Abstract
Secretoneurin (SN), a 33-amino acid neuropeptide, is derived from secretogranin II that is released from sensory afferent C-fibers by capsaicin. Described functions of secretoneurin include chemotaxis of monocytes and endothelial cells, and inhibition of endothelial cell proliferation. Inhibition of monocyte chemotaxis by staurosporine indicated involvement of specific signaling pathways. We have tested effects of SN, substance P (SP), and interleukin-8 (IL-8) on eosinophil migration in modified Boyden chambers including signaling mechanisms of neuropeptide and cytokine stimulation of human eosinophils. Experiments showed SN as eosinophil chemoattractant comparable in its potency to IL-8. Checkerboard analysis, usage of a specific anti–SN-antibody, and receptor desensitization experiments confirmed the chemotactic activity. Preincubation of the cells with effective concentrations of staurosporine or tyrphostin-23 showed no effect, whereas treatment with wortmannin (WTN) or 3-isobutyl-1-methylxantin (IBMX) completely blocked SN-induced migration. Additionally, experiments ruled out tyrphostin-23- and WTN-sensitive signaling pathways for SP-induced chemotaxis of eosinophils. We conclude that SN-stimulated human eosinophil chemotaxis is mediated via a unique and specific signal transduction pathway that involves activation of phosphodiesterases and WTN-sensitive enzymes, ie, phospholipase D and phosphatidylinositol-3-kinase. In contrast, we report that activation of the latter and tyrosine kinases is required for SP-induced chemotaxis of eosinophils.
SENSORY NEUROPEPTIDES are small amino acid molecules released from the afferent c-fibers of peripheral nerve endings.1 Several peptides have been identified and in accordance with the involvement of primary afferent nerve fibers in neurogenetic inflammation,2 the sensory neuropeptides have been identified as potent mediators of inflammatory and immunologic reactions. Moreover, small quantities of some of the peptides, eg, substance P (SP), have been detected in peritoneal mast cells, platelets, and eosinophils, indicating that sensory neuropeptides may also be obtained from nonneuronal sourses during an inflammatory response.3
Secretoneurin (SN) is a newly discovered 33-amino acid peptide derived from secretogranin II (chromogranin C).4 It is widely distributed throughout the central and peripheral nervous systems and can be coreleased with SP and calcitonin gene-related peptide from sensory afferent c-fibers by capsaicin,5 suggesting that it might represent another member of the group of inflammatory neuropeptides. SN may in fact be a regulator of neurogenic inflammation since it was shown to increase monocyte but not lymphocyte chemotaxis, to deactivate neutrophil chemotaxis, and to affect fibroblast, endothelial, and smooth muscle cell migration.6-9 Recently, a receptor for SN has been identified (manuscript submitted)*, but less is known about mechanisms involved in signaling and cell activation by SN.
Many substances including chemokines, neuropeptides, complement fragments, and eicosanoids have been shown to attract eosinophils10-13 or to promote their transendothelial migration.14 With regard to the above-mentioned properties of SN and the knowledge of neuropeptide-induced alteration of eosinophil functions, we investigated the effects of SN on the chemotaxis of human eosinophils, known as prominent inflammatory cells thought to play a major role in the pathogenesis of allergic diseases, and we compared its effects with SP and the cytokine interleukin-8 (IL-8). For reasons of comparison we performed additional experiments with RANTES, a well-known potent eosinophil chemotaxin, and formyl-Met-Leu-Phe (fMLP), and β-endorphin, as another neuropeptide. Checkerboard analysis, usage of specific antibodies, and receptor desensitization experiments served to reveal the specific effect of SN on eosinophil directed migration (chemotaxis). Furthermore, we studied possible signaling pathways in human eosinophils following receptor ligation by the substances investigated.
MATERIALS AND METHODS
Reagents.
