Abstract
Cellular drug resistance is related to a poor prognosis in childhood leukemia, but little is known about the underlying mechanisms. We studied the expression of P-glycoprotein (P-gp), multidrug resistance (MDR)-associated protein (MRP), and major vault protein/lung resistance protein (LRP) in 141 children with acute lymphoblastic leukemia (ALL) and 27 with acute myeloid leukemia (AML) by flow cytometry. The expression was compared between different types of leukemia and was studied in relation with clinical risk indicators and in vitro cytotoxicity of the MDR-related drugs daunorubicin (DNR), vincristine (VCR), and etoposide (VP16) and the non–MDR-related drugs prednisolone (PRD) and L-asparaginase (ASP). In ALL, P-gp, MRP, and LRP expression did not differ between 112 initial and 29 unrelated relapse samples nor between paired initial and relapse samples from 9 patients. In multiple relapse samples, LRP expression was 1.6-fold higher compared with both initial (P = .026) and first relapse samples (P = .050), which was not observed for P-gp and MRP. LRP expression was weakly but significantly related to in vitro resistance to DNR (Spearman's rank correlation coefficient 0.25, P = .016) but not to VCR, VP16, PRD, and ASP. No significant correlations were found between P-gp or MRP expression and in vitro drug resistance. Samples with a marked expression of two or three resistance proteins did not show increased resistance to the tested drugs compared with the remaining samples. The expression of P-gp, MRP, and LRP was not higher in initial ALL patients with prognostically unfavorable immunophenotype, white blood cell count, or age. The expression of P-gp and MRP in 20 initial AML samples did not differ or was even lower compared with 112 initial ALL samples. However, LRP expression was twofold higher in the AML samples (P < .001), which are more resistant to a variety of drugs compared with ALL samples. In conclusion, P-gp and MRP are unlikely to be involved in drug resistance in childhood leukemia. LRP might contribute to drug resistance but only in specific subsets of children with leukemia.
CELLULAR DRUG RESISTANCE is related to a high risk of treatment failure in childhood leukemia. For example, children with acute lymphoblastic leukemia (ALL) with in vitro drug-resistant leukemic cells have a poorer prognosis compared to patients with relatively sensitive cells at initial diagnosis.1,2 Furthermore, leukemic cells of children with acute myeloid leukemia (AML) are in vitro more resistant to several drugs compared with cells of ALL patients.3
Knowledge about mechanisms of multidrug resistance (MDR) in clinical samples is limited, and studies have mainly focussed on the expression of P-glycoprotein (P-gp). P-gp is a transmembrane protein encoded by the MDR1 gene, which transports anthracyclines, vinca alkaloids, and epipodophyllotoxins out of the cell. In contrast to adult leukemia, contradictory results have been reported about the clinical relevance of P-gp in childhood leukemia; in some studies, P-gp expression was higher at relapse compared with initial leukemias4-7 or was related to long-term survival or relapse risk,8,9 whereas in other studies no such associations were found.10-12
Another drug-efflux pump is the MDR-associated protein (MRP).13 Cell lines in which the MRP gene has been deleted were more sensitive to the anthracyclines, vinca alkaloids, and epipodophyllotoxins, whereas the response to cytosine arabinoside remained unchanged.14 In adult AML, differences in MRP expression between initial and relapsed patients were reported.6,15,16 Inconsistent results were found for the relationship between MRP expression at initial diagnosis and response to chemotherapy.15,17,18 Knowledge about MRP in childhood leukemia is limited. Beck et al5 found no difference in MRP mRNA levels between initial and first relapse ALL samples; however, the expression was higher in multiple relapse ALL samples. MRP mRNA levels were also higher in relapsed AML patients compared with initial patients, but these data may be biased because samples from adults and children were analyzed together.6 It is unknown whether the expression of MRP is related to drug resistance and whether it is of clinical importance in childhood leukemia.
The major vault protein/lung resistance protein (LRP) was initially described in non–small-cell lung cancer cell lines that lacked P-gp.19 Recently, it became evident that LRP is present in a variety of human cancer cell lines that have not previously been exposed to drugs. In these cell lines, the expression of LRP correlated with intrinsic resistance to doxorubicin, vincristine, and platinum compounds.20 LRP has been identified as the human homolog of the rat major vault protein, which contributes to 70% of the mass of vault particles.21 The function of these vaults has been associated with nuclear-cytoplasmic transport,22 although direct evidence is lacking. Recently, the number of vaults was shown to be elevated in drug-resistant cell lines.23 Information about the clinical relevance of LRP is limited. LRP expression has been observed in advanced ovarium carcinoma, in melanoma, in non–small-cell lung carcinoma, and in adult AML and CML.24-28 The expression of LRP was related to a poor response to chemotherapy in advanced ovarium carcinoma and adult AML.24 27 In childhood leukemia, the LRP expression and relevance to drug resistance are unknown.
