Abstract
The BCR/ABL gene product of the Philadelphia (Ph) chromosome induces chronic myelogenous leukemia (CML). We generated a hematopoietic cell line, TonB210.1, with tetracycline-dependent BCR/ABL expression to investigate the pathways by which BCR/ABL transforms cells. TonB210.1 demonstrates conditional growth factor independence in tissue culture and rapidly forms tumors in mice fed the tetracycline analog doxycycline. The tumors regress completely upon doxycycline withdrawal, but ultimately reform in all animals. After a long latency, tumors also develop in animals never exposed to doxycycline. Subclones of TonB210.1 established from doxycycline-independent tumors demonstrate distinct mechanisms of transformation. Most subclones manifest increased basal levels of BCR/ABL expression; some have lost the capacity to augment expression upon induction, whereas others remain inducible. More interestingly, some subclones maintain tight conditional expression of BCR/ABL and are therefore transformed by secondary mechanisms that no longer require BCR/ABL expression. These subclones show constitutive phosphorylation of the STAT5 protein, suggesting that activating mutations have occurred upstream in the signaling pathway to STAT5. The tight conditional expression of BCR/ABL in the TonB210.1 cell line affords the opportunity to study several interesting aspects of the biology of BCR/ABL, including activation of critical signaling pathways and transcriptional programs, and its potential role in genomic instability.
CHRONIC MYELOGENOUS leukemia (CML) is a biphasic disease of the hematopoietic system.1 The initial chronic phase is characterized by excessive production of myeloid cells that retain a normal differentiation program. The chronic phase of CML is invariably followed by progression to an acute phase of disease termed “blast crisis,” which resembles acute leukemia.
The presence of the Philadelphia (Ph) chromosome during chronic-phase CML gave the first indication of the molecular basis of the disease.2 The Ph chromosome is formed as the result of a reciprocal translocation of chromosomes 9 and 22. The translocation t(9:22) results in a fusion of two genes, BCR (breakpoint cluster region) from chromosome 223 and ABL from chromosome 9,4 and production of a tyrosine-phosphorylated 210-kD protein, P210 BCR/ABL.5 The oncogenic potential of P210 BCR/ABL has been shown using a number of different systems, including transformation of factor-dependent hematopoietic cell lines6,7 and induction of proliferation in primary bone marrow cultures.37 Transgenic murine model systems have established the ability of P210 BCR/ABL to induce leukemia,9-11 and retroviral transduction of BCR/ABL into murine bone marrow has reproduced a CML-like myeloproliferative disorder,28,38,39 confirming the role of BCR/ABL in inducing chronic-phase disease. Studies of signal transduction pathways have begun to unravel the complex biochemical basis of cell transformation by BCR/ABL, indicating that BCR/ABL has multiple means of activating both RAS and non-RAS signaling pathways.8,12,13 Recent reports have indicated that P210 BCR/ABL and other oncogenic forms of ABL induce the tyrosine phosphorylation and DNA binding activity of signal transducers and activators of transcription (STAT) proteins in hematopoietic cell lines.14 15
The molecular mechanisms involved in the progression of chronic phase to blast crisis in CML are poorly understood. Cytogenetic changes in addition to the Ph chromosome are frequently detected in patients in blast crisis, presumably reflecting the accumulation of genetic mutation.16,17 Mutations leading to a loss of function of the p53 or p16 tumor suppressor genes have been implicated in a minority of cases of blast crisis.18-20 There is a strong theoretic basis and some experimental data to support the role of these mutations in disease progression.21,22 Transgenic animal models of BCR/ABL-induced leukemia demonstrate a high degree of karyotypic instability, and studies in cell culture suggest that BCR/ABL allows cells that have been exposed to genotoxic agents to survive by acting as an inhibitor of apoptotic cell death.23-25 These observations and the clinical certainty with which CML patients progress from chronic phase to blast crisis suggest that the BCR/ABL protein itself may play a role in the genomic instability of chronic-phase disease, but experimental data to support this hypothesis are still lacking.
The generation of cell lines containing conditional oncogene expression is valuable for critical analysis of genetic roles in cellular transformation. Previous hematopoietic cell lines with conditional BCR/ABL expression contained temperature-sensitive mutants of the kinase domain of BCR/ABL.26 27 We have found it difficult to use these temperature-sensitive alleles for studies of cell cycle regulation and signaling pathways, because of suboptimal growth of cells at the permissive temperature. We used an alternative approach to generate a cell line with tightly regulated expression of BCR/ABL using the tetracycline-dependent expression system pioneered by Bujard et al. In this report, we describe the characterization of this cell line, which we believe will be useful for studying various aspects of the biology of BCR/ABL. In the uninduced state, this cell line undergoes rapid apoptosis when deprived of cytokines, proving that the barely detectable level of BCR/ABL expression at baseline is well below a critical threshold for biologic activity. When passaging this cell line in animals, we demonstrated tight antibiotic-dependent development of tumors and their rapid regression following antibiotic withdrawal. However, after a long latency, all animals develop tumors even in the absence of antibiotic, and cell lines established from these tumors are growth factor–independent in vitro. The properties of the subclones suggest several distinct mechanisms by which the TonB210.1 cell line escapes conditional transformation by BCR/ABL. Compared with the parental clone TonB210.1, most subclones demonstrate increased basal levels of BCR/ABL expression, reflecting activation of the tetracycline promoter element in the absence of antibiotic. Some subclones with elevated basal expression have lost the capacity to augment expression in response to antibiotic, while others remain inducible. More interestingly, three subclones are indistinguishable from the parental clone in the pattern of BCR/ABL expression; they maintain virtually undetectable levels of BCR/ABL in the absence of antibiotic and appear to be transformed due to secondary mutation not involving the BCR/ABL locus. All growth factor–independent tumor cell lines were found to have constitutive tyrosine phosphorylation of the STAT5 protein, suggesting that activation of this pathway by mutation is a common mechanism of transformation for this hematopoietic cell line.