RPMI 1640 with phenol-red was from Biological Industries (Kibbutz Beit Haemek, Israel). Bovine serum albumin (BSA) was from Behring Werke AG (Marburg, Germany). SP, IL-8, 3-isobutyl-1-methylxantin (IBMX), wortmannin (WTN), staurosporine, and tyrphostin-23 were from Sigma Chemical Corp (St Louis, MO). Human SN was purchased from Neosystems (Strassbourgh, France) and anti-human SN-antibody was a gift from R. Fischer-Colbrie (Institut of Pharmacology, University of Innsbruck). Monoclonal mouse-anti human IL-8 antibody was from Serotec Ltd (Oxford, UK). MACS magnetic microbeads were from Miltenyi Biotec (Bergisch Gladbach, Germany), and nitrocellulose filters (5-μm pore size) were from Sartorius AG (Goettingen, Germany). All stock solutions, except staurosporine and tyrphostin-23, were stored at −20°C before use. All other reagents not further specified were from Sigma.
Isolation of eosinophils.
For preparation of eosinophils we used MACS (magnetic cell sorting) CD16 microbeads according to the manufacturer's protocol for isolation of untouched human eosinophils by depletion of CD16+ cells. In brief, granulocytes were obtained from buffy coats (mixed with Hanks' balanced salt solution [HBSS] without Ca2+ and Mg2+ in a ratio of 3:1) by dextran sedimentation and centrifugation through a layer of Ficoll-Hypaque (Nycomed Pharma AS, Oslo, Norway). To remove most of the mononuclear cells this step was performed twice and was followed by hypotonic lysis of contaminating erythrocytes using sodium chloride solution.15 After washing, cells were resuspended in 50 μL/5 × 107 cells ice-cold MACS buffer (phosphate-buffered saline [PBS] with 5 mmol/L EDTA and 0.5% BSA) and an equal volume of MACS colloidal superparamagnetic microbeads conjugated with monoclonal anti-human CD16 antibodies was added for an incubation time of 30 minutes at 6°C. Recommended volumes of ice-cold MACS buffer were added to the cell/microbead mixture and the cell suspension was loaded onto the top of the prewashed separation column. The eluate containing CD16− eosinophils was collected, washed, and resuspended in RPMI 1640/0.5% BSA. To increase sensitivity, the separation procedure was repeated. Purity of sorted eosinophils was greater than 98%, as determined by morphology and FACS analysis. Contaminating cells were less than 1% lymphocytes, less than 1% neutrophils and basophils, and monocytes/macrophages at neglectible cell counts.
Eosinophil chemotaxis.
Chemotaxis assays were performed using a modified 48-well Boyden microchemotaxis chamber (Neuroprobe, Bethesda, MD) in which a 5-μm pore-sized cellulose nitrate filter separates the upper and lower chamber.16 Eosinophils were resuspended in RPMI 1640/0.5% BSA to a final concentration of 1 × 106 cells/mL and 50 μL of the cell suspension was placed into the upper chamber. Eosinophils were allowed to migrate toward various concentration gradients of the soluble chemoattractants in the lower chamber for 60 minutes. For checkerboard analysis cells were resuspended in RPMI 1640/0.5% BSA containing various concentrations of SN just before transferring them to the upper chamber. To ensure the specific chemotactic effect of SN, a specific anti-human SN-antibody (dilution 1:1,000) or a monoclonal mouse anti-human IL-8 antibody (10 μg/mL) was added to the chemoattractants in the lower chamber. With the same intention eosinophils were pretreated with SN (0.1 μmol/L) for 10 minutes before they migrated toward chemoattractans. Further to investigate the effects of enzyme blockade, eosinophils were incubated with staurosporine (10 ng/mL), tyrphostin-23 (10 ng/mL), WTN (10 nmol/L), and IBMX (1 μmol/L), respectively, for 30 minutes at 37°C in humidified atmosphere (5% CO2). After washing twice, the assay was proceeded as described above. After the migration period the nitrocellulose filters were dehydrated, fixed, and stained with hematoxylin-eosin. Migration depth of the cells into the filters was quantified by microscopy, measuring the distance (μm) from the surface of the filter to the leading front of cells. Random migration was less the 60 μm in all experiments. Data are expressed as “Chemotaxis Index,” which is the ratio between the distance of directed and undirected migration of eosinophils into the nitrocellulose filters.
