Abstract
Mice with hemophilia B have been engineered using gene targeting techniques. These animals exhibit severe factor IX deficiency and a clinical phenotype that mirrors the human disease. We have bred the founder animals onto two different strains of mice, C57B1/6 and CD-1, and have sought to determine whether adenoviral vectors expressing human factor IX could correct the bleeding diathesis of mice with hemophilia B. Initial experiments showed that purified plasma-derived human factor IX added to murine factor IX–deficient plasma resulted in complete correction of the activated partial thromboplastin time (aPTT), and that injection of 1011 particles of an adenoviral vector expressing human factor IX resulted in normalization of a modified aPTT in mouse plasma. As an additional method of assessing the function of human factor IX in the murine coagulation system, bleeding times were performed in normal, hemophilic, and adenoviral-treated hemophilic mice. By two different bleeding-time techniques, the treated hemophilic mice gave values identical to normal littermate controls, whereas the untreated hemophilic mice exhibited heavy blood loss and prolonged bleeding. There was a marked difference in antibody formation in the two strains of mice; 100% of the hemophilic CD-1 mice formed antibodies to human factor IX, but none of the C57B1/6 mice did. These data suggest that the C57B1/6 hemophilic mice will be more useful for gene transfer studies, while the CD-1 hemophilic mice may be of greater utility in studying the development of inhibitors.
HEMOPHILIA B is the bleeding diathesis resulting from a deficiency of blood coagulation factor IX (F.IX). Genetically engineered hemophilia B mice have recently been produced by gene targeting experiments in ES cells.1 These mice exhibit a severe bleeding diathesis including exsanguination after tail transection, premature death, severe bleeding after trauma, and evidence for spontaneous bleeding in the foot pads that mirrors the disease seen in humans, and should prove useful as a model in efforts to establish an experimental basis for gene therapy of hemophilia in humans.
Despite a high degree of conservation at both the DNA and protein levels, clotting factors from one species are not always fully functional in plasma from another species. Thus, for example, human F.VIIa does not interact with murine tissue factor.2 3 Most currently available gene therapy vectors express human F.IX; new vectors can be constructed expressing murine F.IX, but this is a time-consuming process, and analysis of results would be hampered by the absence of commercially available antibodies to murine F.IX, precluding assays such as enzyme-linked immunosorbent assay (ELISA) and immunofluorescence. Thus, we sought to determine whether vectors expressing human F.IX could correct the bleeding diathesis of mice with hemophilia B. Our results show that human F.IX is functional in the murine coagulation system and that it completely corrects the bleeding diathesis in hemophilia B mice. We also demonstrate a strain-dependent variation in the development of antibodies to human F.IX, suggesting that some strains will be more useful than others in most experimental protocols.
MATERIALS AND METHODS
Recombinant adenoviral vector.
An E1 gene deleted adenoviral vector (Ad-F.IX) containing human F.IX cDNA including the 3′ untranslated region driven by a cytomegalovirus (CMV) immediate early gene promoter/enhancer was constructed as previously described.4 The particle:plaque-forming units ratio of the Ad-F.IX is 30:1 as determined in a plaque assay as previously described.5 The viral preparations were stored at −80°C in phosphate-buffered saline (PBS) containing 3% sucrose.
Animal procedures.
All procedures involving mice were approved by the Institutional Animal Care and Use Committee at The Children's Hospital of Philadelphia in accordance with the Animal Welfare Act. Hemophilia B carrier females were bred with normal male mice of either C57B1/6 or CD-1 background. Hemophilic offspring were identified by Southern blot on tail DNA and by aPTT-F.IX assays.1 Eight- to 9-week-old hemophilia B mice were injected with 1011 particles of Ad-F.IX via the tail vein. Blood was collected directly into citrated tubes (mixed with citrate within 3 seconds) following tail transection performed under anesthesia. Hemostasis was obtained by ligating the tip of the tail with 4-0 Vicryl suture (ETHICON, Inc, Somerville, NJ). For immunofluorescence staining, animals were killed and the left lobe of the liver was removed and frozen in OCT embedding compound (Sakura) in a methylbutane dry ice bath.
