Abstract
Polymorphonuclear neutrophils (PMNs) are essential effector cells in host defense and tissue inflammatory responses. These responses may be initiated after cross-linking of cell surface Fc receptors that bind the constant portion of IgG (FcγR). We evaluated the effect of cross-linking FcγRI or FcγRII on interleukin-6 (IL-6) production by purified PMNs from normal donors or from patients being treated with recombinant human granulocyte colony-stimulating factor (rhG-CSF). In PMNs from normal donors, IL-6 mRNA was detected by reverse transcriptase-polymerase chain reaction only after FcγRI or FcγRII cross-linking. We also found that IL-6 mRNA could be detected in PMNs after either in vitro or in vivo rhG-CSF treatment in the absence of FcγR cross-linking. IL-6 protein was found to be produced intracellularly and secreted by PMNs after cross-linking FcγRI or FcγRII or after rhG-CSF stimulation. Cross-linking FcγRI or FcγRII on PMNs from patients treated with rhG-CSF resulted in a synergistic increase in IL-6 secretion. Upregulation of IL-6 production by PMNs after rhG-CSF treatment may contribute to a clinical engraftment syndrome that occurs during periods of rapid increase in PMN numbers in patients receiving rhG-CSF.
POLYMORPHONUCLEAR neutrophils (PMNs) are the most abundant nucleated cells in blood and are essential for host defense and tissue inflammatory responses. Neutrophils escape the circulation, migrate to sites of tissue inflammation in response to chemotactic factors, and, after activation, function primarily as phagocytic cells.1 The ability of mature neutrophils to mediate phagocytosis and antibody-dependent cellular cytotoxicity (ADCC) is determined largely by the presence of receptors for the constant portion of IgG (FcγR).2,3 Binding of the constant portion of IgG to cell surface Fc receptors (FcγR) initiates neutrophil activation. FcγRII (CD32) and FcγRIII (CD16) are receptor subtypes expressed by a wide variety of cell types, including both neutrophils and monocytes.2,4,5 However, FcγRI (CD64) is primarily expressed on monocytes and macrophages, with little expression on newly produced neutrophils.4,5 Exposure of neutrophils to recombinant human granulocyte colony-stimulating factor (rhG-CSF) results in significant elevation of FcγRI expression.6-8
In addition to functioning as phagocytic cells, recent studies have shown that activated neutrophils also function as a source of cytokines that initiate inflammatory sequelae, including tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and IL-6.9-11 IL-6 is a pleiotrophic cytokine that acts as an endogenous pyrogen, stimulates production of acute-phase proteins by hepatocytes, and enhances both specific humoral and cellular immune responses.12 Serum IL-6 levels are elevated in a number of inflammatory conditions12 and sepsis.13 The studies undertaken here were designed to determine whether cross-linking FcγRI or FcγRII resulted in elevated expression of IL-6 in purified neutrophils. The results show that FcγR cross-linking upregulates expression of both IL-6 mRNA and protein in unstimulated neutrophils.
IL-6 levels are also directly correlated with the rise in neutrophil numbers in the recovery phase after autologous stem cell transplantation.14 It is during the early and steep phase of neutrophil recovery after autologous bone marrow or autologous peripheral blood stem cell transplantation (PBSCT) that an engraftment syndrome has been described in which patients develop noninfectious fever and rash.15,16 Some patients also develop interstitial pulmonary infiltrates and fluid retention.15-18 The underlying etiology of this syndrome has not yet been determined, but the involvement of neutrophils has been suggested. In the course of our studies, we noted that neutrophils derived from patients undergoing rhG-CSF therapy expressed significant levels of IL-6 mRNA in the absence of FcγR cross-linking. This prompted us to determine whether rhG-CSF exposure directly stimulated IL-6 production in neutrophils in vitro or whether the effect of rhG-CSF was indirect and mediated by upregulation and cross-linking of FcγR. The studies presented in this report demonstrate that rhG-CSF directly stimulates the production of IL-6 in the absence of FcγR cross-linking, showing that IL-6 production by PMNs is induced in response to at least two distinct extracellular signals: cross-linking of FcγR or rhG-CSF stimulation. These data contribute to a better understanding of the role of rhG-CSF in the inflammatory reactions that often accompany bone marrow transplantation.
MATERIALS AND METHODS
Isolation of PMNs
PMNs were obtained from heparinized venous blood from normal volunteer donors and patients with metastatic breast cancer undergoing priming with rhG-CSF for peripheral blood progenitor cell collection (WVU IRB Protocol No. 13123) using a double density gradient (Histopaque 1077 and 1119; Sigma Diagnostics, St Louis, MO) purification method as described previously.8 Contaminating red blood cells were removed by hypotonic lysis. Purity of isolated PMNs was greater than 98% as determined morphologically after staining with a modified Wright's-Giemsa stain (Accustain; Sigma Diagnostics) and by flow cytometric analysis. To ensure that the PMN samples were not contaminated with monocytes, mRNA samples from neutrophil preparations were checked by reverse transcriptase-polymerase chain reaction (RT-PCR) amplification of the c-fms cDNA. Thirty-two PCR cycles were performed in the c-fms amplification. We used thec-fms specific primer sequences (5′-TCACTTGGGCTGAATCCCTA-3′) and (5′-TCCCTGTCGTCAACTCCTCA-3′) as described by Valerius et al7 and Kaashoek et al.19
Cross-Linking FcγRI and FcγRII on PMNs
PMNs (106 cells/mL) were incubated for 30 minutes on ice with F(ab′)2 anti-FcγRI 32.2 or Fab′ anti-FcγRII IV.3 (Medarex, Inc, Annandale, NJ). Cells were washed and 100 μg/mL goat F(ab′)2 antimouse IgG and IgM antibody (Ab; Biosource International, Camarillo, CA) was added for 1 hour at 37°C to cross-link FcγRI or FcγRII, respectively. RNA was isolated at the indicated time points.