Statistical analyses.
Data are expressed as mean and standard error of the mean (SEM) of the “Chemotaxis Index.” Means were compared by Kruskal-Wallis analysis of variance and by Mann-Whitney U-test. A difference withP < .05 was considered to be significant. Statistical analyses were calculated using the StatView software package (Abacus Concepts, Berkley, CA).
RESULTS
Migration toward various concentrations of soluble chemoattractants.
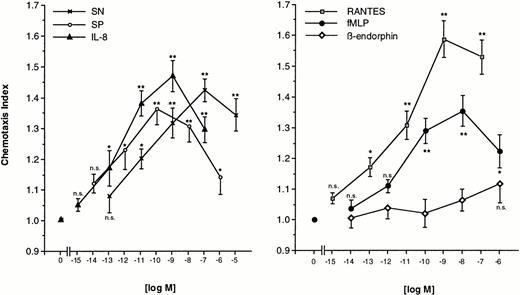
Freshly prepared eosinophils were allowed to migrate toward various concentrations of SN (0.1 pmol/L to 10 μmol/L), SP (0.01 pmol/L to 1 μmol/L), or IL-8 (1 fmol/L to 0.1 μmol/L) for 60 minutes at 37°C in humidified atmosphere. The dose-response curve for each chemoattractant occured as bell-shaped with a maximal response at a concentration of 0.1 μmol/L of SN, 0.1 nmol/L of SP, and 1 nmol/L of IL-8. The lowest doses tested were not able to produce a significant migration. Increasing statistical significance of migration was observed for increasing concentrations, except the highest, of SN and IL-8 (Fig 1, left panel). For comparison, various concentrations of RANTES (1 fmol/L to 0.1 μmol/L), fMLP (0.01 pmol/L to 1 μmol/L) or β-endorphin (0.01 pmol/L to 1 μmol/L) were also used as chemoattractants. RANTES-induced chemotaxis reached significance at a concentration of 0.1 pmol/L and peaked at 1 nmol/L. Eosinophils responded to fMLP, but to a lesser extent as compared to SN. Maximal migration was seen at 10 nmol/L with a chemotaxis index roughly equal produced by a concentration two logarithmic decades below the concentration of maximal response to SN. High-dose β-endorphin slightly stimulated eosinophil migration, but none of the concentrations tested excerted statistically significant migration toward β-endorphin (Fig 1, right panel).
Migration of eosinophils into nitrocellulose micropore filters (5-μm pore size) toward various concentrations of soluble chemoattractants. Various concentrations of SN (0.1 pmol/L to 10 μmol/L), SP (0.01 pmol/L to 1 μmol/L), IL-8 (1 fmol/L to 0.1 μmol/L) (left panel), RANTES (1 fmol/L to 0.1 μmol/L), fMLP (0.01 pmol/L to 1 μmol/L), or β-endorphin (0.01 pmol/L to 1 μmol/L) (right panel) were placed in the lower wells of a modified Boyden chamber. RPMI 1640/0.5% BSA served as control to determine random migration. Thereafter 50 μL of human eosinophils at 1 × 106 cells/mL were added to the upper wells and were allowed to migrate for 60 minutes at 37°C (humidified atmosphere; 5% CO2). After fixing and staining of the filters migration depth was quantified microscopically. Data are expressed as mean ± SEM of the “Chemotaxis Index,” which is the ratio between the distance cells migrate toward test substances and that toward control medium. n = 6. Statistical analyses: Mann-Whitney U-test after Kruskal Wallis analysis of variance (P < .01); n.s., not significant; *, P < .05; **, P < .01.