Assays for antigen levels and biological activity of human F.IX.
Human F.IX (hF.IX) antigen in mouse plasma was determined by ELISA as previously described.4 A polyclonal rabbit anti-human F.IX antibody (Dako Corp, Carpenteria, CA) was used as primary antibody, and a polyclonal goat anti-human F.IX antibody coupled to horseradish peroxidase (Affinity Biologicals, Hamilton, Ontario, Canada) was used as the secondary antibody. The linear range of the standard curve was from 3 to 100 ng/mL. This ELISA does not cross-react with mouse F.IX. All samples were measured in duplicate; the intra- and inter-assay coefficient of variation was 8.7% and 15%, respectively.
APTT-F.IX assays were performed to determine the clotting activity of hF.IX expressed in the treated mice in the presence of hF.IX deficient plasma (Organon Teknika Corp, Durham, NC). In this assay, 50 μL of aPTT reagent (Organon Teknika Corp) and 50 μL of hF.IX-deficient plasma were incubated with 50 μL of a 1:5 dilution of sample mouse plasma. CaCl2was added and time to clot formation was measured with a fibrometer (Fibrosystem; BBL, Cockeysville, MD).1 This assay was developed to limit the amount of mouse plasma required for evaluation.
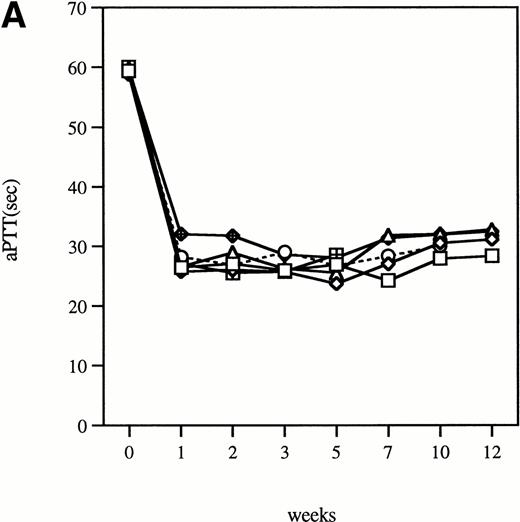
Time course of aPTT-F.IX assays in hemophilia B mice following injection of 1 × 1011 particles of an adenoviral vector expressing human F.IX. Citrated plasma was collected and aPTT-F.IX assays performed at the indicated time points. Each symbol represents an individual animal, with each point an average of duplicate measurements. (A) C57B1/6 hemophilic mice. (B) CD-1 hemophilic mice. Mouse 7 is a normal littermate treated with the same dose of vector. (□), 1; (◊), 2; (○), 3; (▵), 4; (⊞), 5; (⧫), 6; (•), 7.
Time course of aPTT-F.IX assays in hemophilia B mice following injection of 1 × 1011 particles of an adenoviral vector expressing human F.IX. Citrated plasma was collected and aPTT-F.IX assays performed at the indicated time points. Each symbol represents an individual animal, with each point an average of duplicate measurements. (A) C57B1/6 hemophilic mice. (B) CD-1 hemophilic mice. Mouse 7 is a normal littermate treated with the same dose of vector. (□), 1; (◊), 2; (○), 3; (▵), 4; (⊞), 5; (⧫), 6; (•), 7.
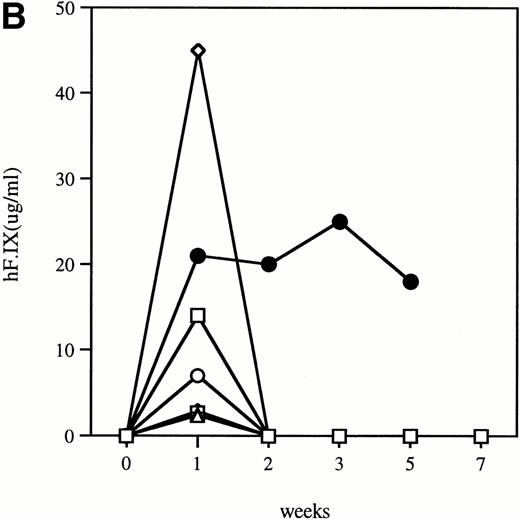
Time course of plasma concentration of hF.IX in hemophilia B mice following intravenous injection of 1011particles Ad-F.IX. Plasma samples were collected at the indicated time points and hF.IX levels determined by ELISA. Each point is an average of duplicate measurements. (A) C57B1/6 hemophilic mice and 1 normal littermate (no. 7). (B) CD-1 hemophilic mice and 1 normal littermate (no. 7). In both cases the normal littermate was treated with the same dose of vector. (□), 1; (◊), 2; (○), 3; (▵), 4; (⊞), 5; (⧫), 6; (•), 7.