This cell line with tight conditional BCR/ABL expression affords the opportunity to study several interesting aspects of the biology of BCR/ABL, including activation of critical signaling pathways and transcriptional programs, and provides an improved conditional model to probe the role of BCR/ABL in cell cycle control and its potential to induce genomic instability.
MATERIALS AND METHODS
Plasmid constructs.
pTetP210 was constructed by digesting pGD21028 with EcoR1 and isolating a fragment containing the P210 BCR/ABL cDNA. This fragment was ligated into EcoR1-digested pTetsplice (a gift from Dr D. Schatz),29 containing a tet-responsive promoter 5′ of the EcoR1 cloning site, forming pTetP210. pUHD172-1neo was a gift from Dr H. Bujard.30
Isolation of BCR/ABL-inducible cell line.
BaF3 cells, a lymphoid cell line dependent on interleukin-3 (IL-3) for survival and proliferation,31 were maintained with 10% fetal bovine serum (FBS) in RPMI supplemented with IL-3 (supplied as 10% conditioned medium [CM] from Wehi3B cells). To generate a BaF3 cell line expressing the reverse tet-transactivator, pUHD172-1neo was linearized with Sca1 and electroporated into BaF3 cells (0.25 kV, 960 μF). Cells were grown for 2 days after electroporation in 10% FBS in RPMI supplemented with IL-3 (supplied as 10% Wehi3B CM) before addition of G418 (2 mg/mL; GIBCO/BRL, Grand Island, NY). G418-resistant cells were designated TonBaF.1. Sca1-linearized pTetP210 was electroporated into TonBaF.1 cells (0.25 kV, 960 μF). Electroporated cells were grown for 2 days in 10% FBS in RPMI supplemented with IL-3 (supplied as 10% Wehi3B CM), 0.5 mg/mL G418, and 1 μg/mL Doxycycline (Sigma, St Louis, MO; 1 mg/mL stock in H2O). Individual IL-3–independent clones were isolated by agar cloning in 10% FBS in RPMI and 1 μg/mL doxycycline. Factor-independent clones were washed extensively with phosphate-buffered saline (PBS) and replica plated to 10% FBS in RPMI in the absence of doxycycline. Most clones remained factor-independent in the absence of doxycycline. However, one clone that remained factor-independent in the absence of doxycycline was designated TonB210.1 and characterized further.
Protein analysis.
For Western blot analysis of ABL protein expression in TonB210.1, cells were grown with 10% FBS in RPMI supplemented with IL-3 (supplied as 10% Wehi3B CM) and varying concentrations of doxycycline for 3 days. Cells were lysed in ABL lysis buffer (1% Triton X-100, 10 mmol/L Tris, pH 7.6, 5 mmol/L EDTA, 50 mmol/L NaCl, 30 mmol/L NaPPi, and 5 mmol/L PMSF) at 108 cells/mL. Protein (50 μg) was run on a 6% acrylamide resolving gel, transferred to nitrocellulose, blocked in 10% milk in PBS and 0.05% Tween-20, and incubated with a rabbit polyclonal antibody to ABL6 o/n in 5% milk in PBS and 0.05% Tween-20. HRP-conjugated goat anti-rabbit IgG secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and ECL were used to detect both BCR/ABL and constitutive cABL protein. Densitometric analysis was performed using MacBas software (Fuji Photo Film Co, Tokyo, Japan). Western blot analysis of ABL protein expression in doxycycline-independent tumor cell lines was made by growing cells with 10% FBS in RPMI in the presence or absence of 2 μg/mL doxycycline. Cells were lysed in ABL lysis buffer and processed as already described.
Signal transduction studies in TonB210.1 cells were performed by growing TonB210.1 cells with 10% FBS in RPMI supplemented with IL-3 (supplied as 10% Wehi3B CM) and in the presence or absence of 2 μg/mL doxycycline for 3 days. Cells were washed extensively in PBS and resuspended in RPMI containing 0.1% bovine serum albumin (in the presence or absence of 2 μg/mL doxycycline). After serum starvation for 6 hours, cells were pulsed for 15 minutes with 10 ng/mL murine IL-3 (a gift from Amgen Inc, Thousand Oaks, CA) or mock-pulsed, followed by addition of ice-cold PBS and lysis in P-Tyr lysis buffer at 108 cells/mL. A quantity of 500 μg of each lysate was immunoprecipitated with 0.5 μg anti-Stat5b antibody (Santa Cruz Biotechnology) o/n at 4°C. Twenty microliters of 50% protein A Sepharose was added for 2 hours at 4°C before centrifugation. Protein A Sepharose–protein complexes were washed three times with P-Tyr lysis buffer, boiled in SDS loading buffer, run on a 6% acrylamide resolving gel, transferred to nitrocellulose, blocked in 3% BSA in Tris-buffered saline (TBS) and 0.05% Tween-20, and incubated with a mouse monoclonal antibody to phosphotyrosine (4G10; Upstate Biotechnology, Lake Placid, NY) o/n in 1.5% BSA in TBS and 0.05% Tween-20. HRP-conjugated goat anti-mouse IgG secondary antibody (Santa Cruz Biotechnology) and ECL were used to detect phosphotyrosine residues. Blots were stripped in 0.2 mmol/L glycine and 0.05% Tween-20 (pH 2.5), blocked in 10% milk in PBS and 0.05% Tween-20, and incubated with a rabbit polyclonal antibody to Stat5b (Santa Cruz Biotechnology) o/n in 5% milk in PBS and 0.05% Tween-20. HRP-conjugated goat anti-rabbit IgG secondary antibody (Santa Cruz Biotechnology) and ECL were used to detect Stat5 expression. Total lysates were also analyzed for BCR/ABL expression as already described.
