Abstract
Despite the widespread usage of hydroxyurea in the treatment of both malignant and nonmalignant diseases and a recent expansion in the recognition of its potential therapeutic applications, there have been few detailed studies of hydroxyurea's pharmacokinetic (PK) behavior and oral bioavailability. Parenteral administration schedules have been evaluated because of concerns about the possibility for significant interindividual variability in the PK behavior and bioavailability of hydroxyurea after oral administration. In this PK and bioavailability study, 29 patients with advanced solid malignancies were randomized to treatment with 2,000 mg hydroxyurea administered either orally or as a 30-minute intravenous (IV) infusion accompanied by extensive plasma and urine sampling for PK studies. After 3 weeks of treatment with hydroxyurea (80 mg/kg orally every 3 days followed by a 1-week washout period), patients were crossed over to the alternate route of administration, at which time extensive PK studies were repeated. Three days later, patients continued treatment with 80 mg/kg hydroxyurea orally every 3 days for 3 weeks, followed by a 1-week rest period. Thereafter, 80 mg/kg hydroxyurea was administered orally every 3 days. Twenty-two of 29 patients had extensive plasma and urine sampling performed after treatment with both oral and IV hydroxyurea. Oral bioavailability (F) averaged 108%. Moreover, interindividual variability in F was low, as indicated by 19 of 22 individual F values within a narrow range of 85% to 127% and a modest coefficient of variation of 17%. The time in which maximum plasma concentrations (Cmax) were achieved averaged 1.22 hours with an average lag time of 0.22 hours after oral administration. Except for Cmax, which was 19.5% higher after IV drug administration, the PK profiles of oral and IV hydroxyurea were very similar. The plasma disposition of hydroxyurea was well described by a linear two-compartment model. The initial harmonic mean half-lives for oral and IV hydroxyurea were 1.78 and 0.63 hours, respectively, and the harmonic mean terminal half-lives were 3.32 and 3.39 hours, respectively. For IV hydroxyurea, systemic clearance averaged 76.16 mL/min/m2 and the mean volume of distribution at steady-state was 19.71 L/m2, whereas Cloral/F and Voral/F averaged 73.16 mL/min/m2 and 19.65 L/m2, respectively, after oral administration. The percentage of the administered dose of hydroxyurea that was excreted unchanged into the urine was nearly identical after oral and IV administration—36.84% and 35.82%, respectively. Additionally, the acute toxic effects of hydroxyurea after treatment on both routes were similar. Relationships between pertinent PK parameters and the principal toxicity, neutropenia, were sought, but no pharmacodynamic relationships were evident. From PK, bioavailability, and toxicologic standpoints, these results indicate that there are no clear advantages for administering hydroxyurea by the IV route except in situations when oral administration is not possible and/or in the case of severe gastrointestinal impairment.
IN THE MORE THAN three decades since the ribonucleotide reductase inhibitor hydroxyurea was first evaluated clinically, a number of diverse applications have been identified for its use.1-4 Although many of these applications were identified from the outset, others in both malignant and nonmalignant diseases are still evolving. The principal use of hydroxyurea has been as a myelosuppressive agent in the treatment of myeloproliferative syndromes, particularly chronic myelogenous leukemia and polycythemia vera.5-8 Although treatment of chronic myelogenous leukemia with hydroxurea for many years had been reserved for patients whose disease was no longer responsive to busulfan, hydroxyurea is currently preferred over busulfan as initial therapy for several reasons.5-7 First, both initial, uncontrolled studies and more recent prospective, randomized trials have suggested that hydroxyurea is more effective than busulfan in prolonging the chronic phase of the disease and possibly overall survival.5-7Second, because hydroxyurea has less effect on hematopoietic stem cells, the prolonged cytopenias that are occasionally observed with busulfan are noted much less readily with hydroxyurea. Finally, the leukemogenic potential of hydroxyurea may be less than that of busulfan.
The use of hydroxyurea as a single agent has never been the mainstay of treatment for advanced solid tumors; however, recent studies indicate that the agent might be effective as a biochemical modulator of the effects of other antimetabolites such as cytosine arabinoside, fludarabine, and 5-fluorouracil, or DNA-damaging agents such as etoposide or cisplatin.10-12 There has also been considerable interest in evaluating the potential radiosensitizing properties of hydroxyurea based on its ability to synchronize cells in radiation-sensitive phases of the cell cycle and inhibit the repair of radiation-induced DNA damage.12-14 More recently, hydroxyurea has been shown to possess antiviral properties against human immunodeficiency virus type I and to accelerate the loss of extrachromosomal amplified genes present in double minute chromosomes.15-17 Treatment of cells in vitro with clinically achievable drug concentrations results in enhanced loss of amplified oncogenes and drug resistance genes, and clinical strategies based on this phenomenon are under consideration.16 17
In the hemoglobinopathies, particularly sickle cell anemia, hydroxyurea stimulates fetal hemoglobin synthesis, which may be caused, in part, by inhibition of DNA synthesis in red blood cell progenitors or a specific alteration of fetal hemoglobin transcription.18-21 In both pilot studies and a randomized, double-blind multicenter study involving patients with sickle cell anemia, treatment with hydroxyurea significantly decreased the incidence of painful sickle cell crises, thereby establishing it as the first clinically acceptable drug with activity in this disorder.9,22 23
Hydroxyurea is conventionally administered by the oral route. Although oral administration has definite advantages in terms of patient convenience, particularly in situations in which protracted, chronic low-dose treatment portends optimal biological activity, there have been concerns about the potential bioavailability of oral hydroxyurea. However, the bioavailability of oral hydroxyurea has never undergone rigorous evaluation and quantitation. Most of these concerns specifically relate to the potential for significant interpatient variability, limiting the predictability of achieving optimal drug concentrations to exploit the schedule dependency that has been observed in preclinical studies.24 For these reasons, an investigational parenteral formulation of hydroxyurea has been evaluated in patients with advanced malignancies.25-28However, the comparative pharmacokinetic (PK) characteristics and bioavailability of intravenous (IV) and oral hydroxyurea have not been rigorously evaluated despite the widespread use of the agent, and the relative indications of these formulations are not clear. The principal objectives of this study were to (1) evaluate and quantitate the bioavailability of oral hydroxyurea; (2) characterize the PK behavior of hydroxyurea administered both orally and IV; and (3) assess and compare the acute toxic effects of both oral and IV hydroxyurea in patients with advanced solid malignancies.