IL-6 Production by PMNs
IL-6 mRNA production.
IL-6 mRNA production by PMNs was evaluated by RT-PCR. RNA was extracted with RNAzol B as described by the manufacturer (Tel-Test Inc, Friendswood, TX). Two micrograms of total RNA was reverse transcribed using 100 μL of Moloneys murine leukemia virus (M-MLV) reverse transcriptase (GIBCO BRL Life Technologies, Grand Island, NY) for 60 minutes at 37°C. IL-6 was amplified using the sense primer (5′-ATGAACTCCTTCTCCACAAGCGC-3′) and antisense primer (5′-GAAGAGCCCTCAGGCTGGACTG-3′). PCR amplification consisted of one cycle of denaturation for 5 minutes at 94°C and primer annealing at 60°C for 5 minutes, followed by 35 cycles of 1.5 minutes at 72°C, 45 seconds at 94°C, and 45 seconds at 60°C, with a final extension of 10 minutes at 72°C. Ten microliters of PCR products was fractionated by 2% agarose gel electrophoresis and stained with ethidium bromide. A 100-bp DNA ladder was loaded on each gel (GIBCO BRL Life Technologies). For each sample, β-actin was amplified to confirm integrity of RNA and efficiency of cDNA synthesis.
Intracellular staining of IL-6 by flow cytometry.
Intracellular staining of IL-6 was performed as previously described.20 Cells were fixed for 10 minutes with 4% paraformaldehyde in phosphate-buffered saline (PBS). PMNs were washed with PBS/bovine serum albumin (BSA) and the pellet was resuspended in 25 μL of permeabilization buffer consisting of 1% fetal calf serum, 0.1% saponin, and 0.1% sodium azide in PBS. Washed cells were incubated with 1 μg of phycoerythrin-labeled anti–IL-6 monoclonal antibody (MoAb; PE-anti–IL-6; Biosource, Camarillo, CA) per 106 cells for 30 minutes at 4°C. Samples were washed three times with PBS/BSA. Ten thousand events were acquired using a FACScan (Becton Dickinson, Thousand Oaks, CA) and analyzed using the software Cellquest Version 1.2 (Becton Dickinson).
Measuring secreted IL-6 by enzyme-linked immunosorbent assay (ELISA).
ELISA plates were prepared as follows. One hundred microliters of goat antihuman IL-6 Ab (10 μg/mL; Biosource International) was added to each well of enhanced protein capturing ELISA plates (Corning Easy Wash; Corning, Corning, NY). The plate was incubated for either 4 hours at room temperature or overnight at 4°C. After two washes with PBS, 200 μL/well of blocking buffer (PBS, 5% fetal bovine serum; GIBCO BRL) was added and the plates were incubated for 2 hours at room temperature. After washing the plates, 100 μL of standard or samples was placed in each well and the covered plate was incubated for 4 hours at room temperature or overnight at 4°C. After four to six washes, 100 μL of biotin-conjugated purified mouse antihuman IL-6 detecting Ab (Biosource International; 0.2 μg/mL in PBS, 5% fetal bovine serum) was added to each well. After 45 minutes of incubation at room temperature, the plate was washed at least six times. One hundred microliters of streptavidin conjugated to horseradish peroxidase (HRP) in dilution buffer (Biosource International) was added per well. After 45 minutes of incubation at room temperature, the plate was washed at least eight times. The covered plate was incubated for 30 minutes at room temperature with stabilized chromogen (100 μL/well; Biosource International). Stop solution (100 μL/well; Biosource International) was added and the plates were read using Microplate Reader (Bio-Rad, Hercules, CA) at OD450.
Samples were run in duplicate and the mean value was taken as the measured concentration. Dilutions of the sample were made to get a reading within the standard range (0 to 10 pg/mL) if the initial concentration was higher than the maximum level of detection.
Effect of G-CSF on Expression of FcγRI and FcγRII on PMNs
Direct immunofluorescence staining using fluorochrome-conjugated MoAbs to FcγRI (clone 32.2; anti-CD64) and FcγRII (clone IV.3; anti-CD32; Medarex, Annandale, NJ) was performed to determine Fc receptor expression on PMNs. Nonspecific Fc receptor binding was blocked by incubation with human IgG (4 mg/mL). Flow cytometric data acquisition was performed using FACScan (Becton Dickinson, San Jose, CA), with 10,000 cells acquired in each measurement and analyzed using the Cellquest Version 1.2 software (Becton Dickinson).
RESULTS
Isolation of PMNs
The isolation method for PMNs yielded cell preparations with greater than 98% purity. Contaminating cells were identified as eosinophils and lymphocytes. To ensure that PMN samples did not have monocyte contamination, neutrophil RNA samples were evaluated by amplification of cDNAs with c-fms–specific primers. c-fms, which encodes the macrophage colony-stimulating factor (M-CSF) receptor, is expressed in monocytes, but not in PMNs.7 The 389-bpc-fms amplicon was not detectable in any PMN cDNA samples used in this study. c-fms–specific amplification was detected in positive control samples prepared from either the monocytic cell line THP-1 or peripheral blood mononuclear cell fractions (data not shown).