Signal transduction studies in doxycycline-independent tumor cell lines were performed by growing cells with 10% FBS in RPMI in the absence of doxycycline and IL-3. Exponentially growing cells were washed in PBS and lysed in P-Tyr lysis buffer at 108 cells/mL. Analysis of Stat5 activation was made as described earlier. A quantity of 500 μg of each lysate was also immunoprecipitated with 4 μg anti-phosphotyrosine antibody (4G10; Upstate Biotechnology) o/n at 4°C. Twenty microliters of 50% protein A Sepharose was added for 2 hours at 4°C before centrifugation. Protein A Sepharose–protein complexes were washed three times with P-Tyr lysis buffer, boiled in SDS loading buffer, run on a 6% acrylamide resolving gel, transferred to nitrocellulose, blocked in 3% BSA in TBS and 0.05% Tween-20, and incubated with a mouse monoclonal antibody to phosphotyrosine (4G10; Upstate Biotechnology) o/n in 1.5% BSA in TBS and 0.05% Tween-20. HRP-conjugated goat anti-mouse IgG secondary antibody (Santa Cruz Biotechnology) and ECL were used to detect phosphotyrosine residues. Total lysates were also analyzed for BCR/ABL expression as already described.
MTT proliferation assay.
Before performing the MTT assay,32 TonB210.1, B210 (constitutively expressing BCR/ABL6), and parental BaF3 cells were grown with 10% FBS in RPMI supplemented with IL-3 (supplied as 10% Wehi3B CM) in the presence of 1 μg/mL doxycycline (Sigma). Cells were washed extensively in PBS to remove IL-3 and resuspended at 8 × 104 cells/mL in 10% FBS in RPMI. The cells (4 × 103) were aliquotted in triplicate to 50 μL growth media containing varying amounts of doxycycline and grown for 3 days at 37°C. A volume of 20 μL 5-mg/mL MTT dye was added (0.83 mg/mL final) and incubated at 37°C for 5 hours. Acidic isopropanol (100 μL, 0.1N HCI) was added and mixed, and the solution was analyzed at OD 570 nm immediately.
Cell viability assay.
TonB210.1 cells were grown with 10% FBS in RPMI in the presence of 1 μg/mL doxycycline. At time 0, cell viability was determined by trypan blue exclusion followed by washing the cells extensively with PBS. Cells were resuspended in 10% FBS in RPMI in the absence or presence of 1 μg/mL doxycycline, and cell viability was monitored every 24 hours for 3 days by trypan blue exclusion assay.
Tumorigenicity studies.
Nude mice (Balb/c) were obtained from Jackson Laboratories (Bar Harbor, ME). For doxycycline induction studies, 400 to 800 μg/mL doxycycline was added to water (containing 1% sucrose) approximately 1 week before cell injection, and the water bottles were light-protected. TonB210.1 cells (107 in 0.25 mL PBS plus 2 μg/mL doxycycline) were injected subcutaneously into each flank of the nude mice. Water containing doxycycline was changed every 3 days. For tumor regression studies, doxycycline was removed from the water and tumor growth was monitored. For detection of doxycycline-independent tumors, TonB210.1 and parental TonBaF.1 cells were grown with 10% FBS in RPMI supplemented with IL-3 (supplied as 10% Wehi3B CM) in the presence of 2 μg/mL doxycycline. TonB210.1 cells (107 in 0.25 mL PBS) or TonBaF.1 cells (107 in 0.25 mL PBS) were injected subcutaneously into the right and left flank, respectively, of the nude mice. Mice were given water without doxycycline or sucrose. Doxycycline-independent tumors were surgically isolated and disrupted to form single-cell suspensions that were cultured in 10% FBS in RPMI.
RESULTS
Generation of a BaF3 cell line with inducible BCR/ABL expression.
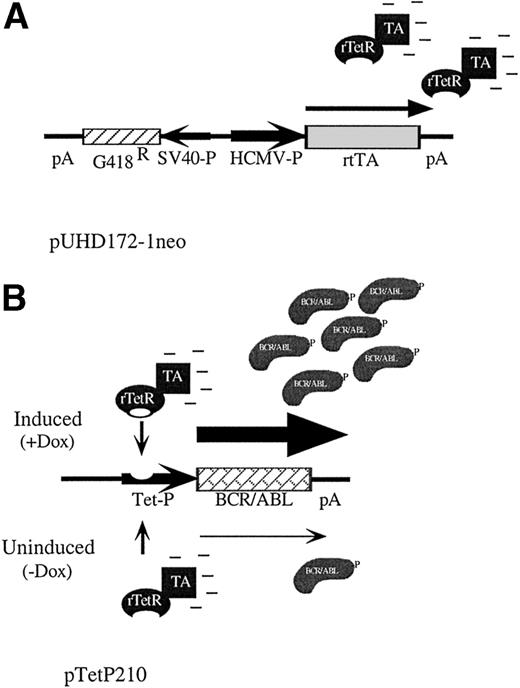
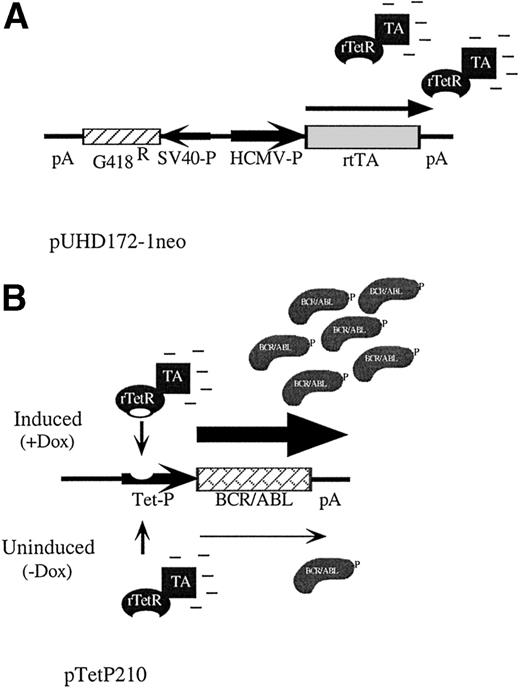
We used the system developed by Gossen et al30,33 to generate a hematopoietic cell line with conditional expression of BCR/ABL. This system uses the control elements of the tetracycline resistance operon encoded in Tn10 of Escherichia coli to regulate gene expression. Briefly, a tetracycline-controlled transactivator protein made by fusing a mutated tet repressor with the activation domain of herpes simplex virus VP16 stimulates transcription from a tet-responsive promoter only in the presence of tetracycline or analogs like doxycycline.30 We initially created a hematopoietic cell line, TonBaF.1, containing the reverse-tet transactivator gene (Fig 1A), and then introduced the P210 BCR/ABL cDNA driven by a tet-responsive promoter (Fig 1B). Taking advantage of the fact that expression of P210 BCR/ABL in BaF3 cells transforms them to factor independence,6single-cell clones were selected in agar in the absence of IL-3 and in the presence of the inducer doxycycline. To identify a clone with the lowest basal levels of BCR/ABL expression and the tightest conditional regulation, cells were replica-plated into medium with or without doxycycline. A cell line that died upon removal of doxycycline was isolated and designated TonB210.1.
