtly healthy young mice (Fig 2B, +/− b). There was a strong correlation between the degree of anemia and splenomegaly, in that mice with the most severe anemia showed the most marked splenomegaly (see below).
We next analyzed peripheral blood taken from 14 GATA-1.05heterozygous mutant mice as well as 6 wild-type female litter mates. The mutant mice displayed varying degrees of anemia and thrombocytopenia (Table 1). Whereas the severity of anemia varied enormously among heterozygous mutant animals, the platelet counts were consistently lower in the GATA-1.05heterozygous mice than in wild-type litter mates (Table 1). Upon necropsy, mice with the lowest hematocrits (hematocrit, 25) uniformly displayed the most pronounced splenomegaly (Fig 2B). These data suggest that the cause of death in GATA-1.05 heterozygous mice is probably due to the anemia and splenomegaly.
Massive accumulation of proerythroblasts and megakaryocytes in the spleens of heterozygous mutant mice.
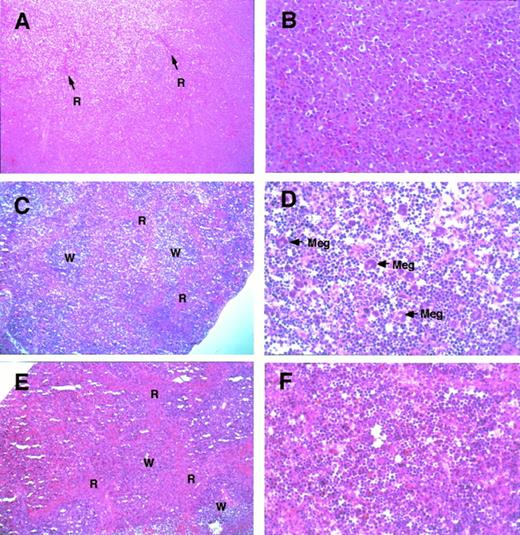
Histological examination of the grossly enlarged spleen (of sample no. 12 in Table 1) showed that the architecture of the spleen was severely affected (Fig 3A; compare C and E). Importantly, proerythroblasts had massively infiltrated the spleen (Fig 3B). Because of proerythroblasts accumulation, there was almost no white pulp and only residual visible red pulp (see arrows). Because the accumulating erythroid cells are immature, whereas in human myeloproliferative disorders the number of mature cells are aberrantly high, these two disorders are different in this respect.
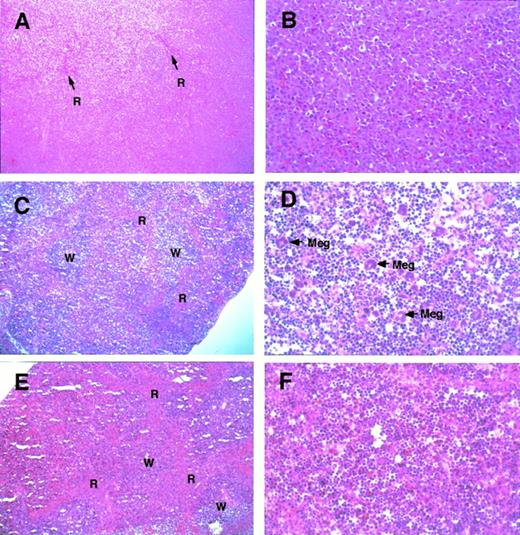
Histological appearance of the spleen from theGATA-1.05 heterozygous mutant and wild-type female mice. Histological sections of the spleens from GATA-1.05heterozygous female mice with severe splenomegaly (A and B;GATA-1.05 heterozygous mouse no. 12 in Table 1) or mild splenomegaly (C and D; heterozygous animal no. 3 in Table 1) are shown. Red pulp (R), white pulp (W), and megakaryocytes (Meg) are indicated. The histological sections of wild-type female mouse spleen (E and F; corresponding to wild-type no. 1 in Table 1) are also shown. Tissues were stained with hematoxyline and eosin. Original magnifications × 20 (A, C, and E) or × 80 (B, D, and F).