Time course of plasma concentration of hF.IX in hemophilia B mice following intravenous injection of 1011particles Ad-F.IX. Plasma samples were collected at the indicated time points and hF.IX levels determined by ELISA. Each point is an average of duplicate measurements. (A) C57B1/6 hemophilic mice and 1 normal littermate (no. 7). (B) CD-1 hemophilic mice and 1 normal littermate (no. 7). In both cases the normal littermate was treated with the same dose of vector. (□), 1; (◊), 2; (○), 3; (▵), 4; (⊞), 5; (⧫), 6; (•), 7.
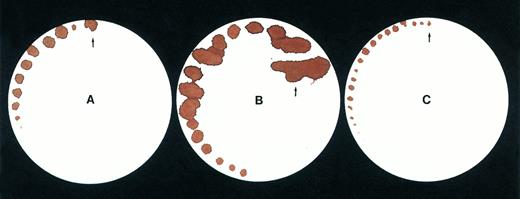
Tail bleeding time by a filter paper method. Data are shown for (A) a normal mouse, (B) a C57B1/6 hemophilic mouse, (C) a treated C57B1/6 hemophilic mouse 1 week after injection of Ad-F.IX vector.
Tail bleeding time by a filter paper method. Data are shown for (A) a normal mouse, (B) a C57B1/6 hemophilic mouse, (C) a treated C57B1/6 hemophilic mouse 1 week after injection of Ad-F.IX vector.
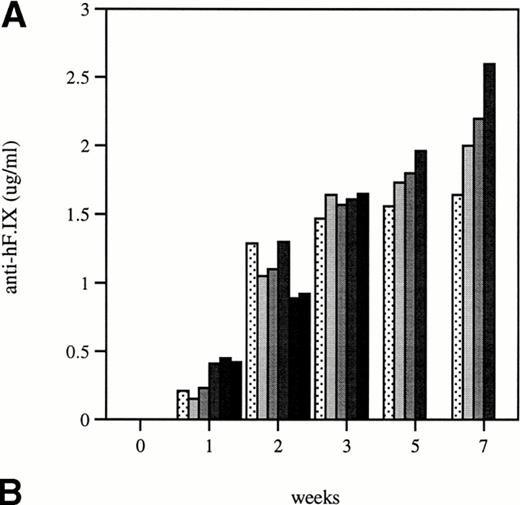
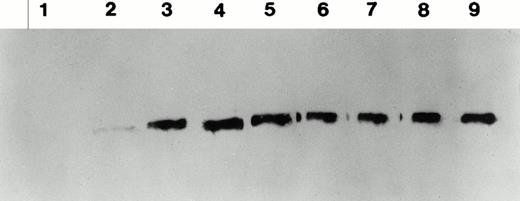
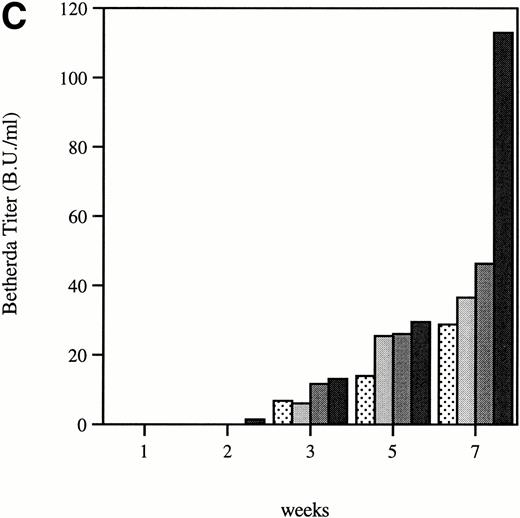
Total and neutralizing antibodies to human F.IX in hemophilic CD-1 mice. Plasma samples were evaluated by (A) ELISA, (B) Western blot, and (C) Bethesda assay at indicated time points. (A) Plates were coated with purified plasma–derived hF.IX, plasma samples from treated mice were applied, and rabbit anti-mouse IgG conjugated with horseradish peroxidase served as a detection antibody. Each result represents duplicate measurements from a single animal. (B) Lanes 1 through 6 represent an immunoblot time course on a single animal, weeks 0, 1, 2, 3, 5, and 7, respectively, after Ad-F.IX injection. Lanes 7 through 9 represent three additional CD-1 hemophilic mice at week 2 postinjection. (C) The presence of antibodies that interfere with coagulation was assessed by the Bethesda assay and reported as Bethesda units. A time course in four representative animals is shown. (▧), 1; (▧), 2; (▩), 3; (▩), 4; (▩), 5; (▪), 6.