Schematic of reverse-tet transactivator construct pUHD172-1neo (A) and tet-inducible BCR/ABL construct pTetP210 (B).
Schematic of reverse-tet transactivator construct pUHD172-1neo (A) and tet-inducible BCR/ABL construct pTetP210 (B).
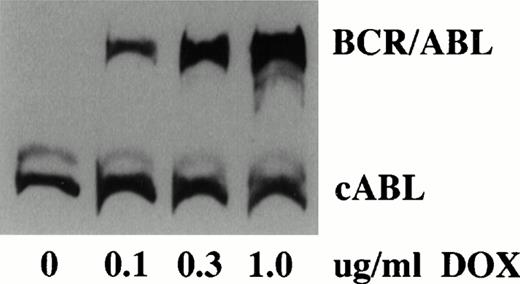
Analysis of TonB210.1 cells grown in varying doxycycline concentrations indicated a titratable dose-dependent increase in expression of the P210 BCR/ABL protein. Western blot analysis of TonB210.1 cells demonstrated virtually undetectable levels of BCR/ABL in the absence of doxycycline (although minute amounts could be detected after long exposure times) and high-level BCR/ABL protein expression at a saturating doxycycline dose of 1 μg/mL (Fig2). No effect of doxycycline on c-ABL expression was found. Densitometric quantitation of Western blot data showed at least a 300-fold increase in BCR/ABL expression upon induction with 1 μg/mL doxycycline.
Analysis of conditional BCR/ABL expression. Western blot analysis of BCR/ABL expression in TonB210.1 cells grown in varying amounts of doxycycline (DOX). Constitutive cABL expression is shown.
Analysis of conditional BCR/ABL expression. Western blot analysis of BCR/ABL expression in TonB210.1 cells grown in varying amounts of doxycycline (DOX). Constitutive cABL expression is shown.
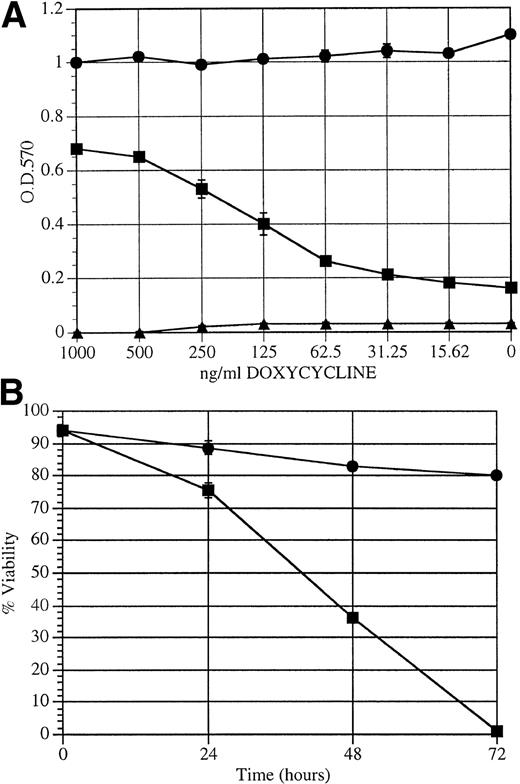
We used a colorimetric cell proliferation assay to determine the effect of doxycycline on the ability of BCR/ABL to transform TonB210.1 cells to factor independence.6,32 In the absence of IL-3, a dose-dependent response to doxycycline was found for proliferation of TonB210.1 cells, with a linear range of effect from 50 to 500 ng/mL doxycycline (Fig 3A). Taking into account the results in Figs 2 and 3A, it appears that BCR/ABL must be expressed at a level comparable to c-ABL for maximal proliferative capacity in this cell line. This threshold is achieved when cells are grown in at least 0.3 μg/mL doxycycline. Below 0.1 μg/mL doxycycline, which corresponds to approximately one third of endogenous c-ABL expression, viability is poorly maintained in the absence of IL-3. In contrast, cells constitutively expressing BCR/ABL (B2106) showed no influence of doxycycline on IL-3–independent growth. BaF3 cells were unaffected by doxycycline, undergoing apoptosis during the 3-day assay. As measured by trypan blue exclusion, TonB210.1 cell viability decreased following doxycycline deprivation, with complete apoptotic cell death by 72 hours (Fig 3B). The slight decrease in viability of TonB210.1 cells in the presence of doxycycline was due to overproliferation of cells in this experiment, leading to a small amount of cell death. To determine if doxycycline-independent subclones of the TonB210.1 cell line develop during passage in culture, 107 TonB210.1 cells were deprived of doxycycline and monitored for the formation of doxycycline and factor-independent cells in vitro. No viable subclones emerged during 7 weeks of in vitro culture, and to date, no such spontaneous factor-independent variants of TonB210.1 have developed while we have maintained these cells in culture.
Effect of doxycycline-induced BCR/ABL expression on factor-independent growth. (A) MTT proliferation assay on (▪) TonB210.1, (•) B210 (constitutively expressing BCR/ABL), or (▴) parental BaF3 cells grown for 3 days with varying concentrations of doxycycline in growth media without IL-3. OD 570 nm is directly related to proliferation. (B) Cell viability of TonB210.1 cells over time upon doxycycline withdrawal. Cell viability was assayed by trypan blue exclusion over a 3-day period following removal (−dox; ▪) or continuation (+dox; •) of doxycycline in the growth media.