Histological appearance of the spleen from theGATA-1.05 heterozygous mutant and wild-type female mice. Histological sections of the spleens from GATA-1.05heterozygous female mice with severe splenomegaly (A and B;GATA-1.05 heterozygous mouse no. 12 in Table 1) or mild splenomegaly (C and D; heterozygous animal no. 3 in Table 1) are shown. Red pulp (R), white pulp (W), and megakaryocytes (Meg) are indicated. The histological sections of wild-type female mouse spleen (E and F; corresponding to wild-type no. 1 in Table 1) are also shown. Tissues were stained with hematoxyline and eosin. Original magnifications × 20 (A, C, and E) or × 80 (B, D, and F).
In one of the slightly enlarged spleens (from heterozygote no. 3 in Table 1), the overall architecture of the spleen was generally preserved, but the mass of red pulp was less prominent (Fig 3C) than that in the wild-type spleen (Fig 3E). Furthermore, the residual red pulp was filled with proerythroblasts (Fig 3D). The megakaryocyte count was also significantly higher (Fig 3D and Table 1) when compared with the number in the spleens of wild-type littermates (Fig 3F). This is in contrast to the observation that the overall number of megakaryocytes was somewhat diminished in the enormously enlarged spleens of the animals that had died spontaneously (Fig 3B).
To assess functional properties of these accumulated megakaryocytes, acetylcholinesterase and glycoprotein IIb expression was examined in the megakaryocytes in the normal and abnormal spleens. Figure 4A and B shows the results of acetylcholinesterase staining in a spleen of a wild-type female, whereas Fig 4C and D shows the comparable staining patterns for the GATA-1.05 heterozygous mouse spleen. The number of megakaryocytes appeared to be greater in the heterozygous mutant mouse spleen, and they were uniformly positive for acetylcholinesterase staining. These megakaryocytes were also positive for glycophorin IIb and displayed normal histological morphology (Fig4E and F). Although these data demonstrated that the megakaryocytes in the spleens of GATA-1.05 heterozygous mutants had progressed quite far in megakaryopoiesis, the terminal steps leading to platelet formation still seem to be impaired in these cells.
GATA-1–negative, neomycin phosphotransferase-positive cells accumulate in the spleens of GATA-1.05 heterozygous mutant mice.
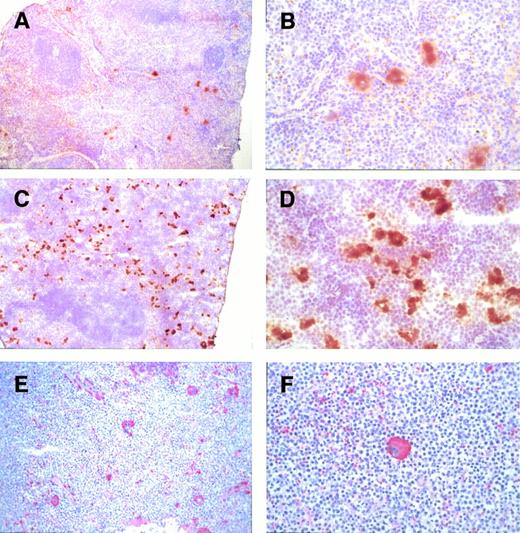
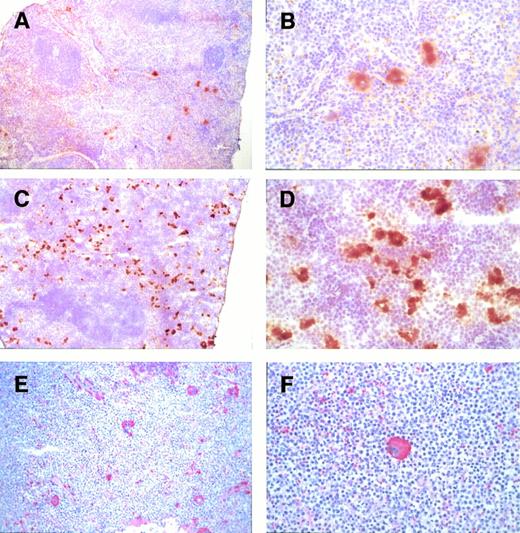
To determine the origin of the cells that accumulate in the spleens ofGATA-1.05 heterozygous mutant mice, we performed immunostaining with an anti–GATA-1 antibody on spleen sections of the mutant and wild-type mice. As shown in Fig 5A, numerous cells in the red pulp of a wild-type spleen were stained brown by the anti–GATA-1 antibody; proerythroblasts and megakaryocytes were shown to express abundant GATA-1 (Fig 5B). In contrast, both the red pulp and white pulp were virtually nonexistent in the enlarged spleen of the heterozygous mutant mouse (Fig 5C). The proerythroblasts and megakaryocytes that had accumulated in these enlarged spleens did not immunoreact with the anti–GATA-1 antibody (Fig 5C); however, a few GATA-1–positive erythroid cells were observed in these same sections in remnants of the red pulp (Fig 5D). These results suggested that the erythroid and megakaryocytic cells that have proliferated and accumulated in the grossly enlarged mutant spleens are derived from parental cells containing an inactivated GATA-1 allele.