Total and neutralizing antibodies to human F.IX in hemophilic CD-1 mice. Plasma samples were evaluated by (A) ELISA, (B) Western blot, and (C) Bethesda assay at indicated time points. (A) Plates were coated with purified plasma–derived hF.IX, plasma samples from treated mice were applied, and rabbit anti-mouse IgG conjugated with horseradish peroxidase served as a detection antibody. Each result represents duplicate measurements from a single animal. (B) Lanes 1 through 6 represent an immunoblot time course on a single animal, weeks 0, 1, 2, 3, 5, and 7, respectively, after Ad-F.IX injection. Lanes 7 through 9 represent three additional CD-1 hemophilic mice at week 2 postinjection. (C) The presence of antibodies that interfere with coagulation was assessed by the Bethesda assay and reported as Bethesda units. A time course in four representative animals is shown. (▧), 1; (▧), 2; (▩), 3; (▩), 4; (▩), 5; (▪), 6.
Immunofluorescence staining for human F.IX on liver sections from treated and untreated hemophilic mice. (A) Untreated C57B1/6 hemophilic mouse. (B) Treated C57B1/6 hemophilic mouse 2 weeks after Ad-F.IX injection. (C) Treated CD-1 hemophilic mouse 2 weeks after Ad-F.IX injection. Original magnification ×200.
Immunofluorescence staining for human F.IX on liver sections from treated and untreated hemophilic mice. (A) Untreated C57B1/6 hemophilic mouse. (B) Treated C57B1/6 hemophilic mouse 2 weeks after Ad-F.IX injection. (C) Treated CD-1 hemophilic mouse 2 weeks after Ad-F.IX injection. Original magnification ×200.
To determine if hF.IX can correct mouse F.IX deficient plasma in vitro, F.IX-deficient plasma was collected from F.IX knockout mice after tail transection. Fifty microliters of murine F.IX-deficient plasma was reconstituted with 1 μL of plasma-derived F.IX (Mononine; Armour Pharmaceutical Co, Kankakee, IL) to reach final concentrations of 5, 0.5, 0.05, 0.005, and 0.0005 μg/mL, representing 100%, 10%, 1%, 0.1%, and 0.01% of the physiological range of hF.IX. Fifty microliters of reconstituted plasma was incubated with 50 μL of aPTT reagent for 3 minutes in the absence of hF.IX-deficient plasma and time to clot formation after addition of CaCl2was measured as described above.
To determine the functional activity of the hF.IX expressed in vivo, 50 μL of diluted plasma from treated hemophilia B mice, diluted to an antigen concentration of 200 to 400 ng/mL in imidazole buffer, was added to 50 μL of hF.IX-deficient plasma, and an aPTT performed as described above. The specific activity level was determined using a standard curve of purified plasma–derived F.IX added at serial dilutions to imidazole buffer containing the same concentration of untreated hemophilia B mouse plasma as treated plasma in the test sample.
Assays for circulating antibody against hF.IX.