Migration of eosinophils into nitrocellulose micropore filters (5-μm pore size) toward various concentrations of soluble chemoattractants. Various concentrations of SN (0.1 pmol/L to 10 μmol/L), SP (0.01 pmol/L to 1 μmol/L), IL-8 (1 fmol/L to 0.1 μmol/L) (left panel), RANTES (1 fmol/L to 0.1 μmol/L), fMLP (0.01 pmol/L to 1 μmol/L), or β-endorphin (0.01 pmol/L to 1 μmol/L) (right panel) were placed in the lower wells of a modified Boyden chamber. RPMI 1640/0.5% BSA served as control to determine random migration. Thereafter 50 μL of human eosinophils at 1 × 106 cells/mL were added to the upper wells and were allowed to migrate for 60 minutes at 37°C (humidified atmosphere; 5% CO2). After fixing and staining of the filters migration depth was quantified microscopically. Data are expressed as mean ± SEM of the “Chemotaxis Index,” which is the ratio between the distance cells migrate toward test substances and that toward control medium. n = 6. Statistical analyses: Mann-Whitney U-test after Kruskal Wallis analysis of variance (P < .01); n.s., not significant; *, P < .05; **, P < .01.
Checkerboard analysis for SN-induced chemotaxis.
To distinguish between SN-induced chemokinesis (increased random migration) and chemotaxis (directed movement of cells along a chemotactic gradient) we performed a checkerboard analysis. Eosinophils were resuspended in medium or medium containing various concentrations of SN (0.01 nmol/L to 0.1 μmol/L) just before transferring the cells to the upper chamber. Addition of 0.1 μmol/L of SN exclusively to the upper compartment resulted in a slightly enhanced migratory response of eosinophils. A gradually increasing concentration gradient of SN between the lower and the upper compartment led to increased and highly significant migration of the cells toward the lower compartment, uncovering the migratory response of eosinophils to SN as true chemotaxis (directed migration) (Table 1).
Specifity of SN-induced chemotaxis.
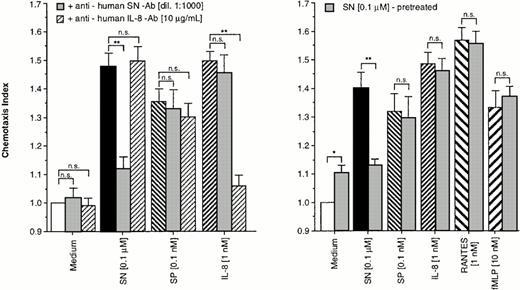
To prove the specificity of the effect of SN on eosinophils, cells were allowed to migrate toward chemoattractants alone or chemoattractants in combination with a specific anti-human SN-antibody (dilution 1:1,000) or a monoclonal mouse anti-human IL-8 antibody in the lower chamber. As shown in Fig 2 (left panel), the SN-antibody was able to block the migratory response to SN (0.1 μmol/L). The IL-8 antibody also nearly completely reduced IL-8–induced chemotaxis, whereas combinations of the antibodies with non–antibody-specific targets had no effect on SN–, SP–, and IL-8–induced eosinophil chemotaxis (Fig 2, left panel).
Specifity of SN on eosinophil chemotaxis into nitrocellulose micropore filters (5-μm pore size). Maximal effective concentrations of SN (0.1 μmol/L), SP (0.1 nmol/L), or IL-8 (1 nmol/L) with or without SN-antibody (dilution 1:1,000) and anti-human IL-8 antibody (10 μg/mL), respectively, were placed in the lower wells of the chamber. RPMI 1640/0.5% BSA alone or with each antibody served as control. Thereafter 50 μL of human eosinophils at 1 × 106 cells/mL were added to the upper wells and were allowed to migrate for 60 minutes at 37°C (humidified atmosphere; 5% CO2) (left panel). In another set of experiments cells were pretreated with SN (0.1 μmol/L) for 10 minutes, washed twice, and thereafter allowed to migrate towards chemoattractants or medium (right panel). After fixing and staining of the filters migration depth was quantified microscopically. Data are expressed as mean ± SEM of the “Chemotaxis Index,” which is the ratio between the distance cells migrate toward test substances and that toward control medium. n = 6. Statistical analyses: Mann-Whitney U-test after Kruskal Wallis analysis of variance (P < .01); n.s., not significant; *,P < .05; **, P < .01.