MATERIALS AND METHODS
Eligibility.
Patients with histologically documented, evaluative or measurable solid tumors refractory to conventional chemotherapy or for whom no effective therapy existed were candidates for this study. Eligibility criteria included:
Age ≥18 years.
A Southwest Oncology Group performance status ≤3.
A life expectancy of at least 12 weeks.
No radiation therapy and/or chemotherapy within 21 days of entering onto protocol (42 days if those treated with mitomycin C or a nitrosourea).
Adequate hematopoietic (white blood cell [WBC] count ≥3,000/μL, absolute neutrophil count [ANC] ≥1,500/μL, platelets ≥100,000/μL, and hemoglobin ≥8 g/dL), hepatic (total bilirubin ≤1.5 mg/dL; serum glutamic-oxaloacetic transaminase [SGOT] and serum glutamic-pyruvic transaminase [SGPT] ≤3-fold times the upper limit of the institutional normal value) and renal (creatinine ≤1.5 mg/dL) functions.
Serum electrolytes within 10% of upper limit of institutional normal values, albumin ≥3.0 g/dL, and glucose ≤250 mg/dL).
No evidence of gastrointestinal impairment, atypical frequency of bowel movements, malignant bowel involvement, or prior surgical excision of the bowel.
No other coexisting medical problems of sufficient severity to limit full compliance with the study.
All patients gave written consent according to federal and institutional guidelines before treatment.
Study design, dosage, and drug administration.
This randomized crossover design study was designed so that patients were initially treated with either 2,000 mg of hydroxyurea either orally or by a 30-minute IV infusion on day 1 of the first course. Patients fasted 8 hours before and 4 hours after the administration of oral hydroxyurea. Water was permitted ad lib except for 1 hour prior to dosing until 15 minutes after drug administration. Coadministration of oral medications were not permitted during this period in the PK phases of the study. Blood and urine samples were collected during this study phase for PK studies. Three days later, all patients continued to receive hydroxyurea at a dose of 80 mg/kg orally every 3 days for 3 weeks. During the fourth week, the patients did not receive any study medication. On the fifth week, the patients were crossed over to receive 2,000 mg of hydroxyurea by the alternate route of administration, and blood and urine samples were also collected for PK studies. Three days later, all patients continued taking hydroxyurea at a dose of 80 mg/kg orally every 3 days for 3 weeks. During the eighth week, the patients did not receive any study medication. Subsequent treatment consisted of hydroxyurea administered at a dose of 80 mg/kg orally every 3 days for at least 6 weeks or until tumor progression was documented, unacceptable toxicity occurred, or the patient refused further treatment. Each 6 weeks of maintenance therapy was considered one complete course. Antacids, H2-histamine antagonists, prostaglandin inhibitors (eg, misoprostrol), and sucralfate were not permitted during the PK portions of the trial. Except for day 1 of the fifth week, at which time oral or IV dosing was to be performed with PK studies, the dose of hydroxyurea was reduced by 25% if the WBC count ranged between 2,000/μL and 3,000/μL and/or platelet counts ranged between 50,000/μL and 100,000/μL on the day of treatment and treatment was held for WBC counts <2,000/μL and/or platelet counts <50,000/μL until recovery to WBC count ≥3,000/μL and platelet counts ≥50,000/μL. Toxicity was evaluated according to the National Cancer Institute (Bethesda, MD), Common Toxicity Criteria.29
Hydroxyurea was supplied as an oral preparation of 500-mg capsules commercially available from Bristol Laboratories Oncology Products (Princeton, NJ). The IV formulation was supplied by the Division of Cancer Treatment, National Cancer Institute, Bethesda, (MD), as a lyophilized powder in 50-mL vials containing 2 g of hydroxyurea with citric acid (56 mg) and sodium phosphate (144 mg). The vials were diluted with 18.6 mL of sterile water United States Pharmacopeia (USP) for injection. The pH of the solution was 6.1. This initial dilution was to be further diluted in 5% dextrose to a total volume of 100 mL and administered over 30 minutes by an infusion pump.
Pretreatment and follow-up studies.
Histories and physical examinations, and routine laboratory studies were performed pretreatment and weekly after treatment. Laboratory studies included a complete blood count, differential WBC count, electrolytes, blood urea nitrogen, creatinine, glucose, total protein, albumin, calcium, phosphorus, uric acid, alkaline phosphatase, SGOT, SGPT, total and direct bilirubin, amylase, prothrombin time, and urinalysis. Twelve-hour urine collections to measure creatinine clearance and electrocardiograms were also performed pretreatment. Formal tumor measurements were performed pretreatment and after every course (every 6 weeks). A complete response was scored if there was disappearance of all active disease on two measurements separated by at least 4 weeks, and a partial response required at least a 50% reduction in the sum of the product of the bidimensional measurements of all measurable lesions on two measurements separated by at least 4 weeks.