Effect of doxycycline-induced BCR/ABL expression on factor-independent growth. (A) MTT proliferation assay on (▪) TonB210.1, (•) B210 (constitutively expressing BCR/ABL), or (▴) parental BaF3 cells grown for 3 days with varying concentrations of doxycycline in growth media without IL-3. OD 570 nm is directly related to proliferation. (B) Cell viability of TonB210.1 cells over time upon doxycycline withdrawal. Cell viability was assayed by trypan blue exclusion over a 3-day period following removal (−dox; ▪) or continuation (+dox; •) of doxycycline in the growth media.
Conditional tumor formation in animals.
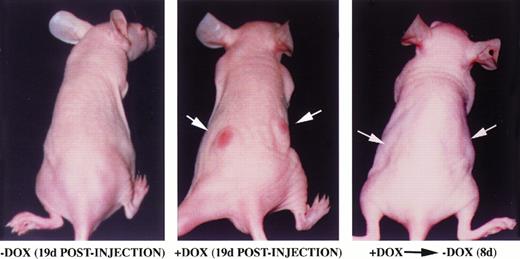
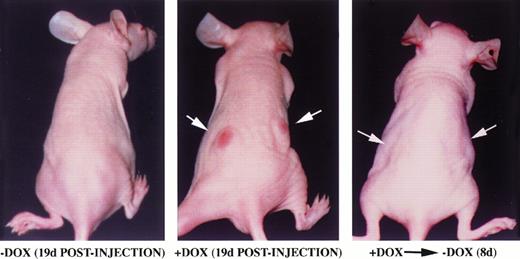
Factor-independent hematopoietic cells expressing BCR/ABL are able to induce tumor formation in mice.6 We analyzed the ability of TonB210.1 cells to form tumors in a doxycycline-dependent manner when injected into mice (Fig 4). TonB210.1 cells (107) grown in the presence of doxycycline were injected subcutaneously into the flanks of nude mice. Mice were given drinking water with or without doxycycline. Tumor formation was evident within 3 weeks in mice given doxycycline (n = 11), while during the same time, no tumors formed in control mice provided plain drinking water (n = 4; Fig 4). When doxycycline was removed from the drinking water of mice with preestablished tumors, the tumors were found to regress within 1 week in all mice (n = 4; Fig 4).
Inducible tumor formation of TonB210.1 cells in nude mice. 107 TonB210.1 cells were injected subcutaneously into the flanks of nude mice given water without (left) or with (middle) 400 μg/mL doxycycline. Tumor regression was observed following removal of doxycycline from the drinking water (right). Sites of TonB210.1 injections resulting in tumors are indicated by arrows.
Inducible tumor formation of TonB210.1 cells in nude mice. 107 TonB210.1 cells were injected subcutaneously into the flanks of nude mice given water without (left) or with (middle) 400 μg/mL doxycycline. Tumor regression was observed following removal of doxycycline from the drinking water (right). Sites of TonB210.1 injections resulting in tumors are indicated by arrows.
Doxycycline-independent tumors arising after a long latency.
Interestingly, all animals in which TonB210.1 tumors regressed ultimately reformed tumors after a long latency period (∼60 days). Animals injected with TonB210.1 cells but never exposed to doxycycline likewise developed tumors in all cases after a similar prolonged latency.
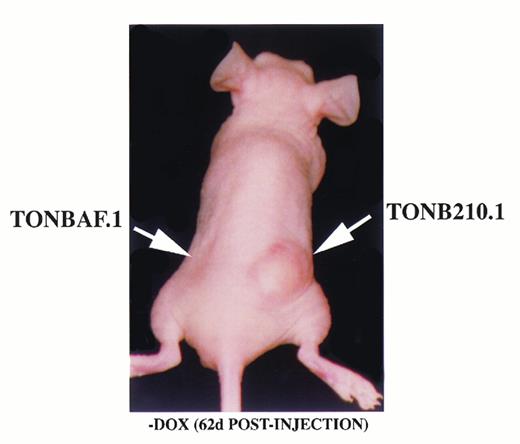
To compare the intrinsic tumorigenic potential of the parental TonBaF.1 cells (lacking the BCR/ABL construct) and TonB210.1, 10 nude mice received subcutaneous injections of 107 TonBaF.1 cells into the left flank and 107 TonB210.1 cells into the right flank. The mice were provided plain drinking water and observed for tumor formation. After approximately 8 weeks, demonstrable tumors had developed at the injection site of TonB210.1 cells in all 10 mice (Fig5), while only a single mouse had a detectable tumor at the site of injection of the parental cells (not shown). This suggested that the parental TonBaF.1 cell line exhibited a minor predisposition to spontaneous tumor formation, while TonB210.1 was tumorigenic in all cases.
Doxycycline-independent tumor formation. TonB210.1 cells (107) and parental TonBaF.1 cells (107) were injected subcutaneously into nude mice given drinking water without doxycycline. Arrows show sites of cell injection.
Doxycycline-independent tumor formation. TonB210.1 cells (107) and parental TonBaF.1 cells (107) were injected subcutaneously into nude mice given drinking water without doxycycline. Arrows show sites of cell injection.
Doxycycline-independent tumors constitutively activate STAT5.