Specifity of SN on eosinophil chemotaxis into nitrocellulose micropore filters (5-μm pore size). Maximal effective concentrations of SN (0.1 μmol/L), SP (0.1 nmol/L), or IL-8 (1 nmol/L) with or without SN-antibody (dilution 1:1,000) and anti-human IL-8 antibody (10 μg/mL), respectively, were placed in the lower wells of the chamber. RPMI 1640/0.5% BSA alone or with each antibody served as control. Thereafter 50 μL of human eosinophils at 1 × 106 cells/mL were added to the upper wells and were allowed to migrate for 60 minutes at 37°C (humidified atmosphere; 5% CO2) (left panel). In another set of experiments cells were pretreated with SN (0.1 μmol/L) for 10 minutes, washed twice, and thereafter allowed to migrate towards chemoattractants or medium (right panel). After fixing and staining of the filters migration depth was quantified microscopically. Data are expressed as mean ± SEM of the “Chemotaxis Index,” which is the ratio between the distance cells migrate toward test substances and that toward control medium. n = 6. Statistical analyses: Mann-Whitney U-test after Kruskal Wallis analysis of variance (P < .01); n.s., not significant; *,P < .05; **, P < .01.
For verification of receptor-regulatory pathways for SN mediated effects, eosinophils were pretreated with 0.1 μmol/L of SN for 10 minutes or remained untreated. After washing twice, cells migrated toward chemoattractants. A difference in the migratory response between the treated and the untreated group was observed only for chemotaxis toward SN (0.1 μmol/L) but not toward SP, IL-8, RANTES, and fMLP (Fig2, right panel).
Effects of enzyme-blockers on eosinophil chemotaxis.
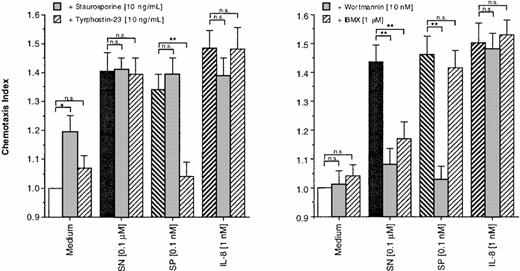
For this purpose, eosinophils were treated either with staurosporine (10 ng/mL), tyrphostin-23 (10 ng/mL), WTN (10 nmol/L), or IBMX (1 μmol/L) or remained untreated for control. After an incubation period of 30 minutes, cells were washed twice. Staurosporine, itself a weak stimulator of migration, had no effect on the migratory response induced by all agents tested. Random migration as well as SN (0.1 μmol/L)– and IL-8 (1 nmol/L)–induced chemotaxis remained unaffected by pretreatment of eosinophils with tyrphostin-23. In contrast, SP (0.1 nmol/L)–induced chemotaxis was reduced to baseline levels by this tyrosine kinase blocker (Fig 3, left panel). WTN and IBMX showed no effect on random migration and IL-8–induced chemotaxis, whereas only WTN completely reduced SP-induced migration. Both drugs highly significant reduced cells response to SN, with higher potency of WTN as compared to IBMX (Fig 3, right panel).