Cross-Linking FcγRI or FcγRII Induced IL-6 mRNA Production by PMNs
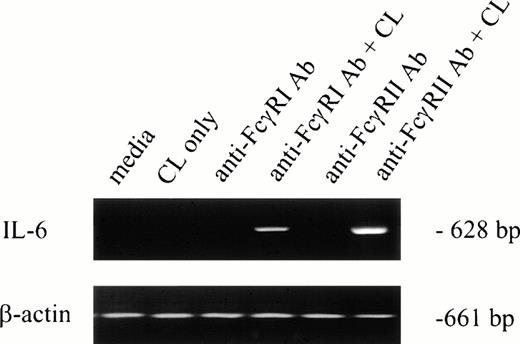
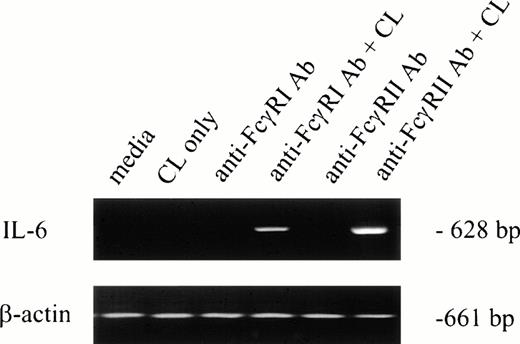
To determine whether IL-6 mRNA was elevated by specific FcγR cross-linking, F(ab′)2 MoAb 32.2 (anti-FcγRI) or Fab′ MoAb IV.3 (anti-FcγRII) was added to neutrophil preparations in vitro. As shown in Fig 1(lanes 3 and 5), IL-6 mRNA was not detectable after MoAb addition. However, cross-linking anti-FcγRI– or anti-FcγRII–coated PMNs with cross-linking antibody resulted in detectable IL-6 mRNA expression in both cases (Fig 1, lanes 4 and 6). Addition of cross-linking antibody alone did not induce IL-6 transcription (Fig 1, lane 2).
IL-6 mRNA expression by PMNs after cross-linking FcγRI or FcγRII. Purified PMNs were obtained from normal donors and IL-6 mRNA expression evaluated by RT-PCR. Data are presented for unstimulated PMNs (lane 1), PMNs after exposure to cross-linker (CL) alone (lane 2), and PMNs after exposure to (Fab′)232.2 (anti-FcγRI Ab) or Fab′ IV.3 (anti-FcγRII Ab) in the absence or presence of cross-linking antibody (lanes 3 through 6). RNA was reverse-transcribed and PCR-amplified using IL-6–specific primers as described in the text. RNA integrity was evaluated by using β-actin primers. The data presented are representative of four nearly identical experiments.
IL-6 mRNA expression by PMNs after cross-linking FcγRI or FcγRII. Purified PMNs were obtained from normal donors and IL-6 mRNA expression evaluated by RT-PCR. Data are presented for unstimulated PMNs (lane 1), PMNs after exposure to cross-linker (CL) alone (lane 2), and PMNs after exposure to (Fab′)232.2 (anti-FcγRI Ab) or Fab′ IV.3 (anti-FcγRII Ab) in the absence or presence of cross-linking antibody (lanes 3 through 6). RNA was reverse-transcribed and PCR-amplified using IL-6–specific primers as described in the text. RNA integrity was evaluated by using β-actin primers. The data presented are representative of four nearly identical experiments.
Cross-Linking FcγRI or FcγRII on PMNs Induced IL-6 Protein Production
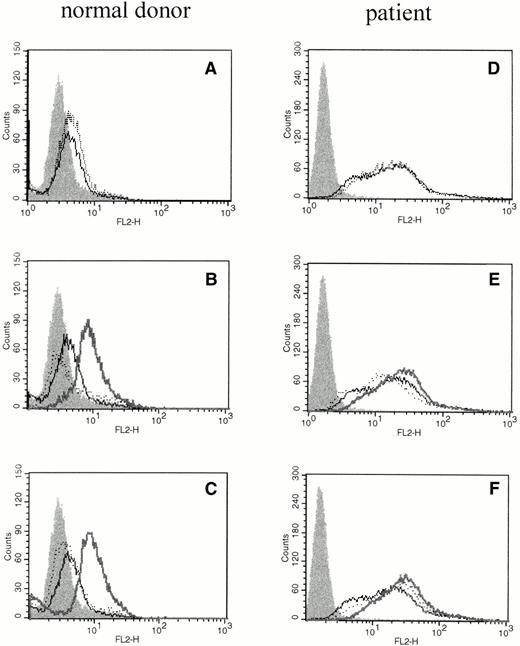
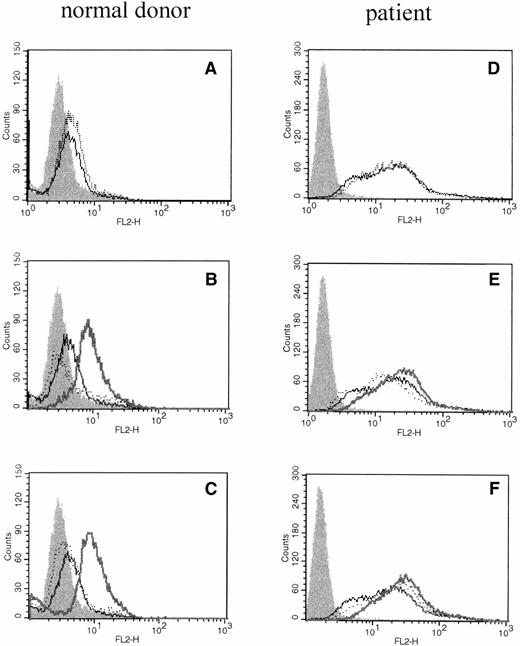
We investigated whether elevations of IL-6 mRNA were accompanied by translation of the protein product using phycoerythrin-conjugated mouse antihuman IL-6 MoAb (Biosource International) and flow cytometric analysis of intracellular proteins. Intracellular IL-6 protein was evident after cross-linking FcγRI or FcγRII (Fig 2B and C), but not when cross-linker (Fig 2A) or primary antibody were added alone (Fig 2B and C). PMNs obtained from patients pretreated with rhG-CSF in vivo showed substantial staining for intracellular IL-6 at baseline, and only moderate increases in the amount of intracellular IL-6 could be seen after cross-linking of FcγRI or FcγRII (Fig 2D through F).
The effect of cross-linking FcγRI or FcγRII on total cellular IL-6 protein production by PMNs. Flow cytometry histograms depict total intracellular IL-6 protein in freshly isolated PMNs from a normal donor (A through C) or PMNs from a patient treated with rhG-CSF in vivo (D through F). In each panel, the solid histogram presents isotype control staining. In (A) and (D), the histograms depict fluorescence staining of freshly isolated PMNs in media (—) and staining of PMNs after exposure to cross-linker alone (···). In (B) and (E), the histograms depict fluorescence staining of PMNs after incubation with media (—) or with (Fab′)2 32.2 anti-FcγRI (···). In (C) and (F), the histograms depict fluorescence staining of PMNs after incubation with media (—) or with Fab′ IV.3 (···). In (B), (C), (E), and (F), the bold line depicts staining after cross-linking anti-FcγRI or anti-FcγRII as listed above. This experiment is representative of four virtually identical experiments.
The effect of cross-linking FcγRI or FcγRII on total cellular IL-6 protein production by PMNs. Flow cytometry histograms depict total intracellular IL-6 protein in freshly isolated PMNs from a normal donor (A through C) or PMNs from a patient treated with rhG-CSF in vivo (D through F). In each panel, the solid histogram presents isotype control staining. In (A) and (D), the histograms depict fluorescence staining of freshly isolated PMNs in media (—) and staining of PMNs after exposure to cross-linker alone (···). In (B) and (E), the histograms depict fluorescence staining of PMNs after incubation with media (—) or with (Fab′)2 32.2 anti-FcγRI (···). In (C) and (F), the histograms depict fluorescence staining of PMNs after incubation with media (—) or with Fab′ IV.3 (···). In (B), (C), (E), and (F), the bold line depicts staining after cross-linking anti-FcγRI or anti-FcγRII as listed above. This experiment is representative of four virtually identical experiments.
IL-6 mRNA Production by PMNs Was Induced by rhG-CSF
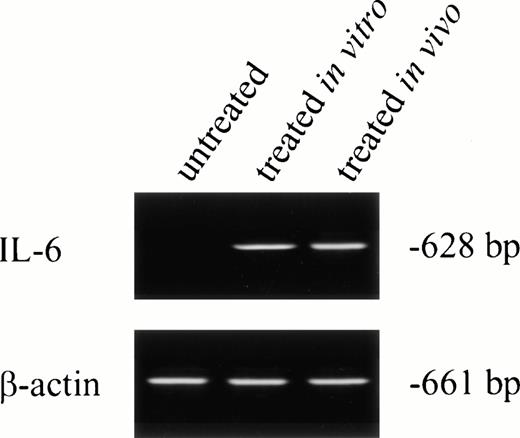
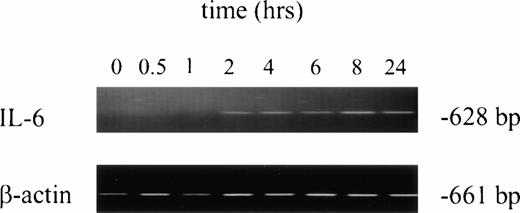
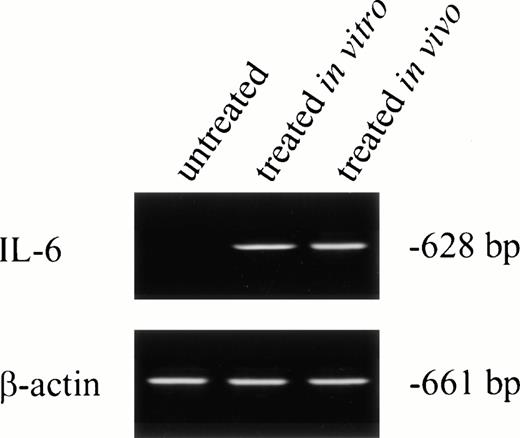
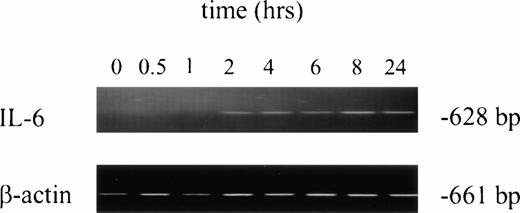
No IL-6 mRNA-amplified product was detectable by RT-PCR from PMNs obtained from normal donors after incubation in medium for 24 hours (Fig 3, lane 1). However, IL-6 mRNA expression was clearly evident after in vitro rhG-CSF treatment of purified PMNs for 24 hours (Fig 3, lane 2). IL-6 mRNA was detected when PMNs were collected from patients undergoing rhG-CSF therapy (Fig 3, lane 3). We investigated this further by evaluating the kinetics of mRNA production by PMNs after in vitro exposure to rhG-CSF for periods ranging from 0 to 24 hours. IL-6 mRNA production was consistently not detectable at baseline when PMNs from normal donors were used. However, amplified IL-6 mRNA was detectable after 2 hours incubation with rhG-CSF (Fig 4) and the 628-bp amplified product remained detectable at 4, 6, 8, and 24 hours with continuous exposure to G-CSF (Fig 4).
IL-6 mRNA expression by PMNs analyzed by RT-PCR. Total cellular RNA was isolated from purified PMNs from a normal donor (the PMNs were maintained in culture media for 24 hours before RNA extraction; lane 1), PMNs from a normal donor treated for 24 hours in vitro with rhG-CSF (lane 2), or PMNs from a patient treated with rhG-CSF in vivo (lane 3). RNA was reverse-transcribed and PCR-amplified using IL-6–specific primers as described in the Materials and Methods. RNA integrity was checked using β-actin primer. This is a representative gel from four experiments performed.
IL-6 mRNA expression by PMNs analyzed by RT-PCR. Total cellular RNA was isolated from purified PMNs from a normal donor (the PMNs were maintained in culture media for 24 hours before RNA extraction; lane 1), PMNs from a normal donor treated for 24 hours in vitro with rhG-CSF (lane 2), or PMNs from a patient treated with rhG-CSF in vivo (lane 3). RNA was reverse-transcribed and PCR-amplified using IL-6–specific primers as described in the Materials and Methods. RNA integrity was checked using β-actin primer. This is a representative gel from four experiments performed.
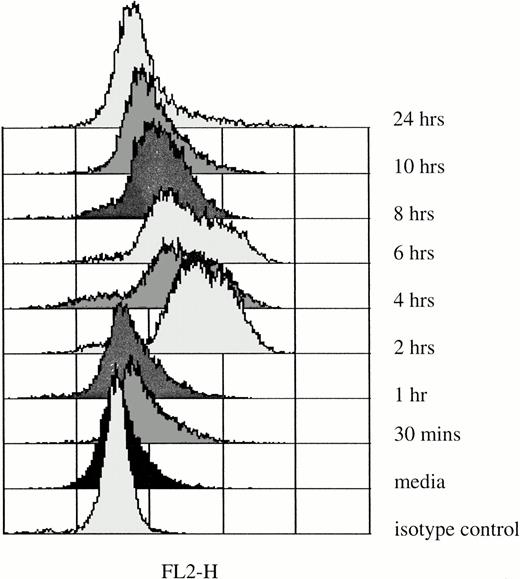
Kinetics of IL-6 mRNA expression after in vitro rhG-CSF stimulation. Purified PMNs from a normal donor were treated in vitro for 30 minutes to 24 hours with 10 ng/mL rhG-CSF. Total cellular RNA was isolated, reverse-transcribed, and PCR-amplified using IL-6–specific primers as described in the Materials and Methods. RNA integrity was evaluated in all experiments using β-actin primers. This is a representative gel from four experiments performed. The trend of IL-6 mRNA induction after rhG-CSF treatment was consistent across all donors evaluated with minor variations in the time of rhG-CSF stimulation necessary before seeing detectable IL-6 mRNA.
Kinetics of IL-6 mRNA expression after in vitro rhG-CSF stimulation. Purified PMNs from a normal donor were treated in vitro for 30 minutes to 24 hours with 10 ng/mL rhG-CSF. Total cellular RNA was isolated, reverse-transcribed, and PCR-amplified using IL-6–specific primers as described in the Materials and Methods. RNA integrity was evaluated in all experiments using β-actin primers. This is a representative gel from four experiments performed. The trend of IL-6 mRNA induction after rhG-CSF treatment was consistent across all donors evaluated with minor variations in the time of rhG-CSF stimulation necessary before seeing detectable IL-6 mRNA.
rhG-CSF Induced IL-6 Protein Production by PMNs
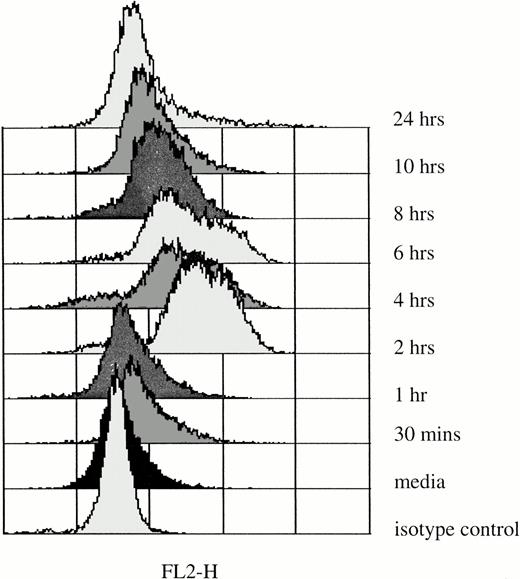
Elevation of IL-6 mRNA in rhG-CSF–treated PMNs does not necessarily correlate with increases in IL-6 protein production in these cells. For that reason, we determined the levels of IL-6 protein production in PMNs immediately after they were obtained from normal donors and after incubation with rhG-CSF in vitro for up to 24 hours (Fig 5). At the initiation of culture, IL-6 levels in PMNs were not different from isotype controls. However, after 2 hours of exposure to 10 ng/mL rhG-CSF, IL-6 protein, detected by elevations of median fluorescence intensities by flow cytometry, was detectable for up to 10 hours (Fig 5).
Intracellular IL-6 protein in PMNs after in vitro rhG-CSF stimulation. Flow cytometric histograms depict staining of freshly isolated PMNs from a normal donor and after incubation with 10 ng/mL rhG-CSF for up to 24 hours. Results presented are from one of four similar experiments. With each time point, simultaneous controls were evaluated and these showed no difference from baseline isotype staining (not shown).