Anti–GATA-1 antibody staining of spleens. Spleens isolated from either a heterozygous mutant female mouse with marked splenomegaly (C and D) or a wild-type mouse (A and B). Many cells in the red pulp (R) of the spleen of the wild-type mouse were clearly GATA-1 positive. Note that both proerythroblasts and megakaryocytes were GATA-1 positive. In contrast, accumulating proerythroblasts and megakaryocytes did not stain for GATA-1 in the spleens of heterozygous mutant female mice (C and D). R, red pulp; W, white pulp; Meg, megakaryocytes. Arrows stand for normal GATA-1–positive cells. Original magnifications × 10 (A and C) and × 80 (B and D).
Anti–GATA-1 antibody staining of spleens. Spleens isolated from either a heterozygous mutant female mouse with marked splenomegaly (C and D) or a wild-type mouse (A and B). Many cells in the red pulp (R) of the spleen of the wild-type mouse were clearly GATA-1 positive. Note that both proerythroblasts and megakaryocytes were GATA-1 positive. In contrast, accumulating proerythroblasts and megakaryocytes did not stain for GATA-1 in the spleens of heterozygous mutant female mice (C and D). R, red pulp; W, white pulp; Meg, megakaryocytes. Arrows stand for normal GATA-1–positive cells. Original magnifications × 10 (A and C) and × 80 (B and D).
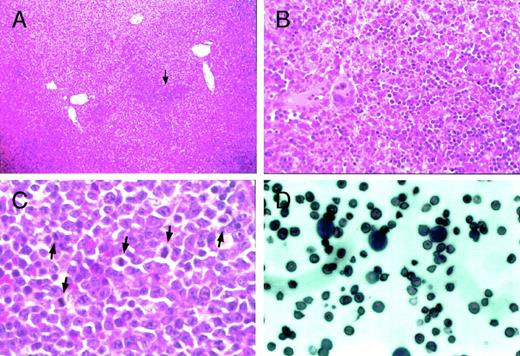
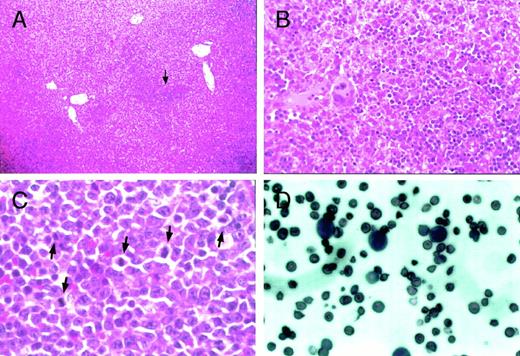
Histological examination of the liver, spleen, and peripheral blood from heterozygous mutant female mice. Proerythroblasts and megakaryocytes accumulate in the sinus of the liver inGATA-1.05 mutant females (arrow in A). (B) is a higher magnification of (A) that contains the region pointed to by the arrow. Original magnifications × 10 (A) and × 80 (B). Mitotic figures were frequently seen in accumulated proerythroblasts in the spleen (arrows in C; original magnification × 200). Proerythroblasts were also observed in the peripheral blood of heterozygous mutant female mice with severe anemia and marked splenomegaly (D; original magnification × 200). The sections (A) through (C) were stained with hematoxylin and eosin reagent, whereas the blood smear was stained with Wright-Giemsa reagent (D).