Effects of enzyme blockers on eosinophil chemotaxis into nitrocellulose micropore filters (5-μm pore size). Eosinophils remained untreated or were treated for 30 minutes at 37°C (humidified atmosphere 5% CO2) with staurosporine (10 ng/mL), tyrphostin-23 (10 ng/mL) (left panel), WTN (10 nmol/L), or IBMX (1 μmol/L) (right panel). After washing twice, 50 μL of human eosinophils at 1 × 106 cells/mL were added to the upper wells and were allowed to migrate for 60 minutes at 37°C (humidified atmosphere; 5% CO2) toward chemoattractants or medium control. After fixing and staining of the filters migration depth was quantified microscopically. Data are expressed as mean ± SEM of the “Chemotaxis Index,” which is the ratio between the distance cells migrate toward test substances and that toward control medium. n = 6. Statistical analyses: Mann-Whitney U-test after Kruskal Wallis analysis of variance (P < .01); n.s., not significant; *, P< .05; **, P < .01.
Effects of enzyme blockers on eosinophil chemotaxis into nitrocellulose micropore filters (5-μm pore size). Eosinophils remained untreated or were treated for 30 minutes at 37°C (humidified atmosphere 5% CO2) with staurosporine (10 ng/mL), tyrphostin-23 (10 ng/mL) (left panel), WTN (10 nmol/L), or IBMX (1 μmol/L) (right panel). After washing twice, 50 μL of human eosinophils at 1 × 106 cells/mL were added to the upper wells and were allowed to migrate for 60 minutes at 37°C (humidified atmosphere; 5% CO2) toward chemoattractants or medium control. After fixing and staining of the filters migration depth was quantified microscopically. Data are expressed as mean ± SEM of the “Chemotaxis Index,” which is the ratio between the distance cells migrate toward test substances and that toward control medium. n = 6. Statistical analyses: Mann-Whitney U-test after Kruskal Wallis analysis of variance (P < .01); n.s., not significant; *, P< .05; **, P < .01.
DISCUSSION
Neutrophils and eosinophils are the major classes of granulocytes that emigrate from the bloodstream but their accumulation patterns in inflammed tissue are strikingly different; neutrophils are rapidly recruited into sites of acute bacterial infection, whereas eosinophils are predominantly recruited into tissues with allergic inflammation or parasitic infection.17 Leukocyte chemotaxis is a complex phenomenon that includes polarization and orientation in the direction of the highest concentration of the chemoattractant; nevertheless, signal transduction pathways that mediate this complicated process are not yet fully understood.18
The goal of our study was to rule out whether and by which mechanisms SN, a novel neuropeptide, elicits eosinophil chemotaxis. First we compared a panel of chemoattractants, including SN, for elicitation of chemotaxis of eosinophils in vitro. Our results are in accordance with findings published previously for eosinophil Boyden chamber- and transendothelial-chemotaxis.12,19-21 Since it has been shown that eosinophils isolated with MACS, in contrast to the density gradient centrifugation method involving cell activation with fMLP, exerted stronger chemotactic response toward chemoattractants,22 it was not surprising that the cells in our system migrated well, even toward gradients of fMLP. For these reasons it is certain that results obtained for SN-induced chemotaxis on eosinophils in our chemotaxis measurement system are reliable. Moreover, results indicate that SN, as compared to RANTES which shows a similar efficacy on eosinophils as the strong chemotactic agonist C5a,19 is a potent stimulator of eosinophil chemotaxis. In fact, a specific anti-human SN antibody was able to completely suppress the chemotactic response to SN, confirming SN as an independent chemoattractant.
There is considerable literature published on homologous and heterologous desensitization to one chemoattractant by another but most previous studies have focused on assays other than chemotaxis. In eosinophils, contrasting to neutrophils, the chemotactic movements induced by fMLP, C5a, or RANTES were not reduced at any concentration by the presence of one of the other chemoattractants. Also, postreceptor signaling pathways are independent and not desensitized by each other, indicating at least three noninterfering receptor-signal transduction pathways for chemotaxis and actin polymerization in eosinophils.21
Preincubation of the cells with an effective concentration of SN for 10 minutes led to a highly significant inhibition of SN-induced but not SP–, IL-8–, RANTES–, or fMLP–induced migration indicating homologous SN receptor downregulation in eosinophils. This result is a hint to a unique and independent receptor and receptor signaling mechanism for SN in eosinophils apart from the noninterfering receptor-signal transduction pathways for chemotaxis mentioned above. Therefore, signal transduction pathways of SN were further investigated.
Many studies focused on the intracellular signaling pathways in eosinophils and described mechanisms involved in superoxide production and granular secretion23-25 but only few were focused on eosinophil migration.26-28 Addition of staurosporine to eosinophils slightly stimulated migration in our study, not surprisingly, since Schweizer et al26 have reported that staurosporine increases the F-actin content in eosinophils, and that PAF-induced chemotaxis and IL-5–induced chemokinesis are independent of protein kinase A and protein kinase C activation.26 The latter finding is in accordance with our observations, where staurosporine failed to influence SN–, SP–, as well as IL-8–induced chemotaxis. Tyrphostin-23, a selective tyrosine kinase inhibitor, blocked SP-induced chemotaxis, which is mediated via specific tyrosine kinase–coupled SP receptors.7 Growth factor–stimulated chemotaxis of neutrophils can be diminished by WTN, a phosphatidylinositol-3-kinase (PI3-kinase) and phospholipase D (PLD) inhibitor, whereas it lacks this effect on neutrophil migration stimulated with classical chemoattractants.29 In eosinophils, we obtained similar results because WTN was able to reduce the chemotactic response toward SN and SP, but not toward IL-8. For affecting cAMP levels in eosinophils we inhibited phosphodiesterase with IBMX, which is reported to inhibit eosinophil's responses with IC50 values equal to the range of the IC50 values obtained with rolipram (type IV-selective).30 Moreover, an in vivo study on the selective type IV phosphodiesterase inhibitor T-440 showed higher potency of the drug in eosinophils rather than in neutrophils.31 Others showed a lack of type III– and type V–selective inhibitors to suppress the chemotactic responsiveness of rat eosinophils in vitro28 and guinea pig eosinophils in vivo.32 To our knowledge, effects of phosphodiesterase inhibitors on eosinophil functions are described only for PAF, leukotriene B4, eotaxin, and C5a activation of the cells.27,28 33 We now observed that IBMX is able to diminish SN-induced migration of eosinophils with no effect on SP or IL-8 stimulation, suggesting that SN uses signal transduction pathways involving cyclic AMP. Additionally we show that SP involve tyrosine kinase and WTN-sensitive enzymes for intracellular signaling in eosinophil chemotaxis.
In conclusion, SN is a potent stimulator of eosinophil chemotacic response, which is mediated via a unique and specific signal transduction pathway that is different from what is described so far and can be affected by phosphodiesterases and WTN-sensitive enzymes, potentially due to PI3-kinase or PLD inhibition. Furthermore, our results suggest that activation of the latter and tyrosine kinases is required for SP-induced chemotaxis of eosinophils. Since phosphodiesterase IV inhibitors has been successfully used in animal models for therapy of eosinophil-related diseases,32 34 and the potent chemotactic activity of SN on eosinophils can be blocked by phosphodiesterase inhibition, this novel neuropeptide might play a role in some of these in vivo models.
ACKNOWLEDGMENT
The authors thank R. Fischer-Colbrie, PhD, for providing the anti-SN antibody and fruitful discussions.
Specific binding sites for 125I-BHSN were identified on human MonoMac 6 cells. Scatchard analysis: single class binding site with kd = 7.3 nmol/L andBmax of 322 fmol/mg protein.
Supported by the Austrian Science Funds (Grant No. 09977 to C.J.W.).
Address reprint requests to Christian J. Wiedermann, MD, Department of Internal Medicine, University of Innsbruck, Anichstrasse 35, A-6020 Innsbruck, Austria.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be here-by marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.