Intracellular IL-6 protein in PMNs after in vitro rhG-CSF stimulation. Flow cytometric histograms depict staining of freshly isolated PMNs from a normal donor and after incubation with 10 ng/mL rhG-CSF for up to 24 hours. Results presented are from one of four similar experiments. With each time point, simultaneous controls were evaluated and these showed no difference from baseline isotype staining (not shown).
Cross-Linking FcγRI or FcγRII on PMNs and rhG-CSF Treatment of PMNs Resulted in IL-6 Protein Secretion
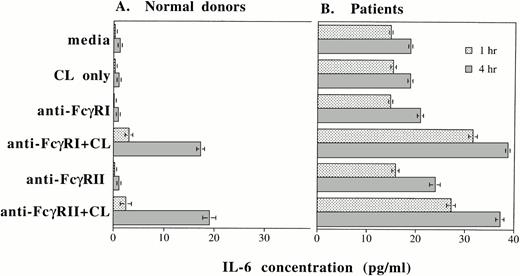
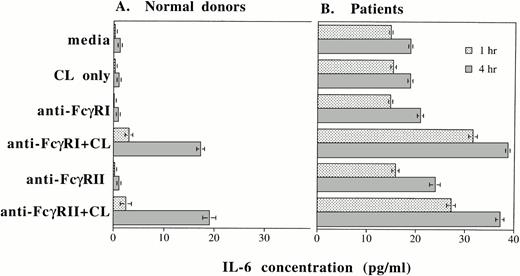
Up to this point, we have shown that IL-6 mRNA production and intracellular IL-6 protein production can be induced by cross-linking FcγRI or FcγRII or by rhG-CSF stimulation. In addition to evaluating intracellular IL-6 production by PMNs, we also determined whether IL-6 protein was secreted by PMNs after cross-linking of FcγRI or FcγRII and after rhG-CSF treatment. Purified PMNs from normal donors were incubated in medium and supernatants were collected after 1 and 4 hours of incubation. At the beginning of the incubation period, IL-6 protein in the supernatants were less than 0.8 pg/mL in all the conditions tested. After 1 hour incubation, increases in detectable IL-6 protein were seen over baseline levels after cross-linking of FcγRI or FcγRII on PMNs from normal donors (n = 3; Fig 6A). A further substantial increase in measured IL-6 protein was recorded 4 hours after FcγRI or FcγRII cross-linked (Fig 6A).
IL-6 protein secretion by PMNs after (A) cross-linking FcγRI or FcγRII and after (B) rhG-CSF stimulation. IL-6 concentrations (in picograms per milliliter) in supernatants after incubation of PMNs cultured in vitro for 1 hour (▪) or 4 hours (▧). IL-6 concentrations were measured by ELISA. Purified PMNs were obtained from three normal donors (A) and from three patients who had been treated with rhG-CSF in vivo (B). Each value depicts the mean ± SEM of three separate experiments. IL-6 concentrations were measured after incubation of PMNs under the conditions listed. CL, cross-linker goat antimouse IgG and IgMF(ab′)2; anti-FcγRI Ab, 32.2 (Fab′)2; anti-FcγRII Ab, IV.3 Fab′.
IL-6 protein secretion by PMNs after (A) cross-linking FcγRI or FcγRII and after (B) rhG-CSF stimulation. IL-6 concentrations (in picograms per milliliter) in supernatants after incubation of PMNs cultured in vitro for 1 hour (▪) or 4 hours (▧). IL-6 concentrations were measured by ELISA. Purified PMNs were obtained from three normal donors (A) and from three patients who had been treated with rhG-CSF in vivo (B). Each value depicts the mean ± SEM of three separate experiments. IL-6 concentrations were measured after incubation of PMNs under the conditions listed. CL, cross-linker goat antimouse IgG and IgMF(ab′)2; anti-FcγRI Ab, 32.2 (Fab′)2; anti-FcγRII Ab, IV.3 Fab′.
In contrast to normal donors, significant baseline concentrations of IL-6 (15 pg/mL) was measured in supernatants from PMNs obtained from patients treated with rhG-CSF. Higher concentrations of IL-6 resulted from cross-linking of FcγRI or FcγRII on these cells (Fig 6B). A further increase in IL-6 protein production measured after 4 hours incubation.
We measured IL-6 concentrations in supernatants from purified PMNs from three normal donors. Supernatants were collected after incubation in media alone or in media with 10 ng/mL rhG-CSF. Supernatants were collected after 0, 0.5, 1, 2, 4, 6, 8, 10, and 24 hours of incubation. The baseline IL-6 concentration at time 0 for PMNs treated with rhG-CSF in vitro was 0.1 pg/mL. However, by 6 hours of incubation, the IL-6 concentration was 0.4 pg/mL; at 10 hours, 4.8 pg/mL; and after 24 hours, 11.9 pg/mL. These data are mean concentrations from three sets of experiments using 3 normal donors and confirm that rhG-CSF treatment stimulates IL-6 protein secretion by PMNs.