Histological examination of the liver, spleen, and peripheral blood from heterozygous mutant female mice. Proerythroblasts and megakaryocytes accumulate in the sinus of the liver inGATA-1.05 mutant females (arrow in A). (B) is a higher magnification of (A) that contains the region pointed to by the arrow. Original magnifications × 10 (A) and × 80 (B). Mitotic figures were frequently seen in accumulated proerythroblasts in the spleen (arrows in C; original magnification × 200). Proerythroblasts were also observed in the peripheral blood of heterozygous mutant female mice with severe anemia and marked splenomegaly (D; original magnification × 200). The sections (A) through (C) were stained with hematoxylin and eosin reagent, whereas the blood smear was stained with Wright-Giemsa reagent (D).
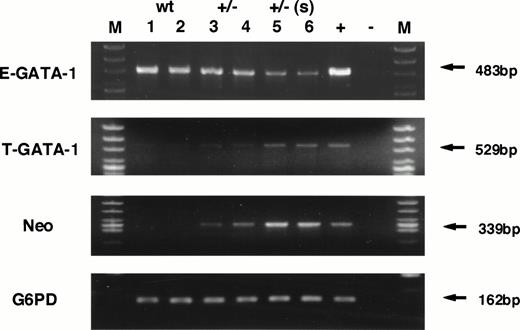
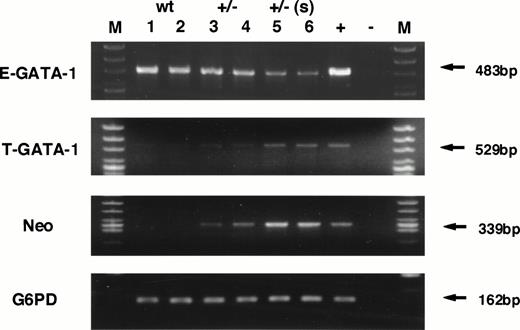
To test this hypothesis, the transcription level of neomycin phosphotransferase (Neo) gene as well as GATA-1 gene, initiating from both testis- and erythroid-specific promoters, were examined by RT-PCR analysis. Because the Neo gene was knocked-in to the GATA-1 locus, we predicted that the presence of Neo mRNA should be detectable in cells in which the GATA-1.05 allele is active, even in the absence of Neo selection. The analysis shown in Fig 6 verified this hypothesis and showed that the level of Neo gene expression increased significantly in the GATA-1.05 heterozygous mouse spleens. The Neo expression level correlates well with the severity of splenomegaly and bears an inverse relationship to the expression of GATA-1 from the erythroid (IE) first exon (E-GATA-1, Fig 6). Interestingly, there is a reciprocal increase in GATA-1 expression from the IT exon (Fig 6, T-GATA-1). These data further support the conclusion that the mutant GATA-1 allele is active in the cells that accumulate in the enlarged spleens of the GATA-1.05 mutant animals.
RNA analysis of wild-type and heterozygous mutant female spleens. Expression of GATA-1 from either the IE promoter (E-GATA-1) or the IT promoter (T-GATA-1) was examined after 35 cycles of amplification using 5′ primers specific for either first exon and a 3′ primer used in common in the GATA-1 third exon. Expression of neomycin phosphotransferase was also examined after 35 cycles of amplification. G6PD was used as the internal control and analyzed after 27 cycles of amplification. The numbers indicate individual animals. Lanes 1 and 2, samples isolated from wild-type spleens; lanes 3 and 4, heterozygous mutant female spleens in animals displaying mild splenomegaly; lanes 5 and 6, heterozygous mutant female spleens having marked splenomegaly. +, positive control; −, no template added; M, marker lane.
RNA analysis of wild-type and heterozygous mutant female spleens. Expression of GATA-1 from either the IE promoter (E-GATA-1) or the IT promoter (T-GATA-1) was examined after 35 cycles of amplification using 5′ primers specific for either first exon and a 3′ primer used in common in the GATA-1 third exon. Expression of neomycin phosphotransferase was also examined after 35 cycles of amplification. G6PD was used as the internal control and analyzed after 27 cycles of amplification. The numbers indicate individual animals. Lanes 1 and 2, samples isolated from wild-type spleens; lanes 3 and 4, heterozygous mutant female spleens in animals displaying mild splenomegaly; lanes 5 and 6, heterozygous mutant female spleens having marked splenomegaly. +, positive control; −, no template added; M, marker lane.