Modified Bethesda assay for hF.IX inhibitors.
A modification of the originally described Bethesda assay7was performed to screen for the presence of antibodies that interfere with coagulation (inhibitors). Ten microliters of serially diluted sample plasma and 10 μL of pooled normal human plasma (Verify 1; Organon Tenika Corp) was added to 30 μL of dilution buffer and incubated at 37°C for 2 hours. This mix was then added to 50 μL of aPTT reagent and 50 μL of human F.IX-deficient plasma and incubated at 37°C for 3 minutes. CaCl2 was added and time to clot formation measured. Pooled normal human plasma incubated in dilution buffer without the test sample was used as a control. The dilution with residual activity closest to 50% was used to calculate the inhibitor titer, in which 50% residual F.IX activity equals 1 Bethesda unit (BU)/mL.
Immunofluorescence staining of liver.
Cryosectioned (6 μm) liver tissues were subjected to an indirect immunofluorescence staining protocol.6 The primary antibody was an affinity-purified goat anti-hF.IX antibody (Affinity Biologicals) and the secondary antibody was a rabbit anti-goat IgG (Dako, Carpinteria, CA) conjugated with fluorescein isothiocyanate (FITC).
Bleeding time assay.
Mice were anesthetized using metofane gas, and positioned horizontally on a platform that allowed the tail to drop about 2 cm from the top of the platform. The tail was pulled through a template with fixed sizes of openings until snug and then transected by cutting the face of the template using a no. 21 surgical blade. This technique results in wounds of identical cross-section of approximately 1.5-mm diameter. To evaluate bleeding from the incision, Whatman filter paper (Whatman International Ltd, Maidstone, UK) was applied to the edge of the forming clot every 30 seconds, taking care not to dislodge the clot. Blood that continued to flow from the cut during the 30-second interval was allowed to fall on the filter paper at the same point. In another set of experiments, blood was collected into a citrated Eppendorf tube for a duration of 5 minutes after the transection. The total volume of whole blood was then measured. For both protocols, hemostasis was achieved following the procedure by closure with Vicryl suture.
RESULTS
Breeding and handling of hemophilia B mice.
Carrier females (ES 129-derived) were bred with males of two different strains, C57B1/6 and CD-1. Breeding into these strains has now occurred for four generations of offspring; complete conversion of one strain to another requires approximately 20 generations. Of 143 mice from the F1-F4 generations, 29 mice (20.3%) were shown to have hemophilia B by Southern blot genotyping (Table 1). This is slightly lower than the expected number (25%), and suggests that there may be some fetal wastage for the affected mice. The number of carrier females was as predicted; these mice appear unaffected clinically. Both hemophilic males and hemophilic females (obtained by breeding carrier females with hemophilic males) are competent breeders, but the mortality rate from bleeding is high among the affected mice, especially in the neonatal period and in association with invasive procedures required for genotyping.
Human F.IX corrects murine F.IX-deficient plasma in an in vitro clotting assay.
Plasma was prepared from three mice with hemophilia B and used to generate pooled murine F.IX-deficient plasma. Purified plasma–derived human F.IX in varying concentrations was added back to F.IX-deficient plasma (either human or murine) and aPTTs determined (Table2). Partial correction of the aPTT of murine F.IX-deficient plasma was demonstrated at concentrations as low as 0.001 U/mL (0.1% of normal human plasma concentration), and the aPTT progressively shortened as a function of the amount of human F.IX added. Thus, human F.IX corrects murine F.IX-deficient plasma in an in vitro clotting assay. For comparison, an identical experiment was performed using human F.IX-deficient plasma as substrate; these results are also shown in Table 2 and are similar to those given for murine F.IX-deficient plasma. The clotting time of 25 seconds in murine plasma reconstituted to 1.0 U/mL of human F.IX is consistent with our observation that the aPTT of pooled normal murine plasma is shorter than that of pooled normal human plasma (data not shown).
In vivo correction of the bleeding diathesis in the hemophilia B mouse model by intravenous injection of an adenoviral vector expressing human F.IX.