Pharmacological studies.
Extensive plasma sampling after both oral and IV drug administration was performed in all patients. For blood samples collected in concert with IV dosing, sampling was performed before treatment, at 15 minutes during the infusion, and immediately before the end of infusion; plasma sampling was performed pretreatment and at 15 and 30 minutes after oral drug administration. For both oral and IV drug administration, plasma sampling was also performed after the initiation of treatment at the following times: 36, 45, 90, 120, and 150 minutes; and at 3, 4, 6, 8, 10, 12, and 24 hours. At each sampling time, 1 mL of blood was withdrawn and then discarded to assure that the heparin solution used to maintain catheterpatency did not dilute the sample. Then 7 mL of blood was removed, and the blood sample was centrifuged immediately. A total of 240 mL of blood was collected over a 2-month period for bioavailability studies. Plasma, divided into two equal aliquots, were transferred to plastic tubes, labeled, and kept frozen at −70°C until analysis. Urine samples were collected before the first dose of study medication and then continuously over the next 24 hours in pooled collections of 0 to 6 hours, 6 to 12 hours, and 12 to 24 hours. Collected urine was shaken to mix thoroughly, and the total volume was measured and recorded. A 20-mL aliquot was taken, and this was labeled and kept frozen at −70°C until analysis.
Hydroxyurea concentrations in both plasma and urine samples were quantitated using a modified colorimetric assay originally described by Fabricus and Rajewsky.30 Briefly, sample preparation and analysis were accomplished as follows. A 1-mL plasma was mixed with 4 mL of water and left to stand for 1 minute. Five milliliters of 1 mol/L perchloric acid was then added, and after being left to stand for 10 minutes, the mixture was centrifuged at 10,000 rpm for 20 minutes at 4°C. The supernatant was then filtered through a Gelman (Ann Arbor, MI) Nylon Acrodisc (0.45 μm) into clean polypropylene tubes. For urine, 2 mL of deionized water was used to dilute a 50-μL aliquot of urine sample. Two milliliters of 1 mol/L perchloric acid was then added, and after being left to stand for 10 minutes, the mixture was centrifuged at 10,000 rpm for 20 minutes at 4°C. The supernatant was then decanted into clean polypropylene tubes. For analysis, 2 mL of the standard or sample was added to 1 mL of the working buffer solution (0.5 mol/L Na2HPO4 + 1.5 mol/L Na2HPO4), 0.1 mL of 10.3 mol/L sodium hydroxide, 1 mL of 1% sulfanilic acid, and 0.1 mL of 0.1 mol/L iodine in 2.5% potassium iodide solution. The mixture was left to stand for 1 minute before adding 0.1 mL of freshly prepared aqueous 0.1 mol/L sodium thiosulfate solution and 1 mL of freshly prepared naphthylenediamine solution. After leaving the mixture to stand for 20 minutes, the absorbance of the resulting solution was measured in triplicate at 540 nm on a Beckman DU650 spectrophotometer (Fullerton, CA) and the average absorbance was recorded.
Hydroxyurea standards for plasma studies were prepared as above in duplicate at the following concentrations: 0, 15, 20, 40, 60, 100, 200, 400, 600, 800, 1,000, and 1,500 μmol/L. For urine studies, hydroxyurea standards for generation of the standard curve were 0, 10, 30, 50, 100, 200, 300, and 500 μmol/L. Standard curves for plasma and urine samples were prepared in duplicate and an average absorbance of each duplicate sample was read in triplicate and an average absorbance was recorded. The lower limits of quantitation for the assay were 15 μmol/L and 10 μmol/L for plasma and urine, respectively. The precision of the assay ranged from 13% at the lower end of the plasma standard curve to 6.8% at the higher end. The accuracy of the assay, as assessed as the closeness of the mean value to the theoretical concentration over the range of the plasma standard curve, was 85.5% (40 μmol/L), 98.1% (100 μmol/L), and 99.4% (800 μmol/L).
Individual hydroyxurea plasma concentration data from all study patients were analyzed by both compartmental and noncompartmental methods. The area under the plasma concentration-versus-time curve (AUC) and the area under the first moment-versus-time curve (AUMC) were calculated using the linear trapezoidal rule. The AUC was extrapolated to infinity by dividing the last measured concentration by the terminal rate constant (λβ), which was determined using nonlinear least square regression as described below. The systemic clearance (Cl) after IV and oral (Cl/F) administration was calculated by dividing the dose by the AUC. Maximum plasma concentration (Cmax) and time to maximum concentration (Tmax) following oral administration were determined by inspection of the concentration-versus-time curve. The mean residence time (MRT) was determined using the equation, AUMC/AUC − (infusion time/2). The mean transit time (MTT) was calculated by dividing the AUMC by the AUC and the mean absorption time (MAT) was derived by subtracting the MRT from the MTT. The half-life of absorption (T1/2a) was calculated by multiplying 0.693 by the MAT. Bioavailability (F) expressed as a percentage was calculated by dividing the oral AUC by the IV AUC normalized to dose (F% = [AUCoral/AUCIV] × [Doseoral/DoseIV, which was equal to 1] × 100%), with the underlying assumption that the drug clearance was the same in each patient during both study periods, and the dose normalization factor was equal to 1.31 The fraction of hydroxyurea dose excreted in urine after either IV or oral administration was calculated by dividing the cumulative quantity of hydroxyurea in the urine samples collected over 24 hours by the dose of hydroxyurea (2,000 mg). Renal clearance of hydroxyurea was estimated by multiplying Cl by the fraction of hydroxyurea that was excreted in the urine. The volume of distribution (VSS) after the administration of IV and oral hydroxurea were calculated by the following equations:
{M}where T represents the infusion duration andKa is 1/MAT. Clearance and volume of distribution terms for oral administration were corrected for F.