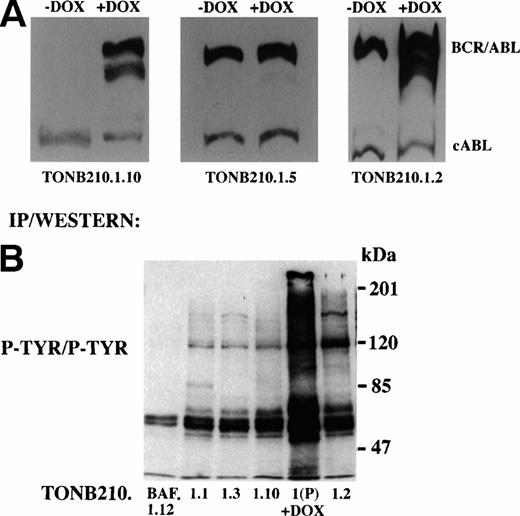
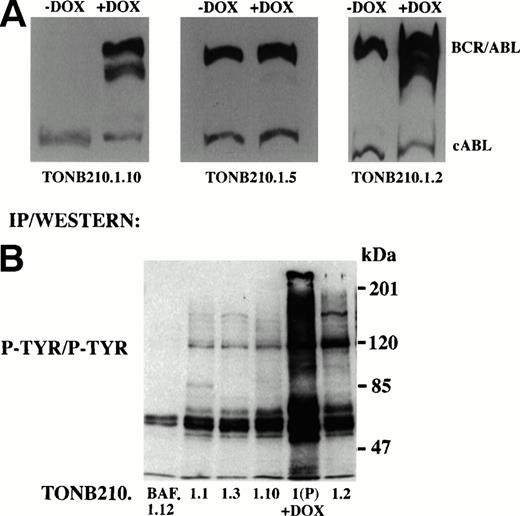
Thirteen cell lines were generated from doxycycline-independent tumors and analyzed for expression of BCR/ABL in the absence and presence of doxycycline. Three specific classes of cell lines were produced. Eight of 13 cell lines, as indicated by TonB210.1.2 (Fig 6A), had elevated baseline levels of BCR/ABL expression in the absence of antibiotic exposure but retained induction of BCR/ABL expression upon addition of doxycycline. Two of 13 cell lines, as indicated by TonB210.1.5 (Fig6A), also had high basal levels of BCR/ABL expression but had lost the ability to augment BCR/ABL expression in response to doxycycline. The remaining cell lines (n = 3), as indicated by TonB210.1.10 (Fig 6A), were the most interesting. In these cells, the regulation of BCR/ABL expression was indistinguishable from the parental TonB210.1 cell line. In the absence of doxycycline, BCR/ABL expression remained virtually undetectable and clearly below the threshold required for factor-independent growth and tumorigenicity. Moreover, the cell lines retained doxycycline-dependent induction of BCR/ABL expression. None of the doxycycline-independent cell lines produced an autocrine growth factor (data not shown). Because BCR/ABL expression is not responsible for the factor-independent proliferation of these subclones, they appear to be transformed by independent genetic events.
Molecular analysis of doxycycline-independent tumors. (A) Western blot analysis to detect expression of BCR/ABL in representative doxycycline-independent tumor cell lines grown in the absence or presence of doxycycline. Constitutive cABL expression is also shown. (B) Immunoprecipitation/Western blot analysis of downstream signaling from doxycycline-independent tumor cells. Protein lysates from doxycycline-independent tumor cell lines grown in the absence of IL-3 and doxycycline were immunoprecipitated with an anti-phosphotyrosine antibody followed by Western blot analysis with the same antibody (P-TYR/P-TYR). Parental TonB210.1 cells grown in the presence of doxycycline were also analyzed (1(P) +DOX). Size markers are indicated at right.
Molecular analysis of doxycycline-independent tumors. (A) Western blot analysis to detect expression of BCR/ABL in representative doxycycline-independent tumor cell lines grown in the absence or presence of doxycycline. Constitutive cABL expression is also shown. (B) Immunoprecipitation/Western blot analysis of downstream signaling from doxycycline-independent tumor cells. Protein lysates from doxycycline-independent tumor cell lines grown in the absence of IL-3 and doxycycline were immunoprecipitated with an anti-phosphotyrosine antibody followed by Western blot analysis with the same antibody (P-TYR/P-TYR). Parental TonB210.1 cells grown in the presence of doxycycline were also analyzed (1(P) +DOX). Size markers are indicated at right.
As a first attempt to identify the signal transduction pathways that were activated in these doxycycline-independent transformed cells, protein lysates were analyzed by immunoblotting with an antibody to phosphotyrosine to detect activation of a distinct tyrosine kinase (Fig6B). With the exception of an 85-kD phosphoprotein detected in TonB210.1.1, no obvious novel phosphotyrosinated proteins were identified using this approach. This 85-kD phosphoprotein is currently under investigation.
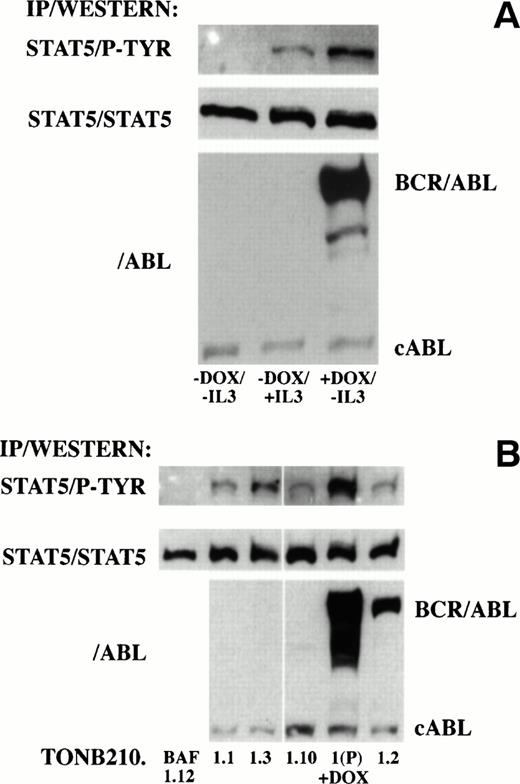
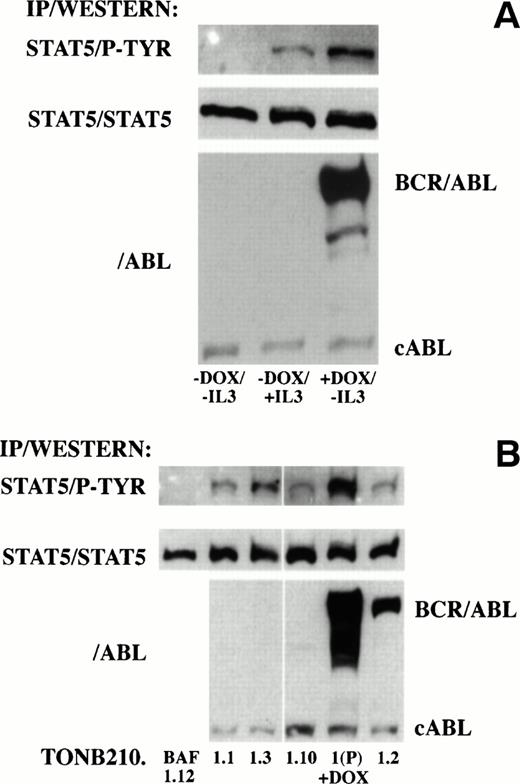
Recent reports have indicated that P210 BCR/ABL and other oncogenic forms of ABL induce the tyrosine phosphorylation and DNA binding activity of STAT proteins in hematopoietic cell lines.14 15To determine if downstream signaling of BCR/ABL through STAT proteins was also doxycycline-dependent in TonB210.1, STAT protein phosphorylation was analyzed in the absence and presence of doxycycline (Fig 7A). Protein lysates were immunoprecipitated with an antibody recognizing STAT5 and blotted with an antibody recognizing phosphotyrosine residues. No phosphotyrosine was detected on STAT5 in the absence of doxycycline or IL-3 stimulation. In contrast, STAT5 phosphorylation was detected in cultures grown in the presence of doxycycline or after IL-3 stimulation. Western blot analysis of protein lysates indicated that STAT5 phosphorylation in the absence of IL-3 correlated with induction of BCR/ABL expression. No phosphorylation of tyrosine residues was detected on STAT1 or STAT3 proteins under these conditions (data not shown).