Although coagulation tests are routinely used to assess hemostasis, the process of coagulation in vivo involves a number of interactions that are not modeled in in vitro testing, including interaction with specific cellular receptors,8-11 and clearance from the circulation.12 13 We sought to assess correction of coagulation in vivo after injection of an adenoviral vector expressing human F.IX (Ad-F.IX). Two strains of mice, CD-1 and C57B1/6, were injected at 8 to 9 weeks of age with 1011 particles of Ad-F.IX, a dose determined in previous studies in normal CD-1 mice to produce supratherapeutic levels of F.IX in the plasma. Blood was obtained at weekly intervals for determination of clotting times and human F.IX antigen levels in treated mice. In addition, bleeding times were determined in hemophilic, normal, and treated mice (1 week after treatment in the latter group).
Clotting times were determined in a modified aPTT assay referred to as an aPTT-F.IX1; this assay is used because it requires only very small amounts of plasma. The baseline clotting time of the hemophilic mice in this assay is 59.9 seconds ± 1.0 seconds and of normal mice is 33.5 seconds ± 3.1 seconds. After a single intravenous injection of Ad-F.IX, the aPTT-F.IX in hemophilic C57B1/6 mice falls into the normal range and remains at that level for the duration of the experiment (Fig 1A).* On the other hand, in hemophilic CD-1 mice the clotting time shortens transiently but then returns to baseline (Fig 1B). Similar results are seen with the hF.IX ELISA, ie, supraphysiologic levels persist (although they are gradually declining) in the C57B1/6 mice for the duration of the experiment (Fig 2A), whereas in the hemophilic CD-1 mice F.IX levels decrease to the undetectable range 2 weeks after injection (Fig 2B).
Tail bleeding times provide an additional measure of coagulation in vivo, since, unlike template bleeding times in humans, tail bleeding times in mice are sensitive to levels of coagulation factors.14-17 Indeed, the first indication that the hemophilia A knockout mice manifested a bleeding phenotype was their 100% mortality rate after tail transection for genotyping.18 We used two different methods to assess bleeding times in the mice. In the first, the tail was transected at a specified diameter (1.5 mm) and filter paper was used to assess blood oozing from the wound without disturbing the forming clot (Fig3A-C). Extensive blood loss in the hemophilic mice necessitated stopping the test after a period of ∼7 to 8 minutes, to prevent exsanguination, but the differences in the findings for normal, hemophilic, and treated mice are readily apparent from visual inspection of the filters. As a second, more quantitative measure of the bleeding time, total blood volume lost over 5 minutes was measured in 12 mice (Table 3). These data document that blood loss in the treated hemophilia mice is similar to that of normal mice, and is 10-fold less than in untreated hemophilia B mice.
The highest levels of F.IX expression, in the range of 50 to 90 μg/mL, are 10- to 20-fold higher than physiological levels. Whether the hepatocyte can synthesize fully active F.IX at these supraphysiologic levels is unknown. We assessed the specific activity of the transgene product in a clotting assay in which serial dilutions of treated hemophilic mouse plasma (containing human F.IX as the transgene product) were added to human F.IX-deficient plasma. Results are compared to a standard curve, and show that, at plasma concentrations in the 20 to 50 μg/mL range, only about half of the material is biologically active (data not shown). These findings are in agreement with our previous studies in hemostatically normal CD-1 mice.4 We have never observed levels of expression of biologically active material of greater than 25 μg/mL, and suspect that this represents an upper limit for biosynthesis of F.IX in the murine liver.
Duration of expression of human F.IX in hemophilic mice is strain-dependent and is limited in CD-1 mice by rapid formation of antibodies to human F.IX.
The structure of the murine F.IX gene deletion in the hemophilia B mice suggests that no protein will be produced.1 In humans with hemophilia B, mutations associated with an absence of any protein production are more likely to result in antibody production when patients are exposed to human F.IX.19,20 We sought to determine whether hemophilic mice from the two strains studied here develop antibodies to human F.IX, and whether strain-dependent differences in antibody formation could account for the difference in duration of expression of the transgene. In the hemophilic C57B1/6 mice, antibodies were undetectable for the duration of the experiment. These data are consistent with previous work in hemostatically normal C57B1/6 mice,21 22 which have not generally produced antibodies to human F.IX after intravenous vector injection (vide infra). The results were quite different in CD-1 hemophilic mice. Figure 4A shows the time course of appearance of antibodies to human F.IX as measured in an ELISA. Data are shown for six mice at weeks 1 and 2, and four mice at later time points; one mouse was killed for immunofluorescence studies and a second mouse died. Antibodies are initially detected 1 week after injection, are present in high-titer beginning 2 weeks after injection, and remain present at high levels for the duration of the experiment. Western blot analysis for the presence of murine antibodies to human F.IX (Fig 4B) confirms these findings and shows that all CD-1 mice tested have readily detectable antibody 2 weeks after injection of Ad-F.IX. Finally, a Bethesda assay on plasma from treated CD-1 mice (Fig 4C) demonstrates the presence of antibodies which inhibit coagulation function (ranging from 6 to 13.1 BU) as early as 3 weeks after injection, with a steady increase documented during the course of the experiment.
Site of transgene expression.
Previous work has shown that intravenous injection of adenoviral vectors into adult mice results in expression primarily in liver.4,21 23 Transgene expression in the liver was also documented in the hemophilia B mice (Fig 5, see page 787). In an immunofluorescence assay using antibodies that do not cross-react with murine F.IX (see Materials and Methods), we show the presence of human F.IX in liver sections from both C57B1/6 and CD-1 hemophilic mice 2 weeks after treatment with Ad-F.IX (Fig 5B and C). Note that hF.IX immunofluorescence staining is comparable for the two strains of mice, despite the fact that human F.IX is not detectable in the plasma of CD-1 mice at this time point. These data, coupled with those shown in Fig 4, suggest that the absence of detectable expression in the plasma of the hemophilic CD-1 mice results from formation of antibodies to human F.IX in this strain. Human F.IX was not detected in liver sections from untreated mice (Fig 5A) or from mice injected with AAV-lacZ (data not shown).
DISCUSSION
The hemophilia B mice are quite fragile in terms of bleeding, and in this respect seem to differ from published reports describing the hemophilia A knockout mice.18,24 As noted earlier, the risk of bleeding in the hemophilia B mice was increased in the neonatal period and in association with invasive procedures required for genotyping, but even routine handling such as occurs in changing cages sometimes led to lethal hemorrhage in these animals. Modifications of routine procedures that resulted in improved survival included deferring tail cutting for genotyping until 8 weeks of age; placing mature males into separate cages, since fighting can result in death from bleeding; avoiding excessive use of the heat lamp (before tail vein injection or blood withdrawal), because hyperactivity can also result in traumatic bleeds; and suturing the tail after tail-cutting, to reduce bleeding from the wound. The reasons for the difference in phenotype between the hemophilia A mice and the hemophilia B mice are unclear. In humans, the two entities are indistinguishable clinically, a fact that is at least partly due to the kinetics of the IXa-catalyzed activation of F.X, the reaction in which F.VIIIa serves as a cofactor. The reaction can be catalyzed by IXa, phospholipid and calcium alone, but the catalytic efficiency is accelerated by more than 4 orders of magnitude when F.VIIIa is present.25 In physiologic terms, then, the reaction does not proceed without the presence of the cofactor. If the murine system is less dependent on the presence of the cofactor, then it may be that small amounts of Xa can be generated in the hemophilia A mice, which lack the cofactor but not the enzyme, whereas the hemophilia B mice, completely lacking the enzyme, will have a virtual absence of function in the intrinsic pathway. Studies are currently underway to assess this possibility by determining levels of activated clotting factors in normal mice and in mice with hemophilia A and hemophilia B.