A pitfall in comparing data on resistance proteins is the use of different techniques and different reference samples such as drug-resistant cell lines and normal cells. A heterogeneous group of patient samples may also limit the interpretation of and comparisons between different studies, such as the use of pooled data of ALL and AML and/or initial and relapse samples. Moreover, comparisons between the expression of resistance proteins and response to combination chemotherapy may underestimate the importance of the protein in question as mechanism of resistance to a single drug. In the present study, we determined the expression of P-gp, MRP, and the novel LRP in a large series of childhood leukemia and normal cells using an optimized and standardized flow cytometrical method.29 The protein expression was compared between different types of leukemia and was related to clinical risk indicators and to the in vitro cytotoxicity of three MDR-related drugs, ie, daunorubicin (DNR), vincristine (VCR), and etoposide (VP16), and two non–MDR-related drugs, ie, prednisolone (PRD) and L-asparaginase (ASP), which are all currently used in the treatment of childhood leukemia.
MATERIALS AND METHODS
Patient samples.
In this study, samples of 168 children with leukemia were examined for resistance protein expression and in vitro drug cytotoxicity (148 fresh and 20 cryopreserved samples). Bone marrow (BM) or peripheral blood (PB) samples were collected from patients of the University Hospital Vrije Universiteit (Amsterdam, The Netherlands) and of hospitals participating in the COALL study group (initial ALL; Prof Dr G. Janka, Hamburg, Germany), the ALL-REZ BFM group (relapsed ALL; Prof Dr G. Henze, Berlin, Germany), and the AML-BFM group (initial and relapsed AML; Prof Dr U. Creutzig and Prof Dr J. Ritter, Münster, Germany). BM and PB samples of 14 nonleukemic children and 3 adults were used to determine the expression of resistance proteins in normal cells. The leukemic patients were classified as follows: 112 initial ALL, 29 relapsed ALL (22 with first relapse and 7 with second or later relapse), 20 initial AML, and 7 relapsed AML (6 with first relapse and 1 second relapse). From 9 patients with ALL, paired samples could be collected at initial diagnosis and at relapse. Within 24 hours of sampling, the mononuclear cells were separated by Lymphoprep (density 1.077 g/mL; Nycomed Pharma, Oslo, Norway) centrifugation at 480g for 15 minutes at room temperature. The mononuclear cells were collected and washed twice in RPMI 1640 (Dutch modification, without L-glutamine; GIBCO BRL, Breda, The Netherlands) supplemented with 1% heat-inactivated fetal calf serum (GIBCO BRL). The percentage of leukemic cells in each sample was determined on cytospin preparations stained with May-Grünwald-Giemsa (Merck, Darmstadt, Germany). When necessary, the percentage of leukemic cells in the sample has been enriched to greater than 80% using monoclonal antibodies linked to magnetic beads (DynaBeads; Dynal, Oslo, Norway) as described previously.30 The immunophenotypes were determined at the central laboratories of the above-mentioned study groups or at the research laboratory of Pediatric Hematology/Oncology, University Hospital Vrije Universiteit. Precursor B-lineage ALL was defined by terminal deoxynucleotidyl transferase (TdT)+/CD19+ and T-lineage ALL by TdT+/cytoplasmic CD3+/CD7+. Precursor B-lineage ALL was further subdivided into proB (CD10−/cytoplasmic μ chain [cμ]−), common (CD10+/cμ−), and preB (CD10+ or −/cμ+).
Antibodies.
P-gp was detected by the monoclonal antibodies C219 (intracellular epitope; Centocor Diagnostics, Malvern, PA) and MRK16 (extracellular; Kamiya Biomedical Co, Thousand Oaks, CA), which are both mouse IgG2a antibodies. MRP was detected by MRPm6 (intracellular, mouse IgG1) and MRPr1 (presumably intracellular, rat IgG2a).31,32 LRP56 (intracellular, mouse IgG2b) was used to determine LRP expression.19 As a positive control, DNA-42 was used (intracellular, mouse IgG2a), which recognizes dsDNA (kindly provided by Dr R. Smeenk, Central Laboratory of Blood Transfusion [CLB], Amsterdam, The Netherlands). Nonspecific isotype-matched antibodies (Dako, Glostrup, Denmark) and omission of the primary antibody were used as negative controls.
Detection of resistance proteins by flow cytometry.