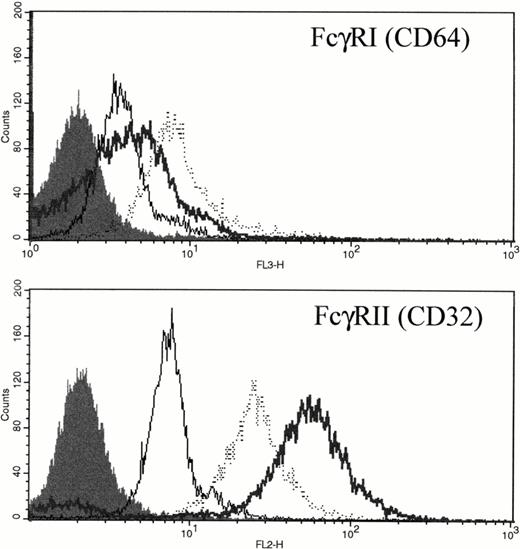
FcγRI and FcγRII Expression on PMNs Was Upregulated by rhG-CSF
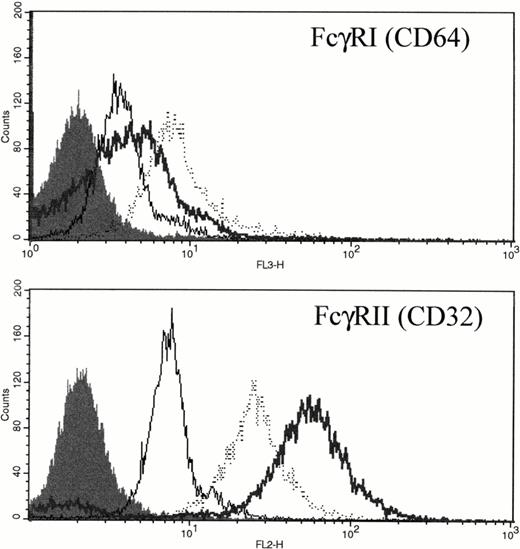
FcγRI is only weakly expressed in unstimulated PMNs (Fig 7 and Gericke et al8). However, after either in vitro incubation with rhG-CSF or in vivo administration of rhG-CSF, FcγRI was consistently elevated compared with unstimulated PMNs (Fig 7A). Unstimulated PMNs express significant levels of FcγRII; however, rhG-CSF treatment, in vitro or in vivo, resulted in elevated FcγRII expression (Fig 7B).
Expression of FcγRI and FcγRII PMNs after exposure to rhG-CSF. Flow cytometric histograms of freshly isolated PMNs from a normal donor (—), PMNs from the same normal donor after treatment with 10 ng/mL rhG-CSF for 24 hours in vitro (···), or PMNs from a patient treated with rhG-CSF in vivo (bold line). Isotype control staining is depicted by the solid histogram. Results are representative examples of four independent experiments.
Expression of FcγRI and FcγRII PMNs after exposure to rhG-CSF. Flow cytometric histograms of freshly isolated PMNs from a normal donor (—), PMNs from the same normal donor after treatment with 10 ng/mL rhG-CSF for 24 hours in vitro (···), or PMNs from a patient treated with rhG-CSF in vivo (bold line). Isotype control staining is depicted by the solid histogram. Results are representative examples of four independent experiments.
DISCUSSION
Inflammatory reactions are regulated by cytokines produced by both monocytes and neutrophils.11 Cytokine release from monocytes after FcγR stimulation has been well characterized21; however, much less is known about the role of FcγR in regulation of neutrophil cytokine production. Previous studies have shown that PMNs produce TNF-α,22,23IL-6,9 and IL-1β9 after stimulation with lipopolysaccharide (LPS) or Candida albicans. IL-6 production by PMNs has also been described after exposure to granulocyte-macrophage colony-stimulating factor (GM-CSF).24 25 However, cytokine secretion by PMNs after FcγR cross-linking or after G-CSF stimulation has not been reported. In this study, we showed that cross-linking either FcγRI or FcγRII on circulating neutrophils resulted in elevation of IL-6 mRNA as well as elevation of both intracellular and secreted IL-6 protein. In addition, we observed that circulating neutrophils from patients undergoing rhG-CSF therapy had detectable IL-6 mRNA as well as detectable intracellular and secreted IL-6 protein in the absence of FcγRI or FcγRII cross-linking and that cross-linking FcγRI or FcγRII on these rhG-CSF–treated PMNs resulted in synergistic increase in the amount of secreted IL-6. Induction of inflammatory cytokine production in patients undergoing rhG-CSF therapy suggests an explanation for clinically significant inflammatory reactions characteristically observed in these patients.
Our studies aimed to determine whether IL-6 mRNA was elevated in PMNs after cross-linking of FcγRI or whether upregulation of FcγRI was required before IL-6 mRNA production occurred. FcγRI expression on PMNs is known to be inducible in vitro with interferon-γ (IFN-γ) or rhG-CSF26-29 and in vivo and in vitro data suggest that FcγRI expression is directly related to plasma G-CSF concentrations.29 In addition, upregulation of FcγRI and FcγRII has been noted by Elsner et al30 in patients with severe congenital neutropenia after rhG-CSF treatment. We found that rhG-CSF elevated FcγRI and FcγRII expression on neutrophils from normal donors or from patients without an underlying neutrophil defect (Fig 7). Upregulation of FcγRI and FcγRII is a hallmark of neutrophil activation.31
We further showed that, although rhG-CSF stimulated IL-6 production by neutrophils, cross-linking FcγRI or FcγRII on rhG-CSF–activated PMNs resulted in further increases in intracellular IL-6 protein expression (Fig 2). It appears that this increase in IL-6 protein production leads to a synergistic increase in IL-6 cytokine secretion after specific cross-linking of FcγRI or FcγRII on rhG-CSF–treated PMNs (Fig 6B). This increased expression of FcγRs may further contribute to inflammatory responses by binding to immune complexes or IgG-coated target cells and cause tissue damage from lysosomal enzyme release and superoxide generation.
It is interesting to note that the addition of the 32.2 F(ab′)2 anti-FcγRI antibody did not result in significant IL-6 production in PMNs from normal donors and that cross-linking of the FcγRI antibody was required before IL-6 mRNA or protein was readily detectable. One possible explanation for the lack of IL-6 production after the addition of antibody 32.2 F(ab′)2 may be antibody excess 32.2 F(ab′)2 was added in high concentrations that would result in most receptors being bound by one arm of F(ab′)2 fragments, precluding cross-linking of adjacent receptors by divalent binding. A second possibility is that the density of FcγRI expressed on the neutrophil cell membrane may be low, particularly in normal donors who have low expression of FcγRI, making cross-linking through 32.2 F(ab′)2 unlikely to occur.
Elevations of IL-6 mRNA following exposure to rhG-CSF occurred in isolated neutrophils within 2 hours or by 1.5 hours after cross-linking FcγRI or FcγRII. Intracellular IL-6 protein production was also seen by 2 hours after G-CSF stimulation of PMNs. This rapid induction of IL-6 mRNA and protein is compatible with results observed in other cell systems.32 Elevation of IL-6 mRNA in osteoblasts was induced as early as 1 hour after stimulation with basic fibroblast growth factor (bFGF) and significantly increased IL-6 protein levels were detected as early as 2 hours.32 Reasons for this rapid increase in detectable mRNA and IL-6 protein production are not investigated here; however, previous studies have shown that regulation of IL-6 mRNA abundance include both rapid transcriptional activation and regulation of mRNA stability.32-35
We did not detect constitutive IL-6 mRNA production in PMNs from normal donors. This contrasts with findings described by Melani et al24 and Palma et al,9 who described constitutive IL-6 mRNA production in PMNs obtained from venipuncture samples. This difference may be the result of slightly different handling of the specimens. Melani et al24 failed to detect amplified IL-6 mRNA in samples obtained from blood bank buffy coats. They suggest this difference may be the result of rapid downregulation of IL-6 after samples were obtained, because, in samples processed within 15 minutes, the IL-6 mRNA amplified product was detectable and in samples processed an hour after it was obtained, IL-6 mRNA was not detected.24
rhG-CSF treatment in vivo resulted in elevated expression of IL-6 mRNA and IL-6 protein production in circulating PMNs. Apoptosis, or programmed cell death, has been implicated in being important in the normal resolution of inflammatory processes and limiting inflammatory tissue injury.36 However, IL-6 has been found to delay PMN apoptosis, thereby increasing the survival of reactive PMNs at sites of inflammation.37 The autocrine production of IL-6 by PMNs could therefore serve to prolong inflammatory responses and result in local tissue injury.
rhG-CSF has increasingly been used in a number of neutropenic conditions, including after high-dose chemotherapy or bone marrow transplantation.38-41 Although rhG-CSF administration results in rapid recovery of circulating PMNs, deleterious effects may also result from growth factor administration. Jain42described cutaneous vasculitis after rhG-CSF administration at a time of increasing ANC, with the cutaneous vasculitis subsiding after the ANC decreased and with the use of topical steroids. A clinical picture resembling Sweet's syndrome (acute febrile neutrophilic dermatosis) has been described after administration of rhG-CSF in a number of hematologic conditions, including myelodysplastic syndrome,43 leukemia,44 and aplastic anemia.45
Similarly, after hematopoietic stem cell transplantation, an engraftment syndrome has been described in which patients develop noninfectious fever and rash during the early and rapid phase of neutrophil recovery.15,16,18 Some transplant patients develop interstitial pulmonary infiltrates and fluid retention.15-18 Steroid administration, along with discontinuing rhG-CSF administration, has been described as resulting in rapid resolution of the clinical findings of fever and rash in patients with this engraftment syndrome.15,16 The pathogenesis of the engraftment syndrome of transplantation has not previously been elucidated, although the involvement of neutrophils has been implicated. The studies presented here provide evidence that PMNs may contribute significantly to the engraftment syndrome by producing the inflammatory cytokine IL-6 after exposure to rhG-CSF. Mianji et al46 found increases in IL-6 mRNA production and IL-6 protein production by PMNs stimulated with LPS could be inhibited in a dose-dependent manner by dexamethasone. Corticosteroid treatment has also been shown to abrogate FcγRI elevation on neutrophils induced in vitro by IFN-γ.47 If IL-6 production by PMNs is a major contributing factor to this clinical syndrome, steroid administration should be effective in inhibiting clinical symptoms.
Taken together, our results show that IL-6 mRNA and protein levels are elevated in neutrophils after exposure to G-CSF or FcγR cross-linking in vitro and in vivo. This elevation of IL-6 in the presence of G-CSF is accompanied by increased expression of FcγRI and FcγRII, which further amplifies inflammatory reactions initiated by increased IL-6. Although additional experiments will be necessary to characterize the inflammatory sequelae of rhG-CSF treatment, it seems reasonable to consider increased IL-6 expression as the underlying cause of the engraftment syndrome that often appears during recovery from blood or marrow transplantation. This clinical complication of rhG-CSF therapy suggests the necessity of optimizing cytokine dosing to allow rapid recovery of neutrophil production in patients with minimal induction of inflammatory reactions.48
ACKNOWLEDGMENT
The authors thank Dr Kenneth R. Meehan for critical review of the manuscript. Flow cytometric studies were performed at the shared Flow Cytometry Core Facility of the Mary Babb Randolph Cancer Center.
S.G.E. was supported by National Institutes of Health Grant No. 1 KO8 AI01183-01A1. This project was supported in part by Grant No. IRG-204 from the American Cancer Society (S.G.E.) and by the DeLynn Bone Marrow Transplantation Research Fund.
Address reprint requests to Solveig G. Ericson, MD, PhD, Blood and Marrow Transplantation Program, Mary Babb Randolph Cancer Center, Robert C. Byrd Health Sciences Center, West Virginia University, PO Box 9162, Morgantown, WV 26506-9162.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.