Analysis of downstream signaling of BCR/ABL through STAT5 in doxycycline-dependent (A) and -independent (B) cells. (A) Protein lysates from serum-starved doxycycline-dependent TonB210.1 cells in the absence of doxycycline and without IL-3 stimulation (−DOX/−IL3), absence of doxycycline pulsed with IL-3 (−DOX/+IL3), or presence of doxycycline in the absence of IL-3 stimulation (+DOX/−IL3) were immunoprecipitated with an anti-STAT5 antibody followed by Western blot analysis with an anti-phosphotyrosine antibody (STAT5/P-TYR). Blots were stripped and reprobed with an anti-STAT5 antibody (STAT5/STAT5). Total lysates were also Western blotted with an anti-ABL antibody (/ABL) for detection of BCR/ABL and constitutive cABL expression. (B) Protein lysates from doxycycline-independent tumor cell lines grown in the absence of IL-3 and doxycycline were immunoprecipitated with an anti-STAT5 antibody followed by Western blot analysis with an anti-phosphotyrosine antibody (STAT5/P-TYR). Parental TonB210.1 cells grown in the presence of doxycycline were also analyzed (1(P) +DOX). Blots were stripped and reprobed with an anti-STAT5 antibody (STAT5/STAT5). Total lysates were also Western blotted with an anti-ABL antibody (/ABL) for detection of BCR/ABL and constitutive cABL expression.
Analysis of downstream signaling of BCR/ABL through STAT5 in doxycycline-dependent (A) and -independent (B) cells. (A) Protein lysates from serum-starved doxycycline-dependent TonB210.1 cells in the absence of doxycycline and without IL-3 stimulation (−DOX/−IL3), absence of doxycycline pulsed with IL-3 (−DOX/+IL3), or presence of doxycycline in the absence of IL-3 stimulation (+DOX/−IL3) were immunoprecipitated with an anti-STAT5 antibody followed by Western blot analysis with an anti-phosphotyrosine antibody (STAT5/P-TYR). Blots were stripped and reprobed with an anti-STAT5 antibody (STAT5/STAT5). Total lysates were also Western blotted with an anti-ABL antibody (/ABL) for detection of BCR/ABL and constitutive cABL expression. (B) Protein lysates from doxycycline-independent tumor cell lines grown in the absence of IL-3 and doxycycline were immunoprecipitated with an anti-STAT5 antibody followed by Western blot analysis with an anti-phosphotyrosine antibody (STAT5/P-TYR). Parental TonB210.1 cells grown in the presence of doxycycline were also analyzed (1(P) +DOX). Blots were stripped and reprobed with an anti-STAT5 antibody (STAT5/STAT5). Total lysates were also Western blotted with an anti-ABL antibody (/ABL) for detection of BCR/ABL and constitutive cABL expression.
Downstream signaling through STAT5 was analyzed in cell lines isolated from doxycycline-independent tumors for possible alterations leading to doxycycline-independent growth. Protein lysates from exponentially growing doxycycline-independent cell lines grown in serum alone were immunoprecipitated with an antibody recognizing STAT5 and immunoblotted with an antibody recognizing phosphotyrosine residues (Fig 7B). In contrast to TonB210.1, where STAT5 phosphotyrosine was not detected in the absence of doxycycline or IL-3 (Fig 7A), all doxycycline-independent cell lines derived from TonB210.1 injection showed constitutive tyrosine phosphorylation of STAT5 even when BCR/ABL expression was undetectable (TonB210.1.1, 1.3, and 1.10; Fig 7B). The level of phosphotyrosine on STAT5 in these cells was lower than in doxycycline-induced TonB210.1 cells, which correlated with slower rates of proliferation of these doxycycline-independent cell lines (data not shown). The doxycycline-independent cell line derived from the single tumor generated from parental TonBaF.1 cells (TonBaF.1.12) had no detectable STAT5 tyrosine phosphorylation.
DISCUSSION
We have created a novel cell line, TonB210.1, with tight conditional regulation of BCR/ABL expression to address the mechanisms of BCR/ABL transformation of hematopoietic cells. Previous conditional alleles of BCR/ABL with temperature-sensitive kinase activity have proved difficult to use because of slow growth at permissive temperatures. The cell line described here, TonB210.1, shows an absolute dependence on the inducer doxycycline for cell survival and proliferation in vitro in the absence of IL-3, correlating with induction of BCR/ABL expression. Furthermore, the induction of BCR/ABL by doxycycline leads to STAT5 tyrosine phosphorylation, recently shown to be correlated with BCR/ABL transformation of hematopoietic cell lines to factor-independent growth.14 15 This cell line will be useful for analysis of signal transduction pathways involved in the transformation of hematopoietic cells by BCR/ABL.