Cells in suspension were fixed using 2% (vol/vol) 37% formaldehyde solution in 100% acetone incubated for 10 seconds at room temperature before incubation with C219, MRK16, MRPr1, and LRP56. For MRPm6, cells were fixed in 100% methanol for 15 minutes at −20°C. These fixation methods resulted in optimal staining intensities and reproducibility of staining, as described elsewhere.29After fixation, cells were washed twice with phosphate-buffered saline supplemented with 0.1% bovine serum albumin (Organon Teknika, Boxtel, The Netherlands) and centrifuged at 4°C (480g for 5 minutes). Purified human Ig (CLB) that contained greater than 90% IgG was used to reduce the background staining especially observed for IgG2a isotypic antibodies in AML samples. To this aim, AML cells were incubated with 0.6% human Ig for 30 minutes on ice and subsequently washed. Blocking of ALL samples with human Ig had no effect on the intensity of the IgG2a isotypic control, because background staining was already low in ALL cells. Next, 0.15 × 106 cells were incubated with the primary antibody for 45 minutes, washed, and incubated with fluorescein isothiocyanate (FITC)-conjugated rabbit antimouse (RAM) F(ab′)2 or rabbit antirat (RAR) antibodies (Dako) for 30 minutes at room temperature. The final antibody concentrations used were 10 μg/mL C219, 5 μg/mL MRK16, 10 μg/mL MRPm6, 1.7 μg/mL MRPr1, 0.6 μg/mL LRP56, 0.2 μg/mL DNA-42, 1:50 FITC-RAM-F(ab′)2, and 1:500 FITC-RAR. Isotypic control antibodies were tested using the same fixative and the same IgG concentration as the specific antibodies. The amount of FITC-labeling was detected by flow cytometry using the 488 nm line of an argon laser (FACScan; Becton Dickinson, Erembodegem, Belgium). Green fluorescence was collected through a 530/30 nm bandpass filter set, using a log mode amplification (FL-1 height). The flow cytometry data were analyzed using LYSYS II software (Becton Dickinson). Leukemic cells were gated based on forward and sideward scatter characteristics, and the fluorescence intensity of this population was expressed in arbitrary units on a log-scale. As a measure for the intensity of staining, the fluorescence index (FI) was used, which represents the ratio between the mean fluorescence intensity of cells stained with the specific antibody and that of cells stained with the isotype-matched control antibody. No difference in the expression of resistance proteins was observed between fresh and cryopreserved samples or between BM and PB samples (both paired and unpaired samples). Therefore, the data were pooled for further analysis.
In vitro drug cytotoxicity assay.
The MTT assay was used to determine the in vitro cytotoxicity of DNR (Cerubidine, Rhône-Poulenc Rorer, Amstelveen, The Netherlands), VCR (Oncovin; Eli Lilly, Amsterdam, The Netherlands), VP16 (Vepesid; Bristol Myers, Weesp, The Netherlands), PRD (Bufa Pharmaceutical Products, Uitgeest, The Netherlands), and ASP (Medac, Hamburg, Germany). The assay conditions were essentially the same as described before.30,33,34 To summarize the test principles, cells were cultured in 96-well plates in the absence (control) or presence of a drug. Each drug was tested at six different concentrations in duplicate. After 4 days of culture at 37°C in humidified air containing 5% CO2, 50 μg 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazoliumbromide (MTT; Sigma, St Louis, MO) was added to each well. Subsequently, cells were incubated with MTT for 6 hours at 37°C. In this period, viable cells can reduce the yellow MTT molecules resulting in a purple formazan product. The formazan crystals were dissolved using acidified (0.04 mol/L HCl) isopropanol, and the quantity of reduced product was measured spectrophotometrically at 562 nm (Bio-Kinetics Reader; Bio-Tek Instruments, Winooski, VT). The optical density value (OD) at 562 nm is linearly related to the amount of viable cells in both ALL and AML samples.33,34 Reproducible test results were obtained when, after 4 days of culture, the control wells without drug contained greater than 70% leukemic cells and the OD of these wells (adjusted for blank values) was higher than 0.050 arbitrary units.30,35 When a sample met these criteria, the leukemic cell survival (LCS) at each drug concentration was calculated by the equation: LCS = (OD drug-containing well/OD wells without drug) × 100% (after subtraction of blank values). The drug concentration lethal to 50% of the cells, ie, the LC50 value, was used as measure for the in vitro drug cytotoxicity. In this study, as in others,33 34 no difference in LC50 values was observed between fresh and cryopreserved cells or between BM and PB samples. Hence, these data were pooled for further analysis.
Statistics.
Differences in the distribution of FI's and LC50 values between unpaired samples were analyzed using the Mann-Whitney U test adjusted for tied ranks. Data of paired samples were analyzed by the Wilcoxon matched pair test. Correlation coefficients were calculated using the Spearman's rank correlation coefficient (Rs). The t-test has been used for significance testing of Rs. A P value ≤.05 was considered statistically significant (two-tailed tested).
RESULTS
Expression of resistance proteins in ALL and AML.
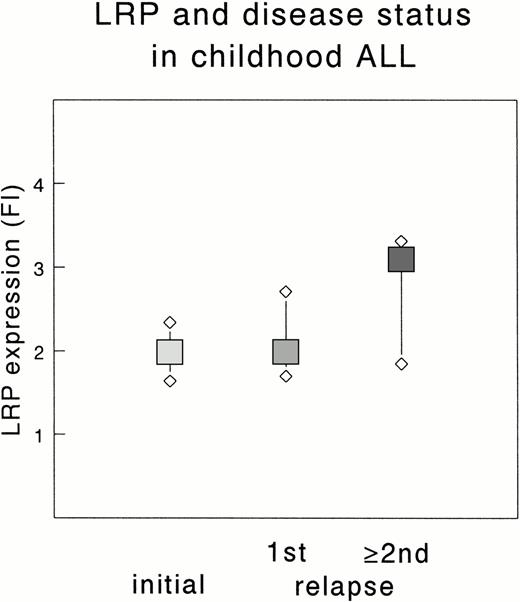
Table 1 summarizes the expression of P-gp, MRP, and LRP in all patients tested, ie, 112 initial ALL, 29 relapsed ALL, 20 initial AML, and 7 relapsed AML patients. The FI of P-gp, MRP, and LRP did not significantly differ between ALL samples taken at initial diagnosis and at (unrelated) relapse. However, the median FI of LRP in multiple relapse samples was 1.6-fold higher compared with samples taken at initial diagnosis or at first relapse of ALL (P = .026 and P = .050, respectively; Fig 1). This difference was not found for P-gp and MRP.
Expression of Resistance Proteins in Childhood ALL, AML, and Normal Cells
| . | P-gp . | MRP . | LRP . | ||
|---|---|---|---|---|---|
| C219 . | MRK16 . | MRPm6 . | MRPr1 . | LRP56 . | |
| ALL | |||||
| Initial | 2.06 (1.63-2.60) | 3.36 (2.51-4.38) | 2.26 (1.98-2.62) | 2.87 (2.49-3.35) | 1.99 (1.64-2.34) |
| Relapse | 2.32 (1.51-2.86) | 3.01 (2.38-3.74) | 2.25 (1.83-2.70) | 2.96 (2.64-3.44) | 2.30 (1.76-3.01) |
| AML | |||||
| Initial | 2.09 (1.38-2.66) | 2.01 (1.76-2.59) | 1.94 (1.68-2.18) | 2.32 (1.50-2.89) | 3.91 (2.86-7.39) |
| Relapse | 1.76 (1.67-2.46) | 2.28 (2.10-2.64) | 2.23 (1.86-2.46) | 2.85 (1.64-3.31) | 2.49 (2.09-2.92) |
| Normal cells | |||||
| PB lymphocytes | 1.88 (1.49-2.01) | 3.24 (2.74-4.06) | 1.88 (1.55-2.02) | 2.70 (2.18-2.99) | 5.14 (4.25-7.22) |
| BM-150 | 1.52/1.82 | 1.57/2.87/3.31 | NT | 1.27/1.35/1.93 | 1.86/2.33/2.37 |
| . | P-gp . | MRP . | LRP . | ||
|---|---|---|---|---|---|
| C219 . | MRK16 . | MRPm6 . | MRPr1 . | LRP56 . | |
| ALL | |||||
| Initial | 2.06 (1.63-2.60) | 3.36 (2.51-4.38) | 2.26 (1.98-2.62) | 2.87 (2.49-3.35) | 1.99 (1.64-2.34) |
| Relapse | 2.32 (1.51-2.86) | 3.01 (2.38-3.74) | 2.25 (1.83-2.70) | 2.96 (2.64-3.44) | 2.30 (1.76-3.01) |
| AML | |||||
| Initial | 2.09 (1.38-2.66) | 2.01 (1.76-2.59) | 1.94 (1.68-2.18) | 2.32 (1.50-2.89) | 3.91 (2.86-7.39) |
| Relapse | 1.76 (1.67-2.46) | 2.28 (2.10-2.64) | 2.23 (1.86-2.46) | 2.85 (1.64-3.31) | 2.49 (2.09-2.92) |
| Normal cells | |||||
| PB lymphocytes | 1.88 (1.49-2.01) | 3.24 (2.74-4.06) | 1.88 (1.55-2.02) | 2.70 (2.18-2.99) | 5.14 (4.25-7.22) |
| BM-150 | 1.52/1.82 | 1.57/2.87/3.31 | NT | 1.27/1.35/1.93 | 1.86/2.33/2.37 |
Values represent the median FI and the 25th and 75th percentiles (in parentheses), which are based on 101 to 112 initial ALL, 23 to 29 relapsed ALL, 13 to 20 initial AML, 5 to 7 relapsed AML, and 8 to 14 normal PB samples. Initial versus relapsed AML: LRP56, P = .041. Initial ALL versus initial AML: MRK16, P < .001; MRPm6,P = .007; MRPr1, P = .01; LRP56, P < .001. Initial ALL versus normal PB lymphocytes: MRPm6, P = .008; LRP56, P < .001.
Abbreviation: NT, not tested.
The FI of 2 to 3 normal BM samples are listed.
Expression of LRP in initial and relapsed childhood ALL. The median FI of each group is depicted by a square; the upper and lower diamonds represent the 75th and 25th percentile, respectively. Data are based on 112 initial, 22 first relapse (1st), and 7 multiple relapse (≥2nd) samples. The difference between the FI of multiple relapse samples and initial or first relapse samples is significant (P = .026 and P = .050, respectively).
Expression of LRP in initial and relapsed childhood ALL. The median FI of each group is depicted by a square; the upper and lower diamonds represent the 75th and 25th percentile, respectively. Data are based on 112 initial, 22 first relapse (1st), and 7 multiple relapse (≥2nd) samples. The difference between the FI of multiple relapse samples and initial or first relapse samples is significant (P = .026 and P = .050, respectively).
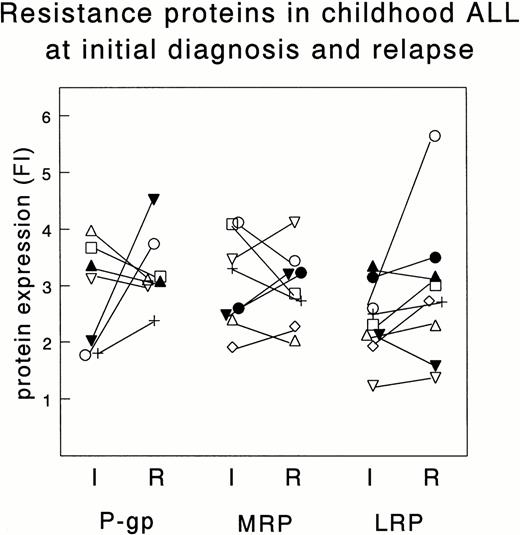
Figure 2 shows the FI of the resistance proteins in paired samples taken at initial diagnosis and at relapse of 9 children with ALL. No significant differences between initial and relapse samples were found: both increased and decreased expression of P-gp, MRP, and LRP occurred at relapse.
Expression of resistance proteins in samples taken both at initial diagnosis (I) and at relapse (R) of the same patients. Depicted are the FI for P-gp, MRP, and LRP using, respectively, MRK16 (n = 7), MRPr1 (n = 8), and LRP56 (n = 9). Each patient is indicated by the same symbol for each resistance protein. Differences in FI are not significant.
Expression of resistance proteins in samples taken both at initial diagnosis (I) and at relapse (R) of the same patients. Depicted are the FI for P-gp, MRP, and LRP using, respectively, MRK16 (n = 7), MRPr1 (n = 8), and LRP56 (n = 9). Each patient is indicated by the same symbol for each resistance protein. Differences in FI are not significant.
In AML patients, the FI of P-gp and MRP did not differ between initial and relapsed patients, but the FI of LRP was lower in the 7 relapse samples tested (P = .041). The expression of resistance proteins was compared between AML and ALL patients at initial diagnosis (Table 1). The FI of P-gp using the C219 antibody did not differ between AML and ALL patients, but the FI using MRK16 was median 1.7-fold lower in AML cells (P < .001). The FI of MRP was slightly lower in AML compared with ALL for both the MRPm6 antibody (median, 1.2-fold; P = .007) and the MRPr1 antibody (median, 1.2-fold; P = .010). AML cells expressed significantly more LRP compared with ALL cells (median, 2.0-fold; P < .001). The unexpectedly lower detection of P-gp using MRK16 in AML samples could not be explained by the blocking procedure before incubation with IgG2a isotypic antibodies, because this decreased the signal of both the isotypic control and the specific antibody to the same extent.
The expression of P-gp, MRP, and LRP was also studied in normal cells (Table 1). For P-gp, the FI using C219 or MRK16 was comparable between normal PB lymphocytes and initial ALL cells. The detection of MRP by MRPm6 showed a slightly lower FI in normal lymphocytes compared with initial ALL patients (P = .008), which was not found for MRPr1. For LRP, the median FI was 2.6-fold higher in PB lymphocytes compared with initial ALL patients (P < .001). The expression of resistance proteins in normal BM cells was studied in only 3 samples. In Table 1, these results are included to give an indication for the expression levels of the resistance proteins in these cells.
Correlation between expression of resistance proteins and immunophenotype, white blood cell count (WBC), and age in childhood ALL.
ALL patients at initial diagnosis were classified by immunophenotype as proB (n = 2), common/preB (n = 90), and T-ALL (n = 20). The expression of the resistance proteins in common/preB and T-ALL patients is shown in Table 2. The expression of P-gp using C219 was 1.3-fold lower in T-ALL compared with common/preB samples (P = .001), whereas for MRK16 no significant difference was found. The median FI using MRPr1 was slightly higher in T-ALL compared with common/preB ALL patients (median, 1.2-fold; P < .001), which was not found using MRPm6. The median FI of LRP was 1.4-fold lower in T-ALL compared with common/preB ALL patients (P < .001).
Correlation Between Expression of Resistance Proteins and Immunophenotype, WBC, and Age in Childhood ALL at Initial Diagnosis
| . | P-gp . | MRP . | LRP . | ||
|---|---|---|---|---|---|
| C219 . | MRK16 . | MRPm6 . | MRPr1 . | LRP56 . | |
| Immunophenotype | |||||
| Common/preB | 2.21 (1.75-2.71) | 3.40 (2.52-4.43) | 2.24 (1.92-2.60) | 2.81 (2.42-3.16) | 2.08 (1.78-2.54) |
| T | 1.67 (1.48-1.78) | 3.11 (2.29-3.97) | 2.35 (2.04-2.78) | 3.49 (3.15-3.82) | 1.49 (1.37-1.65) |
| WBC | |||||
| <25/nL | 2.29 (1.75-2.81) | 3.51 (2.76-4.84) | 2.15 (1.90-2.54) | 2.96 (2.69-3.35) | 2.00 (1.75-2.38) |
| ≥25/nL | 1.89 (1.54-2.42) | 3.28 (2.34-3.97) | 2.35 (2.04-2.67) | 2.86 (2.41-3.35) | 1.98 (1.53-2.50) |
| Age (mo) | |||||
| ≥12-<120 | 2.20 (1.75-2.63) | 3.36 (2.52-4.06) | 2.23 (1.92-2.62) | 2.86 (2.49-3.34) | 2.01 (1.68-2.34) |
| ≥120 | 1.69 (1.42-2.15) | 3.15 (2.26-4.72) | 2.37 (2.07-2.62) | 3.08 (2.48-3.50) | 1.88 (1.57-2.28) |
| . | P-gp . | MRP . | LRP . | ||
|---|---|---|---|---|---|
| C219 . | MRK16 . | MRPm6 . | MRPr1 . | LRP56 . | |
| Immunophenotype | |||||
| Common/preB | 2.21 (1.75-2.71) | 3.40 (2.52-4.43) | 2.24 (1.92-2.60) | 2.81 (2.42-3.16) | 2.08 (1.78-2.54) |
| T | 1.67 (1.48-1.78) | 3.11 (2.29-3.97) | 2.35 (2.04-2.78) | 3.49 (3.15-3.82) | 1.49 (1.37-1.65) |
| WBC | |||||
| <25/nL | 2.29 (1.75-2.81) | 3.51 (2.76-4.84) | 2.15 (1.90-2.54) | 2.96 (2.69-3.35) | 2.00 (1.75-2.38) |
| ≥25/nL | 1.89 (1.54-2.42) | 3.28 (2.34-3.97) | 2.35 (2.04-2.67) | 2.86 (2.41-3.35) | 1.98 (1.53-2.50) |
| Age (mo) | |||||
| ≥12-<120 | 2.20 (1.75-2.63) | 3.36 (2.52-4.06) | 2.23 (1.92-2.62) | 2.86 (2.49-3.34) | 2.01 (1.68-2.34) |
| ≥120 | 1.69 (1.42-2.15) | 3.15 (2.26-4.72) | 2.37 (2.07-2.62) | 3.08 (2.48-3.50) | 1.88 (1.57-2.28) |
Values represent the median FI and the 25th and 75th percentiles (in parentheses), which are based on the following number of ALL samples at initial diagnosis: 80 to 90 common/preB, 19 to 20 T-ALL, 40 to 47 WBC <25/nL, 57 to 62 WBC ≥25/nL, 73 to 80 age ≥12 to <120 months, and 25 to 28 age ≥120 months. Common/preB versus T-ALL: C219,P = .001; MRPr1, P < .001; LRP56, P < .001. Age between 12 and 120 months versus ≥120 months: C219, P = .016.
Initial ALL patients were subgrouped by WBC and age using the risk group stratification criteria of the COALL-92 study (Table 2). The expression of P-gp, MRP, and LRP was comparable between children with a WBC less than 25/nL and those with a WBC ≥25/nL. The expression of MRP and LRP did also not differ between children with an age between 12 and 120 months and those older than 120 months. P-gp detected by C219 was 1.3-fold lower in the oldest group (P = .016), whereas for MRK16 this difference was not found. Within the group of common/preB ALL patients, the expression of P-gp, MRP, and LRP was not related to WBC and age.
Comparison between the expression of resistance proteins and in vitro drug cytotoxicity.
AML patients were more resistant to DNR (median, 1.7-fold; P< .001), VCR (median, 4-fold; P = .015), and PRD (median, >800-fold; P < .001) but not to VP16 and ASP compared with ALL patients. The FI of LRP correlated weakly with the cytotoxicity of DNR in ALL (Rs, 0.25; P = .016) but not in AML samples. No significant correlation was found between LRP and the cytotoxicity of the other 4 drugs. Neither in ALL samples nor in AML samples was the expression of P-gp and MRP correlated with the LC50 values of DNR, VCR, VP16, PRD, or ASP. One exception was found for the FI using MRPm6 in ALL samples, which was weakly related to the LC50 value of VP16 (Rs, 0.22; P = .038).
The FI for P-gp using C219 was weakly related to the FI using MRK16 (Rs, 0.30; P < .001). The cytotoxicity of DNR, VCR, VP16, PRD, or Asp did not differ between patients with the highest and lowest FI for both antibodies. For MRP, the FI using MRPm6 was not significantly related to the FI using MRPr1, and no difference in in vitro drug cytotoxicity was observed between patients with the highest and lowest FI for both antibodies.
A resistance protein profile was made of each ALL patient by combining the results obtained using MRK16, MRPr1, and LRP56. Samples with an FI higher than the median FI were defined positive for the protein in question. The cytotoxicity of DNR, VCR, VP16, PRD, or ASP did not differ between samples that were positive for two or three of the proteins and samples that were positive for one or none of the proteins.
DISCUSSION
The expression of P-gp, MRP, and LRP was studied in childhood ALL and AML and was related to different risk indicators (initial or relapse, immunophenotype, WBC, and age) and to in vitro cytotoxicity of three MDR-related drugs, ie, DNR, VCR, and VP16, and two non–MDR-related drugs, ie, PRD and ASP. In earlier studies we showed that resistance to these drugs was related to the above-mentioned risk indicators, eg, (1) relapsed ALL patients were more resistant to DNR, PRD, and ASP but not to VCR and VP16 compared with patients at initial diagnosis36; (2) T-ALL patients were more resistant to DNR, VCR, PRD, and ASP compared with common/preB ALL patients37,38; and (3) AML patients were more resistant to VCR and PRD than ALL patients.3 In the present study, AML patients were also significantly more resistant to DNR compared with ALL patients (median, 1.7-fold).
P-gp expression was studied using two antibodies, ie, C219 and MRK16. The FI using C219 was only weakly related to the FI using MRK16, which has also been observed in other studies.39,40 The lack of consensus between both antibodies may be explained by the fact that C219 binds to a cytoplasmic epitope and recognizes both MDR1 and MDR3 products, whereas MRK16 binds to an external epitope and is specific for MDR1 products.41-43 Irrespective of which antibody was used, we found no evidence that P-gp expression is an important mechanism of drug resistance in childhood leukemia. (1) No difference was found in P-gp expression between initial and relapsed patients. (2) Expression did not clearly differ between risk groups of patients identified by immunophenotype, WBC, or age. (3) Expression did not differ or was even lower in initial AML compared with ALL samples. (4) Expression of P-gp in normal lymphocytes was comparable with the expression of ALL samples. (5) No association between P-gp expression and cytotoxicity of both MDR and non–MDR-related drugs was found. The lower expression of P-gp in childhood AML samples has also been demonstrated by others; Beck et al44 showed that the MDR1/P-gp mRNA levels were lower in initial AML samples compared with initial ALL and normal BM cells. These levels were increased in relapsed AML and in second or later relapsed ALL, which was not found at the protein level in the present study. In an earlier study, we already showed that the resistance modifiers verapamil and cyclosporin A had no effect on the accumulation and cytotoxicity of DNR and VCR in childhood ALL.11 In summary, our data do not suggest that P-gp expression is related to drug resistance in childhood ALL and AML. Although some studies showed that P-gp expression may be related to a poor prognosis,8,9 other studies10-12 and the present data give no indication that P-gp is clinically important in childhood leukemia.