Ectopic liver hematopoiesis in adult GATA-1.05 heterozygous mutant mice.
The data presented thus far have shown that the cells that proliferate and accumulate in the spleens of GATA-1.05 mutant animals are hematopoietic cells and that they are deficient in GATA-1 synthesis. Erythroid differentiation in the GATA-1.05 heterozygous mutant cells bearing an active GATA-1.05 allele (and an inactive wild-type allele) is arrested at the proerythroblast stage. These data imply that the GATA-1.05 heterozygous mutant may serve as a mouse model for the human disorder, myelodysplastic syndromes (MDS).
MDS is characterized (among other hallmarks) by abnormal accumulation of immature cells in the enlarged spleens of affected individuals, as well as ectopic hematopoiesis. The notion that the GATA-1.05mutant mice might be a good model for the human MDS is further supported by the observation that the heterozygous mutant adult mice that display marked splenomegaly also have ectopic hematopoiesis in the liver, where we found erythroblast-like cells as well as megakaryocytes in the hepatic sinuses (Fig 7A and B). Some hepatocytes were also found to be compressed simply by accumulated hematopoietic cells. In the spleen sections from some heterozygous mutant mice that were found dead, mitotic figures were also often observed (Fig 7C). In addition, high numbers of proerythroblasts were found in the peripheral blood of mice with severe anemia (Fig 7D). These observations suggest that the accumulation of GATA-1–deficient cells leads to a preleukemic state and, therefore, that this mutation has the potential to serve as a useful animal model for human MDS.
DISCUSSION
In this study, we have analyzed the functional contributions of GATA-1 to the growth and differentiation processes of definitive hematopoietic cells in heterozygous GATA-1 knock-down mutant mice. We took advantage of the facts that the murine GATA-1 gene is localized on the X chromosome and that one of the two X chromosomes is inactivated randomly in heterozygous females. The loss of GATA-1 function in this mutant resulted in the stimulation of growth and the arrest of the differentiation in hematopoietic cells, which became manifest as severe anemia, thrombocytopenia, and splenomegaly, thus resulting in a foreshortened life span. The data demonstrate that GATA-1 is necessary for the terminal differentiation of definitive erythroid and megakaryocytic lineage cells in vivo.
GATA-1 has long been thought, and more recently demonstrated, to be a key regulator of erythroid lineage maturation. For instance, we and others recently demonstrated that maturation of primitive erythroid cells is arrested at the proerythroblast stage.14,15Compared with the unequivocal proof supporting the role of GATA-1 in primitive hematopoiesis, the evidence of GATA-1 in definitive hematopoiesis has been quite limited. In this regard, Fujiwara et al15 recently reported that the majority of mice heterozygous for a GATA-1–null allele are born anemic, but they recover during the neonatal period. They speculated that this recovery is presumably due to in vivo selection of progenitor clones with an active normal allele. We have observed the same phenomenon in mice bearing the knock-down GATA-1.05 allele (data not shown). In addition, we report here that mice heterozygous for theGATA-1.05 allele have a significantly shorter life span and splenomegaly. The observation of late onset splenomegaly in theGATA-1.05 heterozygous mutant mice was an intriguing surprise. We demonstrated that the cells that had proliferated and accumulated in these mutant animals are of the erythroid and megakaryocytic lineages. The proliferative cell population was shown to contain an activeGATA-1.05 allele (and an inactive wild-type allele) and undetectable amounts of GATA-1 protein. In summary, these data indicated that, in the absence of GATA-1, hematopoietic progenitors either have, or gradually acquire, a growth advantage over their normal counterparts some time during postnatal development.
Forced expression of GATA-1 protein in avian erythroid progenitor cells was reported to accelerate erythroid cell differentiation with concomitant suppression of proliferation,20 whereas constitutive expression of GATA-1 was reported to interfere with normal cell-cycle progression.21 These reports and our present data lead us to hypothesize that the lack of GATA-1 stimulates proliferation and suppresses differentiation in hematopoietic cells. In this regard, an in vitro ES cell differentiation system developed by Nakano et al22 may serve as an additional useful experimental tool for detailed assessment of the erythroid differentiation process. Our preliminary analysis ofGATA-1.05/Y ES cells14 using this method has showed that the GATA-1.05 mutation directly affects the growth rate of proerythroblasts and megakaryocytes (Suwabe et al, submitted for publication). Because the only resident feedback mechanism that exists in the in vitro ES cell differentiation system is provided by the support cells, which may represent the bone marrow stroma environment (see below), these data further support the hypothesis that GATA-1 acts simultaneously to stimulate hematopoietic cell differentiation and to suppress proliferation.
In contrast to our present finding, Weiss et al12 reported that GATA-1− proerythroblasts simply apoptosed in vitro. We think that this may be accounted for by the difference in in vivo and in vitro experimental systems. A similar discrepancy has been seen previously, in that primitive erythrocytes could not be generated from GATA-1–null ES cells under their in vitro differentiation condition. We14 and others15 have found that primitive erythrocytes are generated, but not terminally differentiated, under the GATA-1 knock-down14 or knock-out condition15 in vivo.
Expression of GATA-1 from the IE promoter was found to be significantly diminished in abnormally abundant hematopoietic cells in the spleen of the GATA-1.05 mutant females. In contrast, GATA-1 expression from the IT promoter concomitantly increased in the anemic mice that displayed splenomegaly. Moroni et al23 recently reported that, when purified hematopoietic progenitors are stimulated by erythropoietin, GATA-1 expression from the IT promoter was upregulated.23 Therefore, the more abundant expression of GATA-1 arising from the IT promoter in the heterozygous mutant mice could be in response to increased levels of erythropoietin in these animals. The data therefore suggest that the expression of GATA-1 from the IT promoter may be under regulatory feedback control in a homeostatic circuit involving the growth control elicited by erythropoietin. However, the amount of GATA-1 from the IT promoter is probably below the sensitivity of histochemical detection, because the normal expression level of GATA-1 from the IT promoter in hematopoietic cells is only about 5% of the total GATA-1 expression in the spleen.
Despite the abnormal accumulation of megakaryocytes in the mutant spleens, a severe and consistent thrombocytopenia was observed in theGATA-1.05 heterozygous female mice. We therefore concluded that GATA-1 may also be important for the terminal differentiation of megakaryocytes. Consistent with this observation, Pevny et al13 previously reported that GATA-1–negative megakaryocytes were abnormally abundant in chimeric fetal livers, suggesting an alteration in the kinetics of their formation or turnover. Furthermore, Shivdasani et al24 have recently demonstrated that GATA-1 plays a critical role in megakaryocyte growth regulation and platelet biogenesis in vivo using a lineage-specific knock-out strategy. All of these results argue that GATA-1 is necessary for terminal differentiation of megakaryocytes.
Megakaryocytes normally express GATA-1, and several genes expressed specifically in this lineage appear to be under the regulation of GATA-1 or other GATA family transcription factors. It has been reported that the glycoprotein IIb gene is directly regulated by GATA-1.25 However, we detected the glycoprotein IIb protein in megakaryocytes that lack GATA-1. This suggests either that GATA-2, which is coexpressed in this cell lineage, could functionally substitute for GATA-1 or that the low level of GATA-1 present in theGATA-1.05 mutant megakaryocytes may be adequate to execute most, but not all aspects, of megakaryocytic differentiation. Regardless of the mechanism used, the results presented here clearly indicate that GATA-2 cannot fully compensate for GATA-1 function in megakaryopoiesis. Thus, the identification of bona fide target genes of GATA-1 remains an important hurdle to extending our understanding of the role of GATA-1 in megakaryocyte differentiation.
ACKNOWLEDGMENT
The authors thank Drs J.D. Engel, N. Kajiwara, N. Kasai, H. Nakauchi, K.-C. Lim, H. Sugiyama, N. Suzuki, N. Takasawa, and K. Yagami for help and discussion.
Supported by the Grants-in-Aid from the Ministry of Education, Science, Sports and Culture, Japanese Society for Promotion of Sciences (RFTF96I00202) and the Ciba-Geigy Foundation (Japan).
Address reprint requests to Masayuki Yamamoto, MD, PhD, Center for TARA and Institute of Basic Medical Institute, University of Tsukuba, 1-1-1 Tennodai, Tsukuba 305, Japan; e-mail: masiya@igaku.md.tsukuba.ac.jp
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.
© 1998 by the American Society of Hematology.