The major purpose of this study was to determine whether human F.IX can correct the bleeding diathesis of hemophilia B mice as measured by in vitro and in vivo clotting assays. The results indicate that human F.IX is fully functional in the murine clotting system. In in vitro clotting assays, purified human F.IX corrects the clotting time of murine F.IX-deficient plasma. In addition, hemophilic mice injected with an adenoviral vector expressing human F.IX exhibit normalization of the aPTT-F.IX. However, coagulation in the whole organism involves a number of interactions that are not tested in in vitro plasma-based clotting assays. Interactions of F.IX/F.IXa with endothelial cell receptors,10 with monocytes,26 and with putative platelet receptors11 all contribute to the in vivo process of coagulation, as do factors such as volume of distribution and mechanisms of clearance. One cannot necessarily assume that human F.IX or IXa will interact with appropriate murine cellular receptors to effect hemostasis in hemophilic mice. To assess this we carried out bleeding times in treated and untreated hemophilic mice. The treated mice received an intravenous injection of an adenoviral vector expressing human F.IX, which had been previously shown to direct high-level expression of human F.IX in hemostatically normal C57B1/6,21 (and unpublished observations, April 1996) and CD-1 mice.4 In the current study, both strains of mice demonstrated correction of plasma-based clotting times and of bleeding times, and both showed phenotypic correction of bleeding, although correction was only transient in the CD-1 mice, due to formation of antibodies to human F.IX.
A wealth of data have shown that hemostatically normal C57B1/6 mice do not generate antibodies to human F.IX21 (and unpublished data, April 1996) or canine F.IX27 when injected intravenously with vectors expressing these transgenes, but duration of expression is limited because of the immune response to adenoviral proteins. (Interestingly, the route of administration of the vector plays a role in whether antibody formation occurs, because C57B1/6 mice do develop antibodies to human F.IX after intramuscular injection of adenoviral or AAV vectors expressing human F.IX.6) In hemostatically normal CD-1 mice, we have previously shown a duration of expression of ∼13 to 16 weeks (during which levels gradually decline) following tail vein injection of Ad-F.IX, and we have documented the presence of antibodies to adenoviral proteins and, based on fall-off curves, the absence of neutralizing antibodies to human F.IX.4 The findings are somewhat different in hemophilic mice of these two strains. Like their hemostatically normal counterparts, the hemophilic C57B1/6 mice do not generate antibodies to human F.IX, but the hemophilic CD-1 mice rapidly develop antibodies to human F.IX, in marked contrast to the hemostatically normal CD-1 mice and to the hemophilic C57B1/6 mice. One may infer from these findings that the presence of murine F.IX, which shares 80% sequence conservation with human F.IX,28produces some level of tolerance, which is strain-dependent to the human F.IX protein. Interestingly, in the CD-1 hemophilic mice, antibodies to the protein were detected by ELISA as early as 1 week after injection, whereas antibodies interfering with function in coagulation were not detected until 2 weeks later.
In humans with hemophilia B, antibodies are more likely to arise in individuals with large gene deletions or nonsense mutations that result in an absence of protein production,19 but the nature of the mutation is clearly not the only determinant of whether antibodies occur, because there are many documented cases where an inhibitor occurs in only a few members of a large cohort all affected with the same mutation.29 The F.IX knockout mice described here have a deletion of genomic sequences containing exons 1-3 of the murine gene; they are thus comparable to humans with large gene deletions, and exhibit a similar phenomenon, ie, in mice with the identical mutation, some (CD-1) develop antibodies whereas others (C57B1/6) do not. A strain-related variability in response to adenoviral vectors has been documented previously, and shown not to correlate with H-2 type.30 We document in these two strains of hemophilic mice a strain-related variability in antibody response to the transgene product. Our findings suggest that C57B1/6 hemophilia B mice will be more useful for studies of gene transfer, whereas the CD-1 strain of hemophilic mice may be of greater utility for studying the development of inhibitors.
The aPTT-F.IX in treated hemophilic mice is shorter than values for normal mice. This is attributed to the higher concentrations of F.IX in the treated mice (samples measured at a 1:5 dilution), and to the presence of human F.IX versus murine F.IX in the assay.
Supported by National Institutes of Health Grants No. R01 HL53668 and P50 HL54500 to K.A.H. and F32 HL09397 to J.N.H.
Address reprint requests to Katherine A. High, MD, Division of Hematology, 310A Abramson Research Center, The Children's Hospital of Philadelphia, 34th St and Civic Center Blvd, Philadelphia, PA 19104.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.