Plasma concentration-time data were modeled using a nonlinear least square regression program (RSTRIP [Version 5.0]; Micromath Inc, Salt Lake City, UT). Concentration data were weighted using 1/concentration2. Goodness of fit of the plasma concentration-time profiles were determined by visual inspection of the plasma profiles and also by using the modified Akaike Information Criteria.32 The oral route model further assumed a first-order input, with a lag time, and first-order output. The IV infusion model assumed constant intravenous input over 30 minutes and first-order output. The secondary pharmacokinetic parameters derived from fitting the plasma hydroxyurea concentration time profiles to the linear compartment model included the following: distribution (α) half-life (T1/2α), elimination (β) half-life (T1/2β), the AUC extrapolated to infinity, and the time delay between drug administration and absorption (Tlag).
The relationships between relevant PK parameters and parameters that reflected myelosuppression were initially explored by visually inspecting the scatterplots. Relevant parameters indicative of myelosuppression that were evaluated included the percentage decrements in the ANCs and platelet counts, which were calculated as follows: % decrease = 100 × ([pretreatment counts − nadir counts]/pretreatment counts).
Statistical considerations.
PK data are presented as the mean ± SD or range unless otherwise stated. Ordinary linear regression was used to compare measures of renal functions and hematopoietic effects with PK parameters for each individual patient. The nonparametric Wilcoxon signed rank test or paired Student's t-test was used to compare PK parameters obtained with oral and IV treatment and different orders of administration.
RESULTS
General.
Of the 29 patients who were treated in the study, 15 patients were randomized to receive oral hydroxyurea as their initial treatment, and 14 patients were randomized to the IV hydroxyurea arm. The characteristics of these patients are displayed in Table1. An additional patient was randomized to the IV hydroxyurea arm, but never received study medication. Two patients did not meet all eligibility criteria. Both subjects previously underwent large bowel resections, but it was determined retrospectively that this deviation neither placed them at increased risk nor rendered their data invalid for analysis. Mean height and weight values were 172.9 cm (range, 149.9 to 198) and 74.2 kg (range, 43 to 136.6), respectively. For the bioavailability studies, the single 2,000-mg dose averaged 29.4 mg/kg and 29.7 mg/kg when given orally and IV, respectively. With few exceptions, the concomitant medications taken by the patients during the oral and IV portions of the study were similar. The largest category of concomitant medications was analgesics, which were taken by 24 patients (83%). Other frequent categories of concomitant medications included: central nervous system drugs in 14 patients (48%), endocrine medications in 13 patients (45%), antianginal or antihypertensive agents in 12 patients (41%), antihistamines or decongestants in 10 patients (34%), antiemetic or antidiarrheal medications in 8 patients (28%), diuretics in 7 patients (24%), and anticoagulants in 3 patients (10%).
There were no objective clinical anti-cancer responses.
Pharmacokinetic studies.
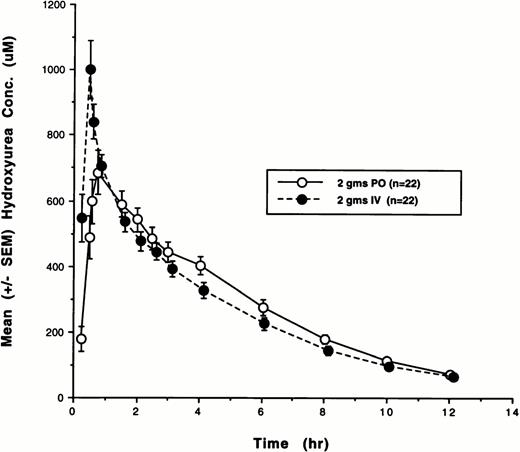
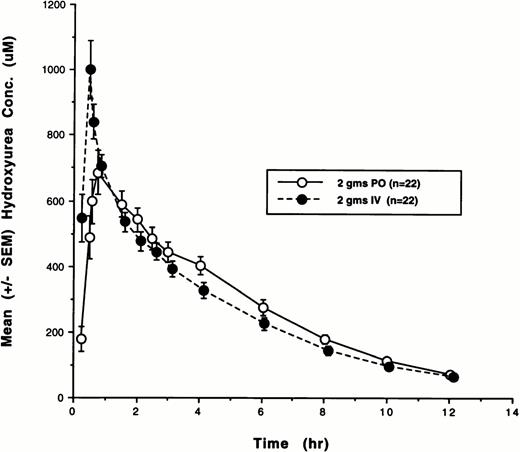
Twenty-two of the 29 patients enrolled in the study received 2,000 mg hydroxyurea by both oral and IV routes and completed all PK and bioavailability studies. Because of early disease progression, four patients received oral hydroxyurea only, and three patients received IV hydroxyurea only. Plasma concentrations from all of these courses were analyzed by both noncompartmental and model-dependent methods. The cumulative plasma concentration-versus-time data for all 22 subjects who were treated with both oral and IV hydroxyurea are shown in Fig1. The plasma disposition of hydroxyurea was well described by a linear two-compartment model with first-order absorption. Hydroxyurea PK parameters and F after IV and oral administration that were determined using both noncompartmental and compartmental methods are listed in Table2. Mean AUC values derived using compartmental methods were in excellent agreement (±10%) with those obtained using noncompartmental methods.
Plasma concentration-versus-time plots for hydroxyurea after both oral (PO) and IV administration. The mean (SE) concentrations as a function of time for all 22 patients are depicted. (–○–), 2 g PO (n = 22); (–•–), 2 g IV (n = 22).
Plasma concentration-versus-time plots for hydroxyurea after both oral (PO) and IV administration. The mean (SE) concentrations as a function of time for all 22 patients are depicted. (–○–), 2 g PO (n = 22); (–•–), 2 g IV (n = 22).
Absorption kinetics.
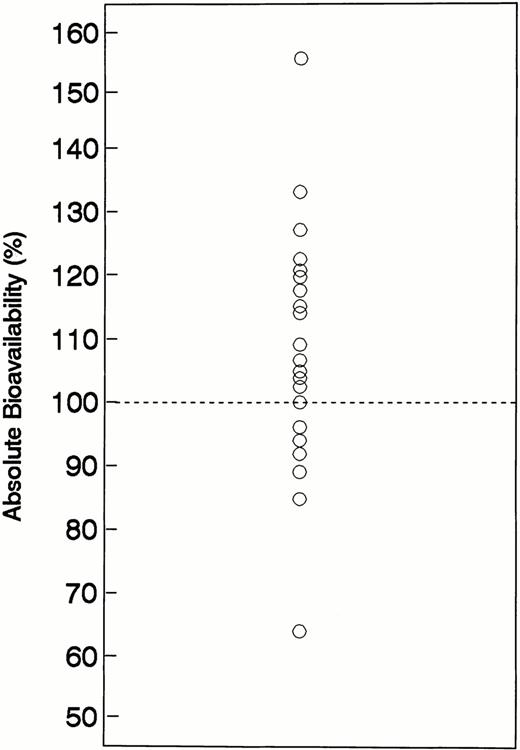
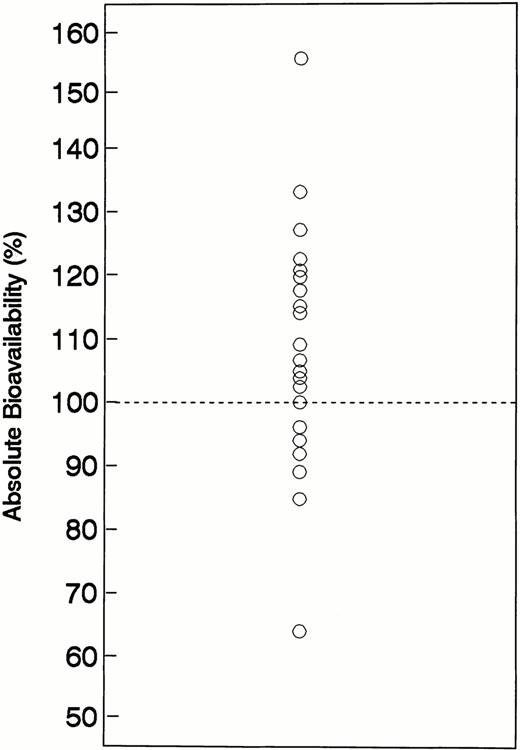
After oral administration, gastrointestinal absorption was relatively rapid. The Tmax averaged 1.22 hours and the Tlag averaged 0.22 hours. The mean T1/2a oral was 0.53 hours and MAT averaged 0.72 hours. The mean value for Cmax after a 2,000-mg oral dose of hydroxyurea was 794 μmol/L. The range of AUC values (noncompartmental) obtained after IV administration of 2,000 mg hydroyxurea was 2,277 to 5,975 μmol/L/h (mean, 3,552), which was similar to the AUC range of 2,209 to 4,726 μmol/L/h (mean, 3,934) after oral administration of hydroxyurea at the same dose. The order of drug administration (IV v oral as first treatment) did not affect the PK behavior of hydroxyurea. The mean (±SD) AUC values for patients receiving oral hydroxyurea as their initial or second treatment were 3,970 (1,330) and 3,810 (900) μmol/L/h (P = .63), respectively, whereas comparable values for patients receiving IV hydroxyurea as their initial or second treatment were 3,450 (700) and 3,910 (1,530) μmol/L/h (P = .26), respectively. Based on both noncompartmental and compartmental methods, the absorption of oral hydroxyurea was high (Table 2). Using noncompartmental methods, the absolute bioavailability determined from 22 patients averaged 108% (range, 64% to 156%) with an interindividual coefficient of variation (CV) of 17%. A scatterplot of the absolute bioavailability values is shown in Fig2. Bioavailability was approximately 100% (range, 85% to 127%) in 19 of 22 subjects, whereas bioavailability was somewhat higher (133% and 156%) in two individuals, and lower (64%) in one subject. No reasonable explanations, including relevant patient characteristics, concomitant medications, or deviation from the standard drug administration scheme, were evident to account for the magnitude of variability in the bioavailability of these three subjects.
Plot absolute oral hydroxyurea bioavailability values for each individual patient. The line represents the line of identity for complete (100%) absolute bioavailability.
Plot absolute oral hydroxyurea bioavailability values for each individual patient. The line represents the line of identity for complete (100%) absolute bioavailability.
Systemic disposition.
The disposition PK parameters of IV and oral hydroxyurea are listed in Table 2. Individual Cmax values after IV administration averaged 19.5% (20.7%) higher than those achieved after oral treatment; mean Cmax values were 1,007 and 794 μmol/L, respectively. Based on compartmental modeling, the initial distribution phase was short, with harmonic mean values for T1/2αivand T1/2αoral of 0.63 and 1.78 hours, respectively. The harmonic mean values for the terminal half-lives, T1/2βivand T1/2βoral were nearly identical, 3.39 and 3.32 hours, respectively. The apparent volumes of distribution VSSivand VSSoral/F were 19.71 and 19.65 L/M2, respectively. The harmonic MRTs for hydroxyurea after IV and oral administration (MRTiv and MRToral) were 4.79 and 5.45 hours, respectively. Based on noncompartmental methods, the mean value for IV clearance (Cliv) was 106 mL/min or 72.16 mL/min/M2 when normalized to body-surface area, whereas oral clearance (Cloral/F) was 124.83 mL/min or 73.16 mL/min/m2.
Urinary excretion.
Sixteen patients had quantitative urine collections for 24 hours after hydroxyurea administered by both the IV and oral routes. The percentage of the hydroxyurea dose excreted in urine following IV and oral treatment averaged 39.2% (range, 21.1 to 62.4%) and 37.8% (range, 26.1% to 45.3%), respectively. The mean (SD) percentages of the total administered oral dose of hydroxyurea excreted during the three timed collection intervals (0 to 6, 6 to 12, and 12 to 24 hours) were 23.4% (7.0), 11.7% (7.0), and 2.9% (11.2), respectively, whereas the respective percentages excreted in these periods after IV dosing were 29% (11.6), 9.2% (4.8), and 2.9% (2.3). The mean ratio of the percentages of hydroxyurea excreted in the urine after oral and IV dosing, another indication of systemic bioavailability, was 0.95 (range, 0.62 to 1.35), with an interindividual CV of 32%. Mean renal clearance rates were 43.54 mL/min (29.03 mL/min/m2) and 47.83 mL/min (27.15 mL/min/m2) following oral and IV administration of hydroxyurea, respectively. The renal clearance of hydroxyurea correlated moderately well with measured creatinine clearance (r = .55, P < .01). In addition, there was a moderate inverse relationship between the AUC and renal clearance of hydroxyurea (r = −.59, P < .01).
Toxicity.
The principal toxicity of hydroxyurea administered orally at a dose of 80 mg/kg every 3 days after an initial oral or IV dose of hydroxyurea at 2,000 mg was myleosuppression, particularly neutropenia. Thrombocytopenia occurred much less frequently, and severe thrombocytopenia was uncommon. Only 6 of 29 (19%) patients required dose modification for hematologic toxicity at any juncture. In this group that consisted largely of heavily-pretreated subjects, nadir ANC counts typically occurred in weeks 2 to 4. Neutropenia was not cumulative, as reflected by a stable incidence of grade 3 or 4 neutropenia with cumulative dosing. The route of administration (oral or IV) of the first dose of hydroxyurea did not influence the rate or severity of the various toxicities of hydroxyurea during courses 1 and 2. Overall, 25 of the 29 patients experienced hematologic toxicity of any severity, including 23 (79%) and 8 (28%) individuals who developed any grade of neutropenia and thrombocytopenia, respectively. Sixteen of 29 patients (55%) experienced severe (grade 3 or 4) myelosuppression sometime in their therapy, with 14 (48%) and 3 (10%) patients developing grade 3 or 4 neutropenia and thrombocytopenia, respectively. Of these patients, 6 subjects (19%) experienced grade 4 neutropenia lasting less than 5 days; an ANC nadir <500/μL that lasted 11 days occurred during a single course administered to one subject. Fever associated with severe neutropenia requiring treatment with parenteral antibiotics never occurred. Only 1 (3.4%) heavily-pretreated individual experienced grade 4 thrombocytopenia of short duration at the initial dose level of 80 mg/kg and recurrent thrombocytopenia did not preclude further chronic dosing at a reduced dose of 60 mg/kg.
Nonhematological effects, possibly attributed to hydroxyurea, occurred infrequently and were generally mild to modest in severity. The most common nonhematological toxicity was nausea and/or vomiting, which usually occurred several hours after dosing. Overall, 15 patients (62%) complained of nausea and 6 patients (21%) experienced vomiting at some time during the study; however, severe (grade 3) nausea and/or vomiting occurred in only 3 of 29 patients (10%) and these toxic effects did not occur during the bioavailability phase of the study. Nausea and/or vomiting were usually treated successfully with phenothiazines and/or metochlopromide. In addition, three individuals experienced elevations in their liver function tests including two patients who developed grade 3 elevations in hepatic transaminases and one subject who developed hyperbilirubinemia. All of these individuals had liver metastases, but these effects could not be definitively attributed to disease progression. One patient developed an atrial arrhythmia during treatment with hydroxyurea, which resolved after treatment with digoxin. Other infrequent toxicities that were mild to modest in severity included diarrhea, anorexia, alopecia, rashes, and malaise.
Pharmacodynamic analysis.
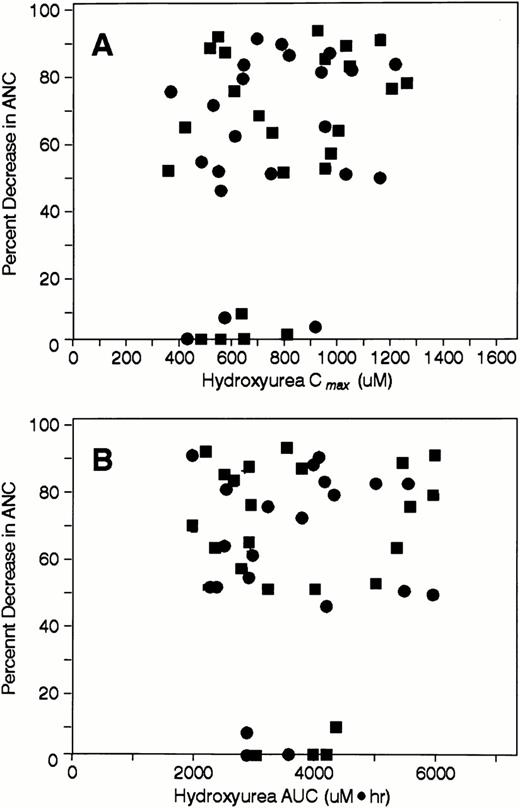
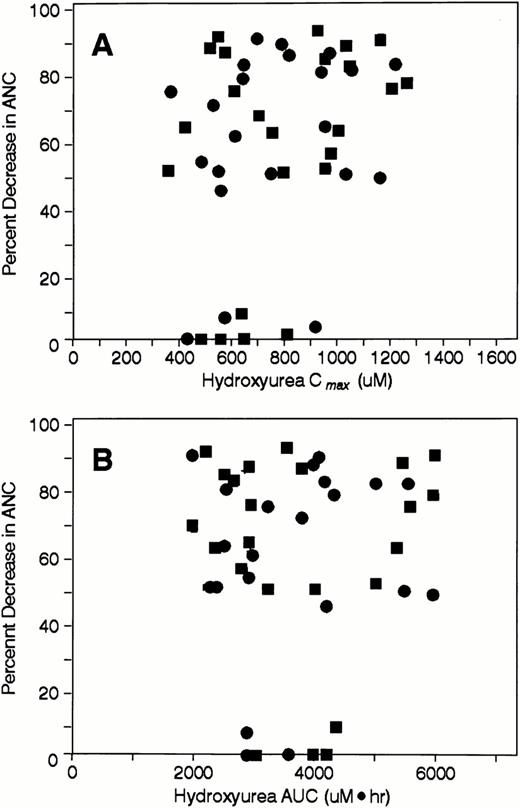
Relationships between hydroxyurea Cmax and AUC and various parameters indicative of drug effects on neutrophils, including the percent decrement in ANCs (Fig 3A and B),the ANC nadir (data not shown), and the grade of neutropenia (data not shown) were sought. Linear relationships were either nonexistent or very weak (all r values <.3 for Cmax; allr values <.1 for AUC). In addition, the relationships between these variables could not be adequately characterized by sigmoidal maximal effect models.
Scatterplots depicting the percent change in ANC during course 1 after IV (•) and oral hydroxyurea (▪) as a function of (A) hydroxyurea Cmax and (B) hydroxyurea AUC.
Scatterplots depicting the percent change in ANC during course 1 after IV (•) and oral hydroxyurea (▪) as a function of (A) hydroxyurea Cmax and (B) hydroxyurea AUC.
DISCUSSION
The recent resurgence of interest in the use of hydroxyurea in the therapy of both malignant and nonmalignant diseases has resulted in concerns about the use of oral administration schedules to simulate optimal pharmacological conditions required for maximal biological activity. Another related, albeit unproven, concern is the greater potential for significant interindividual variability with oral hydroxyurea, which may also hypothetically preclude achieving optimal pharmacologic conditions in some clinical settings and individuals. For these reasons, an investigational parenteral formulation of hydroxyurea has been made available for evaluations of hydroxyurea on a wide variety of dose schedules.1 The investigational formulation has undergone preliminary evaluations, principally on protracted IV infusion schedules in settings where such a formulation and dosing schedules might enable achievement and maintenance of biologically-relevant plasma concentrations, particularly with respect to modulation of the cytotoxic effects of the antimetabolites, DNA-damaging agents, and radiation.1,2,4,10-12,24-28 Using protracted IV infusion schedules, plasma concentrations in excess of 1 mmol/L have been achieved and maintained for 24 to 72 hours, although the biological and clinical relevance of this magnitude of drug concentrations are not known.1 24-28 These concerns regarding the bioavailability of oral hydroxyurea and the potential for significant interindividual variability with oral dosing, coupled with a progressively growing interest in the use of the parenteral formulation to alleviate such concerns, served as the impetus for the present bioavailability and PK study.
In limited preclinical pharmacological studies that were performed several decades ago involving mice and rats, the bioavailability of oral hydroxyurea was determined to be 50% and 72%, respectively.31,33-36 Although the gastrointestinal absorption of hydroxyurea was evaluated in early clinical studies, the numbers of patients studied were small, which resulted in a high level of uncertainty regarding interindividual variability.36-39In these early studies, oral absorption was usually described qualitatively as “rapid” and bioavailability as “high,” but pertinent PK and bioavailability parameters were not rigorously quantitated using conventional, appropriate bioavailability study designs (ie, randomization between IV and oral drug administration with subsequent crossover to the alternate treatment). In fact, except for sporadic reports of individuals who received both oral and IV hydroxyurea several decades ago, formal bioavailability studies have not been performed. In a population PK study in which patients with cancer were treated with a wide range of hydroxyurea dosing schedules, oral bioavailability was determined to be approximately 79% based on intergroup comparisons of PK data.33 The investigators proposed that the lack of complete bioavailability might, in part, be caused by incomplete drug absorption, gut-wall metabolism, and/or first-pass metabolism. However, the determination of F was based on both oral and IV dosing data obtained from different groups of patients who were treated with either oral or IV hydroxyurea using widely disparate doses and schedules. For example, eight patients were treated with oral hydroxyurea at a dose of 20 mmol/m2(1.5 g/m2) every 6 hours, and PK parameters of IV hydroxyurea were derived from a different group of patients who were treated with much higher hydroxyurea doses ranging from 84 to 315 mg/m2/h as a continuous IV infusion for 48 to 72 hours, or as an unspecified IV loading dose followed by 165 to 950 mg/m2/h as a continuous IV infusion for 24 to 48 hours. Furthermore, using nonlinear mixed effect modeling, these investigators determined that the PK behavior of hydroxyurea was nonlinear, which might have further confounded their calculation of F because patients were treated with high IV hydroxyurea doses that approached the nonlinear spectrum and much lower oral doses. In contrast, the PK results of a more recent study that evaluated the feasibility of administering hydroxyurea as a continuous IV infusion for 120 hours at somewhat lower doses (41 to 133 mg/m2/h) than the aforementioned doses were similar to those determined in the present study.40 In that study, the PK behavior of hydroxyurea was shown to be linear; the mean terminal T1/2 was 3.3 ± 0.2 hour, and ClSS and renal Cl averaged 91.6 ± 5 and 35.6 ± 3 mL/min/m2, respectively, resulting in a mean renal excretion of 37.2%.
The present study has shown that the bioavailability of oral hydroxyurea (2,000 mg) is complete or nearly complete. F averaged 108%. Moreover, interindividual variability in F was relatively low, as indicated by the fact that 19 of 22 individual F values were in a narrow range of 85% to 127% and the CV value modest at 17%. Overall, the interindividual variability in bioavailability and other PK parameters with both IV and oral hydroxyurea were much lower than those noted with other oral cytotoxic agents such as melphalan, chlorambucil, busulfan cyclophosphamide, 5-fluorouracil, and vinorelbine.41-49 For example, the profound interindividual differences in the bioavailability of busulfan results in widely disparate AUCs in individuals undergoing high-dose chemotherapy and allogeneic bone marrow rescue, which appears to be a critical determinant for both efficacy and toxicity, specifically venoocclusive disease of the liver.47-49 In contrast, the results of the study discussed in this report indicate that the many apprehensions regarding the potential unpredictability of oral hydroxyurea may be unfounded. Although the data resulting from a clinically relevant hydroxyurea dose (2,000 mg) was well fit by a two-compartment linear PK model and this study evaluated one dose level and was not precisely designed to detect nonlinearity, nonlinear PK behavior has been appreciated in one retrospective analysis of population PK data from patients treated with hydroxyurea on multiple dosing schedules.33 In the retrospective population analysis, the Michealis-Menton constant for hydroxyurea elimination (Km) was reported to be 0.323 mmol/L, which is in the range of Cmax values achieved with both IV (mean, 793.75 μmol/L [range, 503.89 to 1,538.43]) and oral hydroxyurea (mean, 1,006.65 μmol/L [range, 372.54 to 1,279.76]) in the present study.
As indicated by the mean hydroxyurea concentration-time plot depicted in Fig 1, the PK profiles of oral and IV hydroxyurea were nearly identical. An exception was the parameter Cmax which was 19.5% (20.7%) higher on average after IV administration. On the other hand, AUC values achieved with IV and oral hydroxyurea were nearly identical (r = .81, P < .001). The rates of both systemic and renal drug clearance with oral and IV drug administration were also very similar, suggesting that the disposition profiles of IV and oral hydroxyurea are similar, again supporting the notion that the IV route of administration does not portend significant advantages over oral dosing schedules. Although the number of reports in the literature pertaining to the pharmacology of hydroxyurea is scant and the numbers of patients in these reports are small, the results of these limited PK and urinary excretion studies concur with the results in the present study.
The present study also sought to identify pharmacodynamic relationships between pertinent PK parmeters of hydroxyurea, such as AUC and Cmax, and indices that reflect the severity of the principal toxicity, neutropenia (eg, ANC nadir, percent decrement in ANC, and grade of toxicity). Although the design of the study precluded direct comparisons of the severity of neutropenia and other toxicities resulting from repetitive treatment of patients with IV and oral hydroxyurea because only a single IV dose was administered, the acute toxic effects of oral and IV hydroxyurea were similar. Overall, pharmacodynamic relationships were not observed. There have been few pharmacodynamic studies performed with hydroxyurea to date in which efficacy and/or toxicity were related to PK variables. In one study involving 32 patients with sickle cell disease who were treated with a mean daily single oral hydroxyurea dose of 21 mg/kg (range, 10 to 35 mg/kg) for 16 weeks, systemic drug clearance was not useful in predicting for susceptibility to toxicity.22 In addition, neither patient age, baseline serum creatinine, nor creatinine clearance had any bearing on both efficacy and toxicity. However, the most significant determinants, albeit modest, of the final fetal hemoglobin level achieved included the last (“steady-state”) plasma hydroxyurea concentration (r = .39,P = .0001), initial WBC count, and the initial fetal hemoglobin concentration.
From both PK and bioavailability standpoints, the results of this study indicate that there are no clear advantages of administering hydroxyurea by the IV route for the most common dosing schedules used to manage both malignant and benign hematological diseases. In this large study relative to typical bioavailability studies involving patients with advanced malignancies who often have multiple coexisting medical problems and are usually receiving a vast array of concomitant medications that may affect the gastrointestinal absorption and Cl of anticancer agents, there was a relatively minor degree of interindividual variability in the oral bioavailability, as well as the pharmacological disposition, of both oral and IV hydroxyurea. With the exception of patients with significantly impaired gastrointestinal function, there do not appear to be any clear advantages for administering hydroxyurea parenterally, and clinical evaluations of novel uses of hydroxyurea, particularly those targeting and maintaining predefined drug concentrations, should be less wary of using the oral formulation.
Supported in part by National Institutes of Health Grants No. U01-CA-69853, P30-CA-54174, and M01-RR-0136, the Audie Murphy Veteran's Administration Hospital, and NCI Training Grant No. 5T32-CA09434.
Address reprint requests to Eric K. Rowinsky, MD, The Institute for Drug Development, Cancer Therapy and Research Center, 8829 Datapoint Dr, Suite 700, San Antonio, TX 78229.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.