Another transporter protein that might contribute to drug resistance in leukemia is MRP. Recently, besides MRP (now called MRP-1), at least 4 other homologs of this protein have been identified.45 It is unknown yet whether these homologs are also related to multidrug resistance. Sequence analysis showed that the protein segment used to generate the MRPm6 antibody was derived from the most homologous portion located in the C-terminal part of MRP. In contrast, a more MRP-1–specific segment in the N-terminal part of the protein was used for the MRPr1 antibody. This may explain the absence of a significant correlation between the FI using MRPm6 and MRPr1 in our study. Irrespective of which antibody was used, no difference between initial and relapse ALL samples was observed neither compared with the first nor with multiple relapse samples. The latter is in contrast with a study of Beck et al,5 who observed elevated MRP mRNA levels in multiple relapse ALL samples. In our study, the MRP expression was not related to the in vitro cytotoxicity of DNR, VCR, PRD, and ASP. A weak correlation was found between VP16 and the FI using MRPm6 in ALL cells; however, this may not be specific for the resistance-associated MRP-1 homolog, because this relationship was not found using MRPr1. T-ALL cells have a slightly higher expression of MRP compared with precursor B-lineage, but it is unlikely that this small difference can explain the resistance to drugs observed in T-ALL samples.37,38 Moreover, this difference may be lineage-specific, because normal T lymphocytes also express more MRP than B lymphocytes.15,46 Also, in AML samples, we found no evidence that MRP is involved in the resistance to drugs observed in these patients. The expression of MRP in AML patients may be even lower than in ALL patients. Based on these data, it is unlikely that expression of the MRP protein is an important mechanism of drug resistance in childhood leukemia. In other tumor types, MRP may be more important, eg, a high frequency of MRP positivity has been associated with a poor clinical outcome in childhood neuroblastoma.47 48
LRP has been identified as the major component of vaults, which are large ribonucleoprotein particles.21 Approximately 5% of the vaults is associated with the nuclear membrane and nuclear pore complex, but the majority of vaults are located in the cytoplasm.22,49 Knowledge about the role of LRP and vaults in drug resistance is still very limited. Recently, the number of vaults has been shown to be increased in non–P-gp MDR cell lines compared with parental cell lines.23 Although direct evidence is lacking, vaults may contribute to drug resistance by redistributing the drug from the nucleus (drug target) to the cytoplasm. This may explain the lower nuclear-cytoplasmic ratio that was found for doxorubicin in LRP-expressing SW1573/2R120 cells compared with LRP-negative parental cells.19,50 LRP/vaults may also be involved in the sequestration of drugs into vesicles. This may explain the granular staining of LRP found in drug-resistant cell lines, which we and others also observed in leukemic cells using immunocytochemistry (data not shown).19,27 Recently, LRP expression has been related to a poor response to chemotherapy in advanced ovarium carcinoma and adult AML.24,27 In the present study, the expression of LRP was weakly but significantly related to in vitro resistance to DNR in childhood ALL, whereas no significant relationship was found between LRP and the other 4 drugs tested. This illustrates the multifactorial phenomenon of drug resistance; one protein cannot explain resistance to a variety of drugs in all patients. In this respect, drug resistance in T-ALL samples cannot be explained by elevated LRP expression, indicating that other mechanisms should be more important in these cells; eg, an increased expression or activity of glutathione-S-transferases, elevated levels of glutathione, and inhibition of (CD95-mediated) apoptosis.51-54 In AML patients, LRP expression did not correlate with the cytotoxicity of DNR or the other drugs. However, it should be noticed that expression of LRP in initial AML cells, as well as in multiple relapse ALL cells and normal PB lymphocytes, was higher compared with initial ALL cells, all being more resistant to DNR and other drugs compared with ALL cells.3,33 36
In clinical practice, children with leukemia receive a multiagent chemotherapy including corticosteroids, vinca alkaloids, L-asparaginase, antimetabolites, anthracyclines, and epipodophyllotoxins. Treatment failures may be related to the cellular resistance to one or more classes of drugs and to the pharmacokinetics of drugs in each patient. In the present study, we showed that P-gp and MRP are not related to any of the poor-risk indicators and cellular resistance to MDR and non–MDR-related drugs, whereas LRP may contribute to drug (DNR) resistance in subsets of poor-risk patients in childhood leukemia. Further studies are warranted to address the functional role of LRP in cellular drug resistance and its relationship with clinical outcome in childhood leukemia.
ACKNOWLEDGMENT
The authors thank the members of the German COALL study group, ALL-REZ BFM group, and the AML-BFM group for providing the leukemic samples.
Supported by Dutch Cancer Society Grant No. VU 93-641.
Address reprint requests to M.L. den Boer, Department of Pediatric Hematology/Oncology, University Hospital Vrije Universiteit, PO Box 7057, 1007 MB Amsterdam, The Netherlands.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal