Abstract
Protein C inhibitor (PCI), which was originally identified as an inhibitor of activated protein C, also efficiently inhibits coagulation factors such as factor Xa and thrombin. Recently it was found, using purified proteins, that the anticoagulant thrombin-thrombomodulin complex was also inhibited by PCI. The paradoxical inhibitory effect of PCI on both coagulant and anticoagulant proteases raised questions about the role of PCI in plasma. We studied the role of thrombomodulin (TM)-dependent inhibition of thrombin by PCI in a plasma system. Clotting was induced by addition of tissue factor to recalcified plasma in the absence or presence of TM, and clot formation was monitored using turbidimetry. In the absence of TM, PCI-deficient plasma showed a slightly shorter coagulation time compared with normal plasma. Reconstitution with a physiologic amount of PCI gave normal clotting times. Addition of PCI to normal plasma and protein C–deficient plasma resulted in a minor prolongation of the clotting time. This suggested that PCI can act as a weak coagulation inhibitor in the absence of TM. TM caused a strong anticoagulant effect in normal plasma due to thrombin scavenging and activation of the protein C anticoagulant pathway. This effect was less pronounced when protein C–deficient plasma was used, but could be restored by reconstitution with protein C. When PCI was added to protein C–deficient plasma in the presence of TM, a strong anticoagulant effect of PCI was observed. This anticoagulant effect was most likely caused by the TM-dependent thrombin inhibition by PCI. However, when PCI was added to normal plasma containing TM, a strong procoagulant effect of PCI was observed, due to the inhibition of protein C activation. PCI-deficient plasma was less coagulant in the presence of TM. A concentration-dependent increase in clotting time was observed when PCI-deficient plasma was reconstituted with PCI. The combination of these results suggest that the major function of PCI in plasma during coagulation is the inhibition of thrombin. A decreased generation of activated protein C is a procoagulant consequence of the TM-dependent thrombin inhibition by PCI. We conclude that TM alters PCI from an anticoagulant into a procoagulant during tissue factor-induced coagulation.
PROTEIN C INHIBITOR (PCI) is a plasma glycoprotein that belongs to the SERPIN superfamily of serine protease inhibitors, of which α1-protease inhibitor is the prototype.1,2 PCI was initially identified in blood plasma by Marlar and Griffin3 and isolated by Suzuki et al4 as a major regulator of the anticoagulant protease activated protein C (APC). Recently it has been shown that this member of the SERPIN superfamily has a broad target specifity, capable of inhibiting various proteases in coagulation, fibrinolysis, and reproduction.3-12 The lack of documented patients with an abundancy, deficiency, or specific mutation of PCI makes it difficult to determine the true physiologic function for PCI in plasma.
Like the other protease inhibitors, antithrombin (AT) and heparin cofactor II (HCII), PCI is classified as a heparin-binding serpin. Glycosaminoglycans, including heparin, can accelerate the inhibition rate of serine proteases by these serpins (for review see Pratt and Church13).
Two regions of PCI were previously identified as a heparin binding site by homology to a consensus glycosaminoglycan recognition site, and their involvement was demonstrated in protease inhibition assays.14-17 Glycosaminoglycans present either on the cell surface of the endothelial cell or on its basement membrane are believed to be a site of physiologic activity for heparin binding-serpins.
Thrombomodulin (TM) is an endothelial cell receptor (for review see Esmon18), which plays an important modulating role in the anticoagulant response after vascular injury. Binding of thrombin to TM enables thrombin to rapidly cleave the protein C zymogen into the anticoagulant APC. Furthermore, by binding to TM, thrombin is no longer able to clot fibrinogen or to activate platelets. TM possesses a covalently linked glycosaminoglycan chain, which stabilizes the interaction with thrombin.19
Hemostasis involves a series of complex interactions between procoagulant, anticoagulant, and fibrinolytic mechanisms and in each system a role for PCI has been suggested.3 5-7
Rezaie et al20 recently described that PCI is a potent inhibitor of the thrombin-TM complex, thereby providing new clues to the elucidation of a possible physiologic function of PCI in plasma. Although PCI has mostly been implicated to be an inhibitor of APC, it is a much better inhibitor of thrombin. The role of PCI in plasma is therefore intriguing because it can act as a procoagulant by inhibiting APC and as an anticoagulant by inhibiting thrombin. This study was undertaken to investigate this dualistic character of PCI. We studied the effect of the TM-mediated inhibition of thrombin by PCI on tissue factor-induced coagulation in a plasma system. Our results indicate that a main function of PCI in plasma is the inhibition of thrombin, with TM as a cofactor during coagulation.
MATERIALS AND METHODS
All reagents used were analytical grade. Rabbit lung TM and Spectrozyme TH were obtained from American Diagnostica Inc, Greenwich, CT. A recombinant fragment of TM containing the EGF domains 4-6 (TM4-6) was a kind gift of Dr Evan Sadler (Washington University School of Medicine, St Louis, MO). PCI was isolated from fresh frozen plasma. The concentration of plasma PCI was determined by active-site titration and immunoassay as described.16 Protein C was isolated and activated as described by Hackeng et al.21 Recombinant human tissue factor (Innovin) was obtained from Baxter (Unterschleissheim, Germany). Bovine serum albumin (fraction V) was purchased from Sigma (St Louis, MO). The chromogenic substrate S2366 was purchased from Chromogenix (Mölndal, Sweden). Purified human thrombin was a generous gift of Dr Walter Kisiel (University of New Mexico, Albuquerque, NM).
Normal, control, PCI-deficient, and protein C–deficient plasma.
Normal plasma samples were obtained from a donor pool of 40 healthy volunteers. Blood was taken from the antecubital vein, collected into citrate, centrifuged twice for 15 minutes at 2,500g, and stored at −70°C until use. For the preparation of protein C and protein C inhibitor-deficient plasmas, a plasma pool of four healthy donors was prepared as above and subsequently passed over a Sepharose column to which monoclonal antibodies against either protein C (4D1) or PCI (API-39) were immobilized. Control plasma was obtained by passing of plasma over a Sepharose column to which bovine serum albumin had been coupled. The undiluted flow through of these columns was collected separately and used as deficient plasmas. PCI-deficient plasma was tested using an enzyme-linked immunosorbent assay (ELISA) and was found to contain less than 0.5% of PCI. Protein C–deficient plasma was tested with a protein C ELISA and contained less than 1% of protein C. In all assays used, the control plasma was fully comparable to normal plasma, demonstrating that passage of plasma over a Sepharose column had not affected the functional quality of the plasma.
Protease inhibition by PCI was measured using a discontinuous assay method as described by Rezaie et al20 with minor modifications. APC (0.5 nmol/L final concentration [f.c.]) or thrombin (0.5 nmol/L f.c.) were incubated with at least a 10-fold excess of PCI at 37°C in the presence or absence of TM (50 nmol/L). Using the same reaction conditions, the inhibition of thrombin by PCI was also monitored in the presence of 100 nmol/L TM4-6. After incubation for a period of time varying between 15 seconds to 60 minutes, Spectrozyme TH (0.25 mmol/L f.c.) or S2366 (1.0 mmol/L f.c) was added for monitoring the thrombin or APC activity, respectively. Color development was followed at 405 nm using a Spectramax 340 kinetic microplate reader (Molecular Devices, Menlo Park, CA). All experiments were performed in HEPES buffer (25 mmol/L HEPES, pH 7.4, 137 mmol/L NaCl, 3.5 mmol/L KCl, 3 mmol/L CaCl2, 0.1% bovine serum albumin). Rate constants were calculated using the equation: k2=(-ln a)/t[I], where a represents residual protease activity,t is time, and [I] is the PCI concentration. In all experiments the substrate utilization was less than 10% and control assays showed that thrombin, TM, and PCI were stable during the experiments.
Turbidimetry was used to monitor the TM-mediated thrombin inhibition by PCI. The change in turbidity during fibrin formation was monitored at 405 nm in a microplate reader.22-24 An increase in turbidity indicated gel assembly. All experiments were performed in citrated plasma recalcified with CaCl2 (final concentration of 17 mmol/L) resulting in a free Ca2+ concentration of 2.3 mmol/L. A mixture of recombinant tissue factor (Innovin, final dilution 3 × 104) and calcium necessary for recalcification was added to 67.5 μL of plasma to initiate clotting. The volume was adjusted to 125 μL with HEPES buffer, resulting in a final plasma concentration of 54%. After mixing, 100 μL of the reaction mixture was transferred to a microplate and turbidity at 405 nm was monitored at 37°C using a Spectramax 340 kinetic microplate reader.
RESULTS
TM-mediated inhibition of APC and thrombin by PCI.
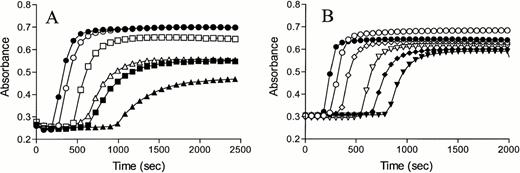
Time courses of inhibition of APC and thrombin by PCI in the absence and presence of an excess of TM in a system with purified components are shown in Fig 1. In agreement with the observations of Rezaie et al,20 we found an inhibition of TM-bound thrombin by PCI. For both proteases identical ratios of protease:inhibitor:thrombomodulin were used. In the absence of TM, thrombin was more rapidly inactivated by PCI compared with APC. Even a 100-fold molar excess of PCI resulted in a minor inhibition of APC (Fig 1A), whereas residual thrombin activity was decreased to 55% under the same conditions (Fig 1B). Addition of rabbit lung TM resulted in modest inhibition of APC by PCI, whereas a strong enhancement of the inactivation rate of thrombin by PCI was observed. The addition of the TM fragment TM4-6 also accelerated thrombin inhibition by PCI (Fig 1C), but to a lesser extent as was observed for rabbit-lung TM.
Inhibition of APC and thrombin by PCI in the absence and presence of TM. APC 0.5 nmol/L (A) or thrombin 0.5 nmol/L (B and C) were incubated with 10 (○,•), 25 (□,▪), or 50 (▵,▴) nmol/L PCI, respectively, in the absence (open symbols) or presence (closed symbols) of TM (50 nmol/L rabbit lung [A and B] and 100 nmol/L TM4-6 [C]). At indicated time points, the chromogenic substrates S2366 (1 mmol/L f.c.) or Spectrozyme TH (0.25 mmol/L f.c.) were added to monitor the remaining activity of APC and thrombin, respectively. Please note the difference in x-axis scales in A, B, and C.
Inhibition of APC and thrombin by PCI in the absence and presence of TM. APC 0.5 nmol/L (A) or thrombin 0.5 nmol/L (B and C) were incubated with 10 (○,•), 25 (□,▪), or 50 (▵,▴) nmol/L PCI, respectively, in the absence (open symbols) or presence (closed symbols) of TM (50 nmol/L rabbit lung [A and B] and 100 nmol/L TM4-6 [C]). At indicated time points, the chromogenic substrates S2366 (1 mmol/L f.c.) or Spectrozyme TH (0.25 mmol/L f.c.) were added to monitor the remaining activity of APC and thrombin, respectively. Please note the difference in x-axis scales in A, B, and C.
Inhibition of APC and Thrombin by PCI in the Absence or Presence of Rabbit Lung TM or Human TM4-6
| . | PCI (M−1 s−1) . | PCI + TM (M−1 s−1) . | Stimulation (fold) . | PCI + TM4-6 (M−1s−1) . | Stimulation (fold) . |
|---|---|---|---|---|---|
| APC | 2.1 × 103 | 4.6 × 103 | 2.2 | ND | |
| Thrombin | 3.7 × 104 | 1.22 × 106 | 33 | 8.8 × 105 | 24 |
| . | PCI (M−1 s−1) . | PCI + TM (M−1 s−1) . | Stimulation (fold) . | PCI + TM4-6 (M−1s−1) . | Stimulation (fold) . |
|---|---|---|---|---|---|
| APC | 2.1 × 103 | 4.6 × 103 | 2.2 | ND | |
| Thrombin | 3.7 × 104 | 1.22 × 106 | 33 | 8.8 × 105 | 24 |
Second order-rate inactivation constants were derived as described in Materials and Methods. Each value represents the average of at least three independent determinations.
Abbreviation: ND, not determined.
A 50% decrease of thrombin activity was observed within 30 seconds when a 50-fold molar excess of PCI was added in the presence of rabbit lung TM or TM4-6, whereas a 15% decrease of the APC activity was observed after 30 minutes under the same conditions. The second order rate constants for the APC and thrombin inhibition by PCI depicted in Table 1 show that rabbit lung TM stimulated the APC inhibition by PCI twofold, whereas a 33-fold enhancement was achieved for the inhibition of thrombin by PCI. When TM4-6 was used, a 24-fold stimulation of the thrombin inhibition by PCI was observed.
In the absence of TM, the inhibition of thrombin by PCI was 18 times more effective compared with the APC-PCI reaction. The addition of TM enhanced this difference in rate inactivation constants of APC-PCI and thrombin-PCI to a factor of 267, favoring the TM-mediated inhibition of thrombin by PCI.
The effect of TM on tissue factor–induced coagulation.
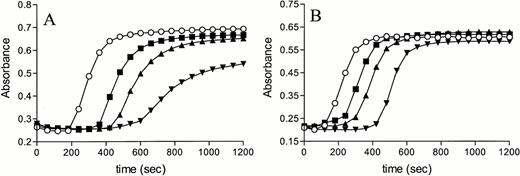
We first studied the effect of TM on tissue factor–induced coagulation in normal plasma to measure its scavenging effect on thrombin and protein C activation. Normal plasma was incubated for 15 minutes with 8.3, 16.7, or 25 nmol/L TM. After recalcification, tissue factor was added and the rate of fibrin formation was followed by measuring the increase in turbidity at 405 nm. Addition of TM to normal plasma resulted in a concentration-dependent delay in coagulation (Fig 2A), which may be due to thrombin scavenging and/or activation of the protein C anticoagulant pathway.
Effect of TM on tissue factor-induced coagulation. Normal plasma (A) and protein C–deficient plasma (B) were incubated with 8.3 (▪), 16.7 (▴), or 25 nmol/L (▾) TM or with buffer (○). Clotting was initiated by adding recombinant tissue factor (Innovin, diluted by a factor 3 × 104) and calcium required for recalcification, and the formation of fibrin was measured in time as the change in turbidity at 405 nm.
Effect of TM on tissue factor-induced coagulation. Normal plasma (A) and protein C–deficient plasma (B) were incubated with 8.3 (▪), 16.7 (▴), or 25 nmol/L (▾) TM or with buffer (○). Clotting was initiated by adding recombinant tissue factor (Innovin, diluted by a factor 3 × 104) and calcium required for recalcification, and the formation of fibrin was measured in time as the change in turbidity at 405 nm.
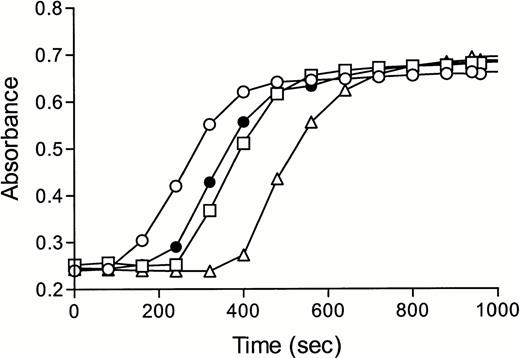
To monitor the thrombin scavenging effect of TM alone, we repeated the experiment with protein C–deficient plasma. A similar, but less dramatic pattern, was observed suggesting that the anticoagulant effect of TM is indeed partly caused by thrombin scavenging and partly by protein C activation (Fig 2B). Reconstitution of protein C–deficient plasma had only a minor effect on coagulation in the absence of TM, whereas in the presence of TM, a concentration-dependent anticoagulant effect of protein C was observed (Fig 3). These results indicated that TM was needed for activation of the protein C anticoagulant pathway during tissue factor-induced coagulation and excluded the possibility that the presence of traces of APC in our preparations were responsible for the observed effects.
Reconstitution of protein C–deficient plasma in the absence or presence of TM. Plasma deficient in protein C was incubated with buffer (○,•) or 40 (□,▪), 80 (▵,▴) or 160 (▿,▾) nmol/L protein C in the absence (A, open symbols) or presence (B, closed symbols) of TM (25 nmol/L). Fibrin formation was monitored as the change in turbidity at 405 nm after addition of recombinant tissue factor (Innovin, diluted by a factor 3 × 104) and calcium required for recalcification. Please note the difference in x-axis scales in (A and B).
Reconstitution of protein C–deficient plasma in the absence or presence of TM. Plasma deficient in protein C was incubated with buffer (○,•) or 40 (□,▪), 80 (▵,▴) or 160 (▿,▾) nmol/L protein C in the absence (A, open symbols) or presence (B, closed symbols) of TM (25 nmol/L). Fibrin formation was monitored as the change in turbidity at 405 nm after addition of recombinant tissue factor (Innovin, diluted by a factor 3 × 104) and calcium required for recalcification. Please note the difference in x-axis scales in (A and B).
Although the maximal turbidity signal was affected by the addition of TM in a concentration-dependent manner, this effect was the same in normal and deficient plasmas and did, therefore, not exclude comparison of turbidity measurements between the different plasmas when the same concentration of TM was used.
The role of PCI in tissue factor-induced fibrin formation in plasma.
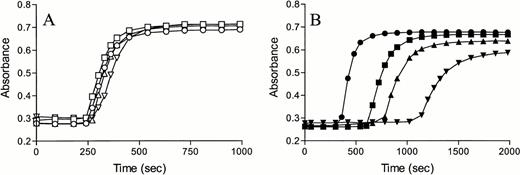
The role of PCI in tissue factor-induced coagulation was studied using turbidimetry. In this assay, PCI-deficient plasma was reconstituted with 80 or 160 nmol/L PCI and compared with normal plasma. The results shown in Fig 4 demonstrate that PCI has a concentration-dependent anticoagulant effect on tissue factor-induced coagulation. PCI-deficient plasma displayed a slightly shorter coagulation time that could be restored to normal by the addition of a plasma concentration of PCI. To rule out any effects due to complex formation between APC and PCI, the experiments were repeated with protein C–deficient plasma. Addition of 40, 80, and 160 nmol/L PCI to protein C–deficient plasma resulted in a minor delay in coagulation identical to the delay observed when normal plasma was used (data not shown), indicating that PCI acts as an inhibitor of coagulation under these conditions.
Effect of protein C inhibitor on tissue factor-induced coagulation. Normal plasma (closed symbols) and PCI-deficient plasma (open symbols) were incubated with buffer (○,•), 80 (□), or 160 (▵) nmol/L of protein C inhibitor. After incubation, coagulation was started by the addition of recombinant tissue factor (Innovin, diluted by a factor 3 × 104) and calcium. The formation of fibrin was measured in time as the change in turbidity at 405 nm.
Effect of protein C inhibitor on tissue factor-induced coagulation. Normal plasma (closed symbols) and PCI-deficient plasma (open symbols) were incubated with buffer (○,•), 80 (□), or 160 (▵) nmol/L of protein C inhibitor. After incubation, coagulation was started by the addition of recombinant tissue factor (Innovin, diluted by a factor 3 × 104) and calcium. The formation of fibrin was measured in time as the change in turbidity at 405 nm.
The role of PCI in tissue factor-induced fibrin formation in plasma in the presence of TM.
Fibrin formation was monitored after addition of tissue factor to recalcified plasma, which was preincubated for 15 minutes with 10 nmol/L TM. In contrast to the observed anticoagulant effect of PCI in the absence of TM, the addition of 80 or 160 nmol/L PCI to normal plasma resulted in a procoagulant effect in the presence of TM (Fig 5A). To study the possibility that the procoagulant PCI effect was caused by a TM-stimulated inhibition of APC by PCI, the experiment was repeated using protein C–deficient plasma. The effect of PCI was monitored by enriching protein C–deficient plasma with 80 and 160 nmol/L PCI, followed by monitoring the tissue factor-induced fibrin formation (Fig 5B).
Effect of protein C inhibitor on tissue factor-induced coagulation in the presence of TM. Normal plasma (A) and protein C–deficient plasma (B) were incubated with buffer (○), 80 (▪), or 160 (▴) nmol/L protein C inhibitor in the presence of TM (10 nmol/L). After incubation, recombinant tissue factor and calcium were added, and the change in turbidity was monitored at 405 nm.
Effect of protein C inhibitor on tissue factor-induced coagulation in the presence of TM. Normal plasma (A) and protein C–deficient plasma (B) were incubated with buffer (○), 80 (▪), or 160 (▴) nmol/L protein C inhibitor in the presence of TM (10 nmol/L). After incubation, recombinant tissue factor and calcium were added, and the change in turbidity was monitored at 405 nm.
The addition of PCI to protein C–deficient plasma in the presence of TM resulted in a PCI-dependent delay in fibrin formation, making PCI an inhibitor of coagulation under these conditions, in contrast to the procoagulant PCI effect in the presence of TM observed in normal plasma (Fig 5A). This indicated that PCI plays a major role in the inhibition of the protein C pathway by either preventing the activation of protein C or by inhibiting APC.
Comparison of the TM-mediated PCI reactivity between normal plasma and PCI-deficient plasma.
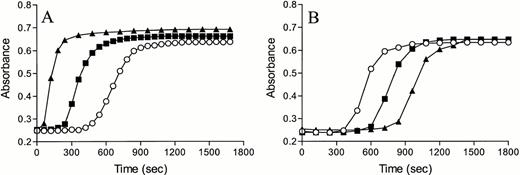
The effect of PCI was also monitored using PCI-deficient plasma, which was directly compared with normal plasma, both in the absence or presence of rabbit lung TM (Fig 6A) or TM4-6 (Fig 6B). In the absence of TM, fibrin formation occurred earlier in PCI-deficient plasma compared with normal plasma, as was already observed (Fig 4). However, in the presence of 10 or 25 nmol/L TM, PCI-deficient plasma displayed a delay in coagulation compared with normal plasma. The same phenomenon was observed when normal plasma was compared with PCI-deficient plasma in the presence of TM4-6. Figure 6B shows that addition of the TM fragment resulted in a concentration-dependent delay in coagulation. Compared with rabbit lung TM, higher concentrations of the recombinant TM4-6 fragment (50 and 100 nmol/L) were necessary to enable comparison between TM and TM4-6. Figure 6A and B show that the overall effect of PCI in tissue factor-induced coagulation is procoagulant in the presence of TM or TM4-6.
The effect of TM on tissue factor-induced coagulation of normal plasma and PCI-deficient plasma. Normal plasma (open symbols) and PCI-deficient plasma (closed symbols) were incubated with buffer (○,•) or 10 (□,▪) or 25 (▵,▴) nmol/L rabbit lung TM (A) or 50 (◊,⧫) or 100 (▿,▾) nmol/L TM4-6 (B). Clotting was initiated by adding recombinant tissue factor and calcium, and the formation of fibrin was measured in time as the change in turbidity at 405 nm.
The effect of TM on tissue factor-induced coagulation of normal plasma and PCI-deficient plasma. Normal plasma (open symbols) and PCI-deficient plasma (closed symbols) were incubated with buffer (○,•) or 10 (□,▪) or 25 (▵,▴) nmol/L rabbit lung TM (A) or 50 (◊,⧫) or 100 (▿,▾) nmol/L TM4-6 (B). Clotting was initiated by adding recombinant tissue factor and calcium, and the formation of fibrin was measured in time as the change in turbidity at 405 nm.
DISCUSSION
In this report, we studied the inhibition of thrombin and APC by PCI in the absence and presence of TM in a purified system and in a plasma system. The interaction between PCI and TM was studied to search for an explanation for the dual role of PCI in plasma, in which it displays both procoagulant and anticoagulant properties. Previous studies indicated that binding of thrombin to TM induces a change in the conformation, which prevents thrombin to clot fibrinogen and to activate platelets,18,25,26 but which results in a stimulation of protein C activation. Rezaie et al20suggested that this TM-induced change in thrombin conformation stimulated the inhibition of thrombin by PCI.
Our data show that thrombin, both in the absence and presence of TM, is a much better target for PCI than APC. In the absence of TM, the rate inactivation constant for thrombin by PCI was 18 times more favorable compared with the APC-PCI reaction using identical stoichiometry between protease and PCI. In the presence of rabbit lung TM, we observed a modest (twofold) stimulation of the APC-PCI reaction compared with a 33-fold enhancement of the thrombin inhibition by PCI. When the human TM fragment TM4-6 was used, a 24-fold stimulation was observed for the inhibition of thrombin by PCI. Comparison of the second order rate constants of the thrombin inhibition by PCI in the presence of rabbit lung TM or TM4-6 suggests that the inhibition reaction is merely dependent on conformational changes in thrombin when bound to TM.
Earlier studies indicated stimulating effects varying between 2- and 140-fold20 27 for the inhibition of TM-bound thrombin by PCI. Our data indicated that the net result of the rate inactivation constant for the TM-mediated thrombin-PCI reaction was 267 times more favorable compared with the TM-dependent APC-PCI reaction. Because thrombin, especially in the presence of TM, was a much better target for PCI than APC, we studied the role of PCI on tissue factor-induced coagulation in plasma in the presence and absence of TM. In the absence of TM, PCI acted as a modest inhibitor of tissue factor-induced coagulation. Comparison of the plasma concentration of PCI to the concentration of antithrombin (80 nmol/L and 2.3 μmol/L, respectively), another thrombin inhibitor with similar affinity for thrombin in the absence of a glycosaminoglycan, confirmed the minor role of PCI in coagulation in the absence of TM. In the presence of TM, however, addition of PCI exhibited a strong procoagulant effect on tissue factor-induced coagulation, provided protein C was present. This suggests that PCI either inhibits the activation of protein C by TM-bound thrombin or that PCI inhibits APC, although the latter mechanism is unlikely because of the unfavorable kinetics. In both scenarios, PCI inhibits the anticoagulant activity of the protein C pathway. When generation of APC cannot take place such as in normal plasma in the absence of TM or vice versa in the presence of TM, but in protein C–deficient plasma, PCI exhibits an anticoagulant effect presumably due to the inhibition of thrombin. The overall impact of PCI is procoagulant because the impact of the inhibition of protein C activation is stronger than the modest direct inhibitory effect of thrombin.