As predicted, nude mice injected with TonB210.1 cells rapidly develop tumors that are doxycycline-dependent and reversible, confirming that BCR/ABL expression can be conditionally regulated in vivo to influence tumor formation. Following doxycycline withdrawal and tumor regression, tumors reappeared in all animals after a long latency. Furthermore, tumors developed after a long latency in animals injected with TonB210.1 cells but never exposed to doxycycline. We have characterized these spontaneously transformed subclones of TonB210.1 and demonstrate in most cases an elevated basal expression of BCR/ABL, clearly above the threshold for full proliferative activity. Some subclones fail to express higher levels of BCR/ABL when exposed to doxycycline (eg, TonB210.1.5), while others remain inducible (eg, TonB210.1.2; Fig 6A). Since the TonB210.1 cell line was derived from a single cell by cloning in soft agar, the subclones with elevated basal expression represent novel variants with stable, reproducible, and distinct BCR/ABL expression patterns that emerged following passage in animals and reestablishment in cell culture. These cell lines do not reflect emergence of subclones from a population of cells with “leakiness” of conditional promoter function. We cannot rigorously exclude the possibility that epigenetic, as opposed to mutational, mechanisms account for the emergence of these variants. However, given the predisposition for integration of concatamers upon electroporation of constructs into cells, rearrangements of the transduced pTetP210 or pUHD172-neo sequences might provide an explanation for the frequent escape from conditional regulation that we observed.
Of the doxycycline-independent tumors that arose from passage of TonB210.1 cells in mice, the most interesting subclones maintain tight doxycycline-dependent expression of BCR/ABL (eg, TonB210.1.10; Fig 6A). These subclones behave similarly to the parental TonB210.1 cells with respect to BCR/ABL expression, which remains virtually undetectable in the absence of doxycycline. However, without induction, the cells are fully transformed, factor-independent in vitro, and tumorigenic in vivo. The argument that independent secondary mutations maintain the transformed state in the absence of BCR/ABL expression is most compelling for these subclones. All subclones of this class exhibited STAT5 phosphorylation in the absence of BCR/ABL expression, suggesting that a mutational event that activates this signal transduction pathway (and perhaps others) is responsible for cell proliferation.
Our observations of late-emerging doxycycline-independent tumors from TonB210.1 cells are reminiscent of a recent report demonstrating time-sensitive reversal of ductal hyperplasia in transgenic mice with tetracycline-regulated expression of the SV40 large T antigen.34 When expression of the SV40 T antigen was switched off after 4 months, ductal hyperplasia of the submandibular gland was reversible. However, following 7 months of SV40 T antigen expression, a transformed phenotype persisted even after expression of SV40 T antigen was completely suppressed. These investigators concluded that secondary genetic changes maintained the transformed phenotype in these mice, supporting a model of time-dependent multistep tumorigenesis.
A previous study of the biologic effects of a temperature-sensitive allele of BCR/ABL by Kabarowski et al26 concluded that the primary consequence of BCR/ABL expression in BaF3 cells was an enhanced cell survival in the absence of IL-3, and that emergence of factor-independent cell lines required prolonged exposure to BCR/ABL tyrosine kinase activity by propagation of cells at the permissive temperature. They argued that cooperating genetic events were required for the complete abrogation of growth factor dependence by BCR/ABL. Our observation of late-emerging tumors in mice injected with TonB210.1 but never exposed to doxycycline begs the question of whether BCR/ABL expression is required for doxycycline-independent tumors to arise. However, consistent with the prior study, isolation of the TonB210.1 cell line entailed selection of cells in the induced state, during which time secondary mutations might have accumulated. Although no spontaneous factor-independent clones emerged following doxycycline withdrawal of TonB210.1 cells in vitro, injection of the cells into the mouse likely provides a richer source of proliferation and survival factors than can be supplied by serum in cell culture conditions, and therefore a more permissive environment for the outgrowth of clonal variants in vivo.
Griffiths et al35 and Voncken et al36speculated that BCR/ABL kinase expression might accelerate mutagenic events in cells through induction of genetic instability, a notion that has gained significant experimental support through studies of karyotypic instability in strains of mice carrying BCR/ABL transgenes35,36 and demonstrable effects of BCR/ABL on cell cycle checkpoints in cultured cells.25 Our study did not directly determine whether BCR/ABL expression contributed to genetic instability in TonB210.1, but the results suggest the hypothesis that the emergence of doxycycline-independent tumorigenic subclones is a consequence of BCR/ABL expression in BaF3 cells. TonB210.1 cells appear to have an increased rate of conversion to factor independence when passed through mice (generating doxycycline-independent tumors in all cases from 108 cells), but the precise role of BCR/ABL is unclear because the parental factor-dependent BaF3 cells show some spontaneous conversion to factor independence and tumorigenicity when propagated in mice (1 tumor per 108 cells). In future studies, the TonB210.1 cell line will be valuable for directly measuring the mutational frequency in a defined clone of cells in which BCR/ABL expression can be turned on and off. TonB210.1 cells should be helpful in defining the precise role of BCR/ABL in the genomic instability hypothesized to be responsible for the progression of chronic-phase disease to blast crisis. The secondary mutations that arise in subclones of TonB210.1 to produce doxycycline-independent tumors have been selected for the ability to transform BaF3 cells. We are attempting to identify these secondary mutations using an expression cloning strategy to determine whether analogous mutations play a role in the progression of human CML to acute blast crisis.
ACKNOWLEDGMENT
We gratefully acknowledge Professor H. Bujard and D. Schatz for providing plasmids for the tet-expression system, and Eugene Y. Koh for helpful comments on the manuscript.
Supported by a grant (IRG-173F) from the American Cancer Society (G.Q.D.) and in part by a Career Award from the Burroughs Wellcome Fund (G.Q.D.).
Address reprint requests to George Q. Daley, MD, PhD, Whitehead Institute, 9 Cambridge Center, Cambridge, MA 02142.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.