The similarity between the reactive site regions of PCI and ATIII may be viewed as an additional indication that PCI reactivity is directed towards the inhibition of TM-bound thrombin. The P1 residue of the serpin primarily dictates serpin-serine protease interactions with additional contributions of neighboring residues.28-30Whereas the reactive site bonds for PCI and α1-antitrypsin, two major inhibitors of APC differ, both PCI and ATIII possess an Arg-Ser reactive site bond.28,29,31 In addition, mutagenesis studies have shown that glycine at the P2 position as in ATIII, is preferred, but that phenylalanine at the P2 of PCI is tolerated for thrombin inhibition.28,29 On the other hand, a P2 glycine is not well tolerated for APC inhibition in contrast to the hydrophobic phenylalanine at P2 of PCI.28,29 This may explain why PCI can inhibit both APC and thrombin, two enzymes with apparent opposite functions. Whereas ATIII is mainly responsible for the inactivation of free thrombin, the role of PCI as thrombin inhibitor is directed to the inactivation of TM-bound thrombin. The ability of TM4-6 to enhance the inhibition of thrombin by PCI shows that the inhibition reaction is not primarily dictated by the chondroitin sulfate. This is an important distinction between the reactivities of PCI and ATIII towards thrombin, as it has been demonstrated that the inhibition of TM-bound thrombin by ATIII is dictated by this glycosaminoglycan.32 The inhibitory role for PCI on APC and TM-bound thrombin reflects the importance of PCI as regulator of the anticoagulant protein C pathway.
In conclusion, a major function of PCI in plasma during coagulation is the inhibition of TM-bound thrombin. The modest anticoagulant behavior of PCI as a thrombin inhibitor is counteracted by the downregulation of protein C activation resulting in a procoagulant effect.
ACKNOWLEDGMENT
We thank Dr A.R. Rezaie for helpful suggestions, Dr J.E. Sadler for the gift of TM4-6, Dr W. Kisiel for the gift of human thrombin, and the personnel of the Red Cross Blood Bank for providing us the donor plasma.
Supported in part by Grant No. 92.306 from The Netherlands Heart Foundation, and a fellowship from the Royal Netherlands Academy for Arts and Sciences. J.C.M.M. is an Established Investigator of The Netherlands Heart Foundation.
Address reprint requests to Marc G.L.M. Elisen, PhD, Department of Haematology (G03.647), University Hospital Utrecht, PO Box 85500, 3508 GA Utrecht, The Netherlands.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 1. Inhibition of APC and thrombin by PCI in the absence and presence of TM. APC 0.5 nmol/L (A) or thrombin 0.5 nmol/L (B and C) were incubated with 10 (○,•), 25 (□,▪), or 50 (▵,▴) nmol/L PCI, respectively, in the absence (open symbols) or presence (closed symbols) of TM (50 nmol/L rabbit lung [A and B] and 100 nmol/L TM4-6 [C]). At indicated time points, the chromogenic substrates S2366 (1 mmol/L f.c.) or Spectrozyme TH (0.25 mmol/L f.c.) were added to monitor the remaining activity of APC and thrombin, respectively. Please note the difference in x-axis scales in A, B, and C.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/5/10.1182_blood.v91.5.1542/3/m_blod4050201.jpeg?Expires=1769375429&Signature=t3n5dpkj5eLDf~uxwBS~xG1pA9GfgW3DF2j7G9UeIIWF7R1SxxOjQEnqwCwqjvkHnZKzJs3EY~Iq0HlBPW4A7UnjKT1SbJfhkJuotwcs8Mu7GG-iOTIrAt4S5XUPj1XJ74N9FTMfhnPJ8W5a6QnADah3ErpfekIioetFKtL9LsBXZMp8irik-j2u4EutVEOR57BTXyKQvIMKnAVSBiMST7j9-28SCBwb3GYb2W7lYgMjfjREJEPZ2ZN~oxF3ud-H6dpZYHPrvkYF9t4~D6Ff~QrDPzDBsIKqmelXOcldAXuDaR1Bzwv3CBMknwti0BBdDulDkI7hVtIxzKMgmqqUQw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal