Abstract
Tumor angiogenesis, the development of new blood vessels, is a highly regulated process that is controlled genetically by alterations in oncogene and tumor suppressor gene expression and physiologically by the tumor microenvironment. Previous studies indicate that the angiogenic switch in Ras-transformed cells may be physiologically promoted by the tumor microenvironment through the induction of the angiogenic mitogen, vascular endothelial growth factor (VEGF). In this report, we show Ras-transformed cells do not use the downstream effectors c-Raf-1 or mitogen activated protein kinases (MAPK) in signaling VEGF induction by hypoxia as overexpression of kinase-defective alleles of these genes does not inhibit VEGF induction under low oxygen conditions. In contrast to the c-Raf-1/MAP kinase pathway, hypoxia increases phosphatidylinositol 3-kinase (PI 3-kinase) activity in a Ras-dependent manner, and inhibition of PI 3-kinase activity genetically and pharmacologically results in inhibition of VEGF induction. We propose that hypoxia modulates VEGF induction in Ras-transformed cells through the activation of a stress inducible PI 3-kinase/Akt pathway and the hypoxia inducible factor-1 (HIF-1) transcriptional response element.
TUMOR DORMANCY can be defined as a state of angiogenic independent growth in which solid tumors often do not exceed 2 mm in diameter.1-6 The molecular mechanisms that disrupt the balance between positive and negative regulators of angiogenesis to shift cells into an angiogenic dependent state are beginning to be defined. Among the group of pro-angiogenic factors, vascular endothelial growth factor (VEGF), an endothelial cell specific mitogen, has been shown to be upregulated in cell lines expressing constitutively activated oncogenic forms of Ras,6-10 suggesting a link between accumulation of oncogenic alterations and the switch to angiogenic-dependent growth. In addition to its Ras responsiveness, VEGF expression is also markedly elevated under a low oxygen environment in cell culture and in regions of tumors located near necrotic areas.11,12 Induction of VEGF by hypoxia has been reported to involve a c-Src/Raf/MAP kinase pathway in some cell lines,13 and severe hypoxia has been shown to stimulate Src activity. However, studies by Gleadle and Ratcliffe in which Src expression was manipulated genetically and physiologically suggest that Src is not critical for the induction of VEGF, erythropoietin, or glucose transporter-114 by hypoxia. These results somewhat obscure the role of Src kinase in the regulation of VEGF even though Src kinase is rapidly and potently induced under hypoxic conditions.15
We have focused our studies on how oncogenic Ha-ras alone or in combination with low oxygen conditions in the tumor microenvironment stimulates VEGF activity. Because VEGF activity can be regulated both by transcriptional induction as well as by mRNA stabilization,16,17 we have used VEGF promoter-reporter gene constructs to distinguish between these two endpoints. Clearly, in some cells, both hypoxia and hypoglycemia increase the rate of VEGF mRNA transcription as well as decrease the rate of VEGF mRNA degradation. Although the trans- and cis-acting factors that control VEGF stabilization are still under investigation, deletional analysis of the human VEGF promoter has identified a 8-bp (5′-TACGTGgg-3′) region that differs by two nucleotides from the binding site for the hypoxia inducible transcription factor-1 (HIF-1, 5′-TACGTGCT-3′) originally identified in the erythropoietin (EPO) gene.18 This heterodimeric protein composed of two subunits, HIF-1α and HIF-1β (known as aryl hydrocarbon receptor nuclear translocator, ARNT),19 has also been found to regulate the transcription of some glycolytic enzyme genes such as aldolase-A and phosphoglycerate kinase 1.20 Promoter constructs containing HIF-1 are not only highly responsive to hypoxia, but they also respond to an oncogenic form of Ras (Ha-ras). Recently, Arbiser et al6 have elegantly shown that the introduction of Ha-ras into endothelial cells is capable of increasing the growth rate and angiogenic profile of these cells into a more aggressive form. If these transformed cells were treated with wortmannin, a specific inhibitor of phosphoinositol 3-kinase (PI 3-kinase), they exhibited both decreased expression of VEGF activity in cell culture and in transplanted tumors. PI 3-kinase, a lipid kinase consisting of an 85-kD regulatory subunit (p85) and an 110-kD catalytic subunit (p110), is a key component of growth factor signal transduction cascades.21-24 Although the exact signaling pathways downstream of PI 3-kinase are still under investigation, recent studies have shown that the proto-oncogene Akt is activated by lipid products of PI 3-kinase.25
We investigated whether a PI 3-kinase/Akt pathway signaled VEGF induction through the HIF-1 transcription factor under hypoxic conditions in cells expressing oncogenic Ha-ras (NIH3T3R). Because at least two potential pathways involving Ras have been implicated in the response of VEGF in cells to low oxygen conditions, one involving Src/Raf-1/MAPK and a second involving Ras and PI 3-kinase, we examined what contribution each potential pathway had on transcriptional activation of VEGF.
MATERIALS AND METHODS
Cell culture and hypoxia treatment.NIH3T3 cells were maintained in culture in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% (vol/vol) fetal calf serum. NIH3T3R and Rat-1 R (Ha-ras transformed)-, RasN17-, Raf301-cells were maintained in culture with DMEM media that was additionally supplemented with 400 μg/mL G418. NIH3T3R cells that were stably transfected with a mutant inhibitory allele of Ras [Ras(N17)] or Raf-1 [Raf301] were tested for functionality by assaying for inhibition of NF-κB activation by hypoxia and Ha-ras–induced anchorage independent cell growth in soft agar.26 Those stably transfected cell lines which showed the strongest suppression of both phenotypic responses by Ras and Raf-1 mutant inhibitory alleles were used in transient transfection experiments to determine their effect on VEGF. Cells were cultured in a well-humidified 95% air, 5% CO2 incubator at 37°C and were subconfluent at the beginning and end of hypoxia incubation. For hypoxic stimulation, the culture plates were placed into specially designed aluminum hypoxia chambers that were prewarmed at 37°C overnight. These chambers were flushed with a gas mixture containing 5% CO2 , and 95% N2 to achieve the desired oxygen level, then sealed and incubated for 5 hours at 37°C followed by 2 hours of reoxygenation.26
Plasmid constructs and transfections.A Sac I-Sac I fragment (1,511 bp) from the human VEGF gene, including 1,175 bp of the promoter from the start site of transcription and 336 bp of untranslated mRNA, and a 385-bp deletion fragment derived from the 1,511-bp fragment (located at position −1175 to −790) were inserted into pGL2 basic (Luciferase Expression System; Promega, Madison, WI). A hypoxia inducible factor-1 polymer (HIF-1 polymer) was generated by polymerase chain reaction (PCR) amplification from the Sac I-Sac I fragment using the forward primers, HIF-1 wild-type (HIF-1 wt, 5′-ccacagtgcatacgtgggctcc-3′) and HIF-1 mutant (HIF-1 mut., 5′-ccacagtgcataAAAAggctcc-3′), and a reverse primer at position −782 (Pst I; 5′-ctggcctgcagacatc-3′).7 In transfection experiments, 3 × 106 cells were electroporated with the indicated plasmid DNA and incubated for 17 hours in complete DMEM before treatment. Total quantity of plasmid DNA was kept constant by the addition of pBluescript plasmid (Stratagene, LaJolla, CA). Twenty-four hours after transfection, cells were washed with phosphate-buffered saline and lysed in 100 μL of luciferase lysis buffer (Luciferase Expression System, Promega, Madison, WI) before measuring luciferase activity as previously described.7 Five micrograms of each plasmid containing either the HIF-1α or HIF-1β coding sequence, generously provided by Dr G. Semenza (Johns Hopkins University School of Medicine, Baltimore, MD), were cotransfected with 5 μg of HIF-1 wt polymer- or HIF-1 mut. polymer-reporter plasmids. pK71R-ERK1 and pK52R-ERK2, plasmids expressing the dominant negative mutant forms of Mitogen Activated Protein Kinases (MAPKs), also referred to as extracellular signal-regulated kinases (ERKs), and pFos-luc were kindly provided by Dr David Brenner (University of Texas Southwestern Medical Center, Dallas). Mutant ERKs (0.1 μg) were cotransfected in NIH3T3R cells with 1 μg of Fos-luc reporter plasmid and assayed for luciferase activity. One or 5 μg of VEGF reporter plasmid was cotransfected with 0.1 and 1 μg of the mutant ERK expression vectors. A mutant form of the p85 (Δp85) subunit of PI 3-kinase that lacks amino acids from 479 to 513 and contains two extra amino acids (Ser-Arg), was generously provided by Dr M. Kasuga (University of Kobe School of Medicine, Kobe, Japan),27 was cotransfected with 5 μg of 385-bp-, HIF-1 wt polymer- and HIF-1 mut. polymer-reporter plasmids. One-tenth, 1, or 10 μg of a plasmid containing the wt p110 coding sequence, kindly provided by Dr Julian Downward (Imperial Cancer Research Fund, London, UK),28 was cotransfected with 5 μg of HIF-1 wt polymer-reporter plasmid. One-tenth, 1, 5, or 10 μg of a plasmid containing a defective kinase allele of Akt [HA-Akt(K179M)], generously provided by Dr T. Franke (Harvard Medical School, Boston, MA), was cotransfected with 2.5 μg of 1,511-bp-, 385-bp-, and HIF-1 wt polymer-reporter plasmids. Overexpression of wt or dominant negative constructs were verified for activity by assaying the appropriate downstream effector.
Cell treatment.NIH3T3R cells were left untreated or treated with hypoxia (HYP), tumor necrosis factor-α (TNF-α; 200 U/mL), epidermal growth factor (EGF; 30 ng/mL), anisomycin (Anis.; 30 ng/mL), sodium chloride (NaCl; 0.1 mol/L), and sorbitol (0.2 mol/L) for 7 hours before luciferase measurement. UV light was applied at 40 J/m2 and assayed 24 hours later for luciferase activity. These treatment schedules were initially emulated after published protocols29-35 but were later modified to determine the optimal conditions of activation of known downstream effectors. Except for hypoxia, shorter or longer incubation times also did not increase VEGF induction.
JNK/SAPK activity assay.The JNK/SAPK activity assay was performed as described previously by Sanchez et al32 with GST tagged c-Jun as a substrate. JNK/SAPK activity induced by anisomycin, 0.02% oxygen (HYP 0, 30, and 60 minutes) and 3.9% oxygen or 0.02% oxygen with or without reoxygenation after 0 minutes or 30 minutes was analyzed using immune complex kinase assays to quantitate increases in JNK/SAPK activity. The GST-c-Jun plasmid was kindly provided by Dr James R. Woodgett (Ontario Cancer Institute, Toronto, Ontario, Canada).
PI 3-kinase assay.PI 3-kinase assays were performed as previously described by Franke et al.22 Briefly, NIH3T3 and NIH3T3R cells were exposed to air (C), PDGF-BB (10 ng/mL) for 15 minutes, or 0.02% oxygen for 5 and 15 minutes. After a phosphate-buffered saline (PBS) wash, cells were lysed in buffer containing 137 mmol/L NaCl, 20 mmol/L Tris-HCl, pH 7.5, 1 mmol/L MgCl2 , 1 mmol/L CaCl2 , 10% glycerol, 1% NP-40, 1 μg/mL leupeptin, 1 μg/mL aprotinin, 1 μg/mL pepstatin, 1 mmol/L phenylmethylsulfonyl fluoride (PMSF), and 1 mmol/L sodium orthovanadate. Lysates were assayed for protein concentration using the bicinchoninic acid technique (Pierce Biochemicals, Rockford, IL). Equal amounts (300 μg) of lysate from control or treated cells were incubated with 5 μg of antiphosphotyrosine antibody (UBI, Lake Placid, NY) and immunoprecipitated with Protein A-Sepharose beads (Sigma, St Louis, MO). Kinase reactions were performed using PI as the substrate and labeling with γ32P-ATP. Thin-layer chromatography (TLC) was performed with a CHCl3 :MeOH:water:ammonium hydroxide (40:48:10:5) solvent system. Results were visualized by autoradiography.
RESULTS
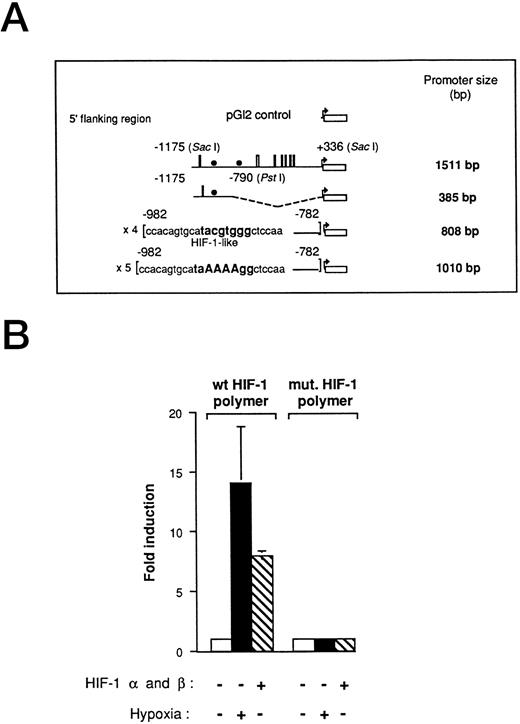
Hypoxia-responsive element of the human VEGF promoter in Ha-ras–transformed cells.We have previously used VEGF reporter constructs to demonstrate that a hypoxia inducible factor-1–like (HIF-1-like) sequence promotes VEGF activity under low oxygen conditions in Ha-ras–transformed cells.7 In this study, we used the 1,511-bp VEGF promoter-reporter gene that includes 1,175 bp from the start site of transcription and 336 bp of the 5′ untranslated region of VEGF promoter (Fig 1A). To further investigate VEGF promoter activity under hypoxic conditions in cells expressing an oncogenic form of Ha-ras (NIH3T3R cells), we also used the 385-bp deletion fragment of the 1,511-bp promoter, located at position −1175 (Sac I) to −790 (Pst I), as well as the HIF-1 polymer ligated to the luciferase reporter gene. They both contain at least one HIF-1 element (385-bp, one copy; HIF-1 polymer, four copies). This series of promoter-reporter genes allows us to investigate the effect of hypoxia on the full-length VEGF promoter as well as on specific regulatory elements. As with many regulatory elements, the addition of multiple copies increases the magnitude of the readout. The HIF-1 polymer construct was induced to comparable levels by either cotransfection with HIF-1α- and HIF-1β-expression vectors or by hypoxia alone (Fig 1B). A mutant HIF-1 element was unresponsive to either hypoxia or cotransfection with HIF-1α and HIF-1β.
Functional demonstration of a hypoxia inducible factor-1 (HIF-1) element in the human VEGF promoter region in Ha-ras–transformed cells (NIH3T3R). (A) Promoter deletion constructs derived from a 1,511-bp fragment of the VEGF promoter. The HIF-1–like wt and mutant polymers were generated by PCR amplification from the 1,511-bp fragment. Thin lines, 5′ sequences used (numbers refer to the distance from the start site of transcription); boxes, SP-1-binding sites, and solid circles, HIF-1–like element. (B) Transient transfections with HIF-1α (5 μg)- and HIF-1β (5 μg)-expression vectors with 5 μg of the HIF-1 polymer- and the HIF-1 mutant-VEGF reporter plasmid. NIH3T3R cells were transfected with reporter plasmid, allowed to recover for 17 hours in air, treated in air or hypoxia (0.02% O2) for 5 hours before being assayed for luciferase activity. The relative fold induction refers to the ratio of luciferase activity measured in treated cells relative to the activity observed in the untreated controls. Values represent the means of at least three independent transfections. Error bars represent one standard deviation of the mean.
Functional demonstration of a hypoxia inducible factor-1 (HIF-1) element in the human VEGF promoter region in Ha-ras–transformed cells (NIH3T3R). (A) Promoter deletion constructs derived from a 1,511-bp fragment of the VEGF promoter. The HIF-1–like wt and mutant polymers were generated by PCR amplification from the 1,511-bp fragment. Thin lines, 5′ sequences used (numbers refer to the distance from the start site of transcription); boxes, SP-1-binding sites, and solid circles, HIF-1–like element. (B) Transient transfections with HIF-1α (5 μg)- and HIF-1β (5 μg)-expression vectors with 5 μg of the HIF-1 polymer- and the HIF-1 mutant-VEGF reporter plasmid. NIH3T3R cells were transfected with reporter plasmid, allowed to recover for 17 hours in air, treated in air or hypoxia (0.02% O2) for 5 hours before being assayed for luciferase activity. The relative fold induction refers to the ratio of luciferase activity measured in treated cells relative to the activity observed in the untreated controls. Values represent the means of at least three independent transfections. Error bars represent one standard deviation of the mean.
A Raf/MAPK or SAPK pathway is not used in signaling VEGF induction under hypoxia.Ras represents a critical junction in the downstream transmission of signals from growth factor receptors and from exposure of cells to stress-inducing agents.36 In many different cell types, activated Ras triggers a kinase cascade involving the serine-threonine kinase Raf-1 which phosphorylates and activates the dual-function specificity kinase MEK (MAP Kinase Kinase) which then phosphorylates and activates MAP Kinase via tyrosine and threonine phosphorylation.37,38 To determine if Ha-ras–transformed cells use an Raf-1/MAP Kinase pathway to signal VEGF induction under low-oxygen conditions, we transfected NIH3T3R cells either stably or transiently with a dominant inhibitory mutant of c-Raf-1, Raf301.39 We found that neither stable overexpression of Raf301 (Fig 2A), nor transient overexpression of an Raf301 plasmid (Fig 2B), which have both been shown to be active in inhibiting NF-κB signaling,15 had a significant effect on VEGF reporter gene induction in response to hypoxia. In comparison, the dominant inhibitory allele of Ras, N17,40 was able to completely inhibit VEGF induction by hypoxia7 (Fig 2A). These findings indicate that in Ha-ras–transformed cells, the Raf-1 kinase is not used as a downstream effector of Ras to signal VEGF induction under hypoxic conditions even though it is activated and able to transmit signals to other transcriptional regulators.
Raf-1 is not a downstream effector in the signal transduction pathway used to signal VEGF induction in Ha-ras–transformed cells (NIH3T3R). (A) NIH3T3R cells were stably transfected with a mutant inhibitory allele of Ras [Ras(N17)] or Raf-1 [Raf301] before transient transfection with 5 μg of the 385-bp VEGF reporter plasmid and exposure to hypoxia. Values represent at least five independent transfections. Error bars represent 1 SD of the mean. (B) Transient transfections with Raf301 (0.1, 1, and 10 μg) and 385-bp reporter construct (2.5 μg) and exposure to hypoxia. (C and D) The effect of MAPKs on signaling VEGF induction by hypoxia. Kinase defective alleles of ERK1 and ERK2 were cotransfected with VEGF reporter constructs to examine their effect on inhibiting VEGF induction by low-oxygen conditions. The relative fold induction refers to the ratio of luciferase activity measured in treated cells relative to the activity observed in the untreated controls. Values represent the means of at least three independent transfections. Error bars represent 1 SD of the mean.
Raf-1 is not a downstream effector in the signal transduction pathway used to signal VEGF induction in Ha-ras–transformed cells (NIH3T3R). (A) NIH3T3R cells were stably transfected with a mutant inhibitory allele of Ras [Ras(N17)] or Raf-1 [Raf301] before transient transfection with 5 μg of the 385-bp VEGF reporter plasmid and exposure to hypoxia. Values represent at least five independent transfections. Error bars represent 1 SD of the mean. (B) Transient transfections with Raf301 (0.1, 1, and 10 μg) and 385-bp reporter construct (2.5 μg) and exposure to hypoxia. (C and D) The effect of MAPKs on signaling VEGF induction by hypoxia. Kinase defective alleles of ERK1 and ERK2 were cotransfected with VEGF reporter constructs to examine their effect on inhibiting VEGF induction by low-oxygen conditions. The relative fold induction refers to the ratio of luciferase activity measured in treated cells relative to the activity observed in the untreated controls. Values represent the means of at least three independent transfections. Error bars represent 1 SD of the mean.
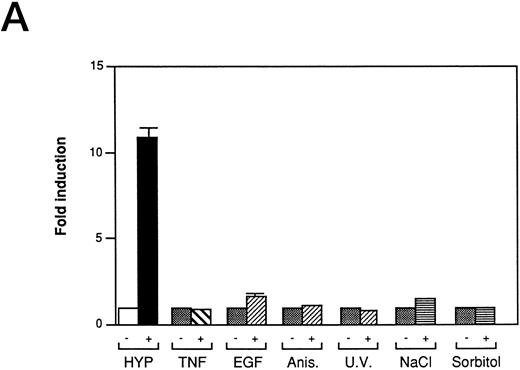
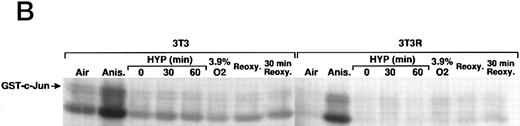
We also investigated the role of MAPK in VEGF induction by hypoxia in Ha-ras–transformed cells. Kinase dead alleles of MAP Kinases (also referred to as extracellular signal-regulated kinases, ERK-1 and ERK-2)41 are functionally able to inhibit c-fos promoter reporter (Fos-Luc plasmid derived from human c-fos promoter38 ) activity induced by Ha-ras42 (Fig 2C), but do not block hypoxic inducibility of VEGF (Fig 2D). These observations argue against the involvement of ERK-1 and ERK-2 in VEGF signaling in Ha-ras–transformed cells. The mammalian MAP Kinase family (see Fig 7) also includes the members of the stress response pathway, p3829,43 and c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK).29,30,32,43,44 They all require phosphorylation on threonine and tyrosine residues for activation by different upstream effector kinases. The dual phosphorylation of each of MAP Kinase is regulated by a family of dual-function specificity kinases, MAP Kinase Kinase. In some cell types, p38 (MKK) is phosphorylated by MKK3/MKK629 and JNK/SAPK by MKK4/SEK1.29,30,32,44 The stress response pathways can be activated by cytokines such as TNF-α and interleukin-1 (IL-1),29,32,45 growth factors, and UV light.31,32 The mechanism of JNK/SAPK activation by mitogenic stimuli is complex. Because the activation of JNK/SAPKs by growth factors is blocked by a dominant negative allele of Ha-ras (N17),46 growth factor signaling of JNK/SAPK activation might also be mediated through Ras. Treatment in our NIH3T3R cells with stimuli known to activate the JNK/SAPK pathway,29-33 the p38 kinase pathway,34,35 or the NF-κB pathway,15 indicated that these signaling pathways are not used to induce VEGF promoter activity through HIF-1 transcriptional regulatory sequences (Fig 3A). Furthermore, neither short-term hypoxia nor the combination of hypoxia and reoxygenation stimulates c-jun phosphorylation when compared to a treatment with the protein synthesis inhibitor anisomycin, a known inducer of the JNK/SAPK pathway (Fig 3B). Thus, JNK/SAPK and p38 kinases do not modulate the Ras-dependent VEGF signaling pathway under low-oxygen conditions.
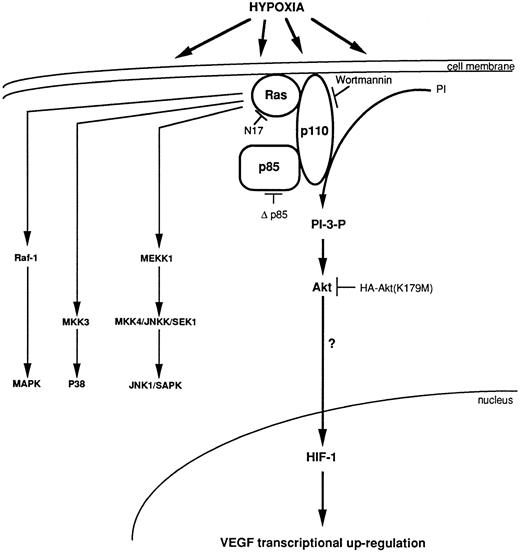
Model for transcriptional regulation of human VEGF gene through an HIF-1 element under a synergistic effect of Ha-ras and hypoxia, via the activation of the PI 3-kinase, which activates Akt.
Model for transcriptional regulation of human VEGF gene through an HIF-1 element under a synergistic effect of Ha-ras and hypoxia, via the activation of the PI 3-kinase, which activates Akt.
Inducers of JNK/SAPK and p38 kinase do not activate the same hypoxia-responsive elements that stimulate VEGF promoter activity in Ha-ras transformed cells (NIH3T3R). (A) Effect of JNK/SAPK and p38 kinase activators on the induction of a 385-bp VEGF reporter construct in NIH3T3R cells. NIH3T3R cells were left untreated or treated with 0.02% oxygen (HYP), TNF-α (200 U/mL), epidermal growth factor (EGF, 30 ng/mL), anisomycin (Anis, 30 ng/mL), sodium chloride (NaCl, 0.1 mol/L), or sorbitol (0.2 mol/L) for 7 hours before luciferase measurement. UV light was applied at 40 J/m2 24 hours before luciferase measurement. The relative fold induction refers to the ratio of luciferase activity measured in treated cells relative to the activity observed in the untreated controls. Values represent the means of at least three independent experimental results. Error bars represent 1 SD of the mean. (B) Comparison of JNK/SAPK activity induced by anisomycin with JNK/SAPK activity induced by 0.02% oxygen (HYP 0, 30, and 60 minutes) and 3.9% oxygen or 0.02% oxygen with or without reoxygenation after 0 minutes or 30 minutes. Values represent at least three independent experiments.
Inducers of JNK/SAPK and p38 kinase do not activate the same hypoxia-responsive elements that stimulate VEGF promoter activity in Ha-ras transformed cells (NIH3T3R). (A) Effect of JNK/SAPK and p38 kinase activators on the induction of a 385-bp VEGF reporter construct in NIH3T3R cells. NIH3T3R cells were left untreated or treated with 0.02% oxygen (HYP), TNF-α (200 U/mL), epidermal growth factor (EGF, 30 ng/mL), anisomycin (Anis, 30 ng/mL), sodium chloride (NaCl, 0.1 mol/L), or sorbitol (0.2 mol/L) for 7 hours before luciferase measurement. UV light was applied at 40 J/m2 24 hours before luciferase measurement. The relative fold induction refers to the ratio of luciferase activity measured in treated cells relative to the activity observed in the untreated controls. Values represent the means of at least three independent experimental results. Error bars represent 1 SD of the mean. (B) Comparison of JNK/SAPK activity induced by anisomycin with JNK/SAPK activity induced by 0.02% oxygen (HYP 0, 30, and 60 minutes) and 3.9% oxygen or 0.02% oxygen with or without reoxygenation after 0 minutes or 30 minutes. Values represent at least three independent experiments.
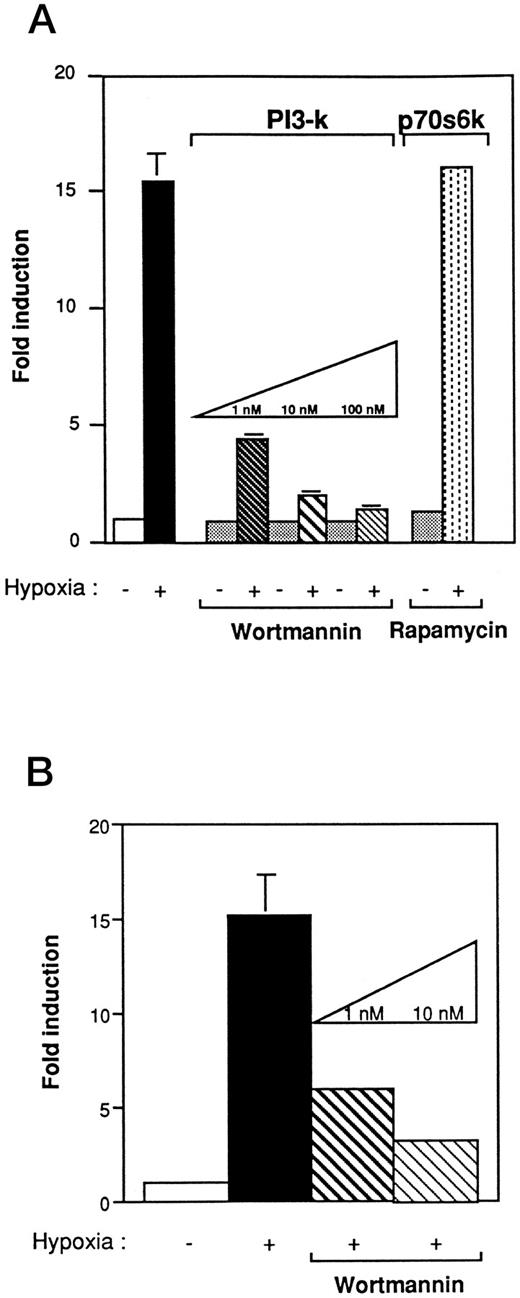
PI 3-kinase plays a pivotal role in VEGF induction under hypoxic stress.Studies have shown that Ras, in the active GTP-bound conformation, interacts with PI 3-kinase.24 To determine whether PI 3-kinase was involved in the VEGF signaling cascade, we first used wortmannin, a specific inhibitor of PI 3-kinase activity. Wortmannin binds to the 110-kD subunit and irreversibly inhibits PI 3-kinase activity when added at nanomolar concentration to mammalian cells.23 NIH3T3R cells were pretreated 2 hours before hypoxia with increasing concentrations of wortmannin (1 to 100 nmol/L). As shown in Fig 4A, wortmannin inhibited VEGF induction by hypoxia in a concentration-dependent manner. At 1 nmol/L, VEGF response to hypoxia was reduced to 77%. Results found with the 385-bp VEGF reporter construct (Fig 4A) were similar to those obtained with a reporter construct containing a polymer of HIF-1 in NIH3T3R or Rat-1R cells that were treated with increasing concentrations of wortmannin (Fig 4B). To determine if PI 3-kinase stimulated VEGF induction through the p70 ribosomal protein S6 kinase (p70S6k), one of the putative targets of PI 3-kinase activation, we examined the effects of rapamycin, a specific inhibitor of the p70S6k pathway, on VEGF induction (Fig 4A).47 In contrast to cells treated with wortmannin, cells pretreated with concentrations of rapamycin up to 20 ng/mL still exhibited VEGF induction under hypoxic conditions.
The effect of wortmannin and rapamycin on VEGF promoter activity in Ha-ras–transformed cells (NIH3T3R). (A) Wortmannin inhibits the induction of the hypoxia-responsive element in the VEGF promoter in NIH3T3 Ras-transformed cells. Cells that were transiently transfected with 5 μg of a 385-bp VEGF reporter plasmid were preincubated in varying concentrations of wortmannin or rapamycin for 2 hours before hypoxic exposure. Values represent at least five independent transfections. Error bars represent 1 SD of the mean. (B) Wortmannin inhibits the induction of the hypoxia-responsive element in the VEGF promoter in Rat-1 Ras-transformed cells. Transient transfection with 5 μg of the HIF-1 expression vector. Cells were preincubated with varying concentrations of wortmannin for 2 hours before hypoxic exposure. The relative fold induction refers to the ratio of luciferase activity measured in treated cells relative to the activity observed in the untreated controls. Values represent the means of at least three independent transfections. Error bars represent 1 SD of the mean.
The effect of wortmannin and rapamycin on VEGF promoter activity in Ha-ras–transformed cells (NIH3T3R). (A) Wortmannin inhibits the induction of the hypoxia-responsive element in the VEGF promoter in NIH3T3 Ras-transformed cells. Cells that were transiently transfected with 5 μg of a 385-bp VEGF reporter plasmid were preincubated in varying concentrations of wortmannin or rapamycin for 2 hours before hypoxic exposure. Values represent at least five independent transfections. Error bars represent 1 SD of the mean. (B) Wortmannin inhibits the induction of the hypoxia-responsive element in the VEGF promoter in Rat-1 Ras-transformed cells. Transient transfection with 5 μg of the HIF-1 expression vector. Cells were preincubated with varying concentrations of wortmannin for 2 hours before hypoxic exposure. The relative fold induction refers to the ratio of luciferase activity measured in treated cells relative to the activity observed in the untreated controls. Values represent the means of at least three independent transfections. Error bars represent 1 SD of the mean.
To genetically demonstrate a role for a PI 3-kinase signaling pathway in VEGF induction by hypoxia, we cotransfected either wt p11028 or mutant p85 (Δp85) expression vectors48,49 with VEGF reporter constructs. Previous reports showed that overexpression of p110, the catalytic subunit of PI 3-kinase, results in increased PI 3-kinase activity.50 Using a similar approach, we examined the involvement of PI 3-kinase in VEGF induction by coexpression of wt p110 and a HIF-1 reporter construct in NIH3T3R cells (Fig 5A). Ectopic overexpression of the p110 subunit resulted in the enhancement of the HIF-1 reporter expression, suggesting a link between PI 3-kinase activity induced by the p110 subunit and the hypoxic response element in the VEGF promoter. Coexpression of the p85 regulatory subunit of PI 3-kinase did not increase the activity of VEGF reporter constructs (data not shown). This result is consistent with several studies showing that overexpression of p85 might uncouple the signal-dependent receptor/PI 3-kinase interaction resulting in an inhibitory effect.50 However, cotransfection of a dominant negative mutant Δp85 suppressed the activity of the 1,511-bp-, 385-bp-, and HIF-1-VEGF reporter constructs under hypoxic conditions (Fig 5B), but had little effect on Fos-luciferase reporter activity induced by Ha-ras. To more directly investigate the relationship between hypoxia, Ha-ras, and PI 3-kinase, we first compared the levels of PI 3-kinase activity in NIH3T3- and NIH3T3R-cells under oxic conditions. PI 3-kinase activity, as measured by an in vitro immune complex kinase assay that uses antiphosphotyrosine to immunoprecipitate activated PI 3-kinase through the SH2 domains of p85, in control untransformed NIH3T3 cells was low (Fig 5C). In contrast, PI 3-kinase activity in Ha-ras–transformed cells (NIH3T3R) under control conditions was already elevated because it is a downstream effector of Ha-ras. However, when NIH3T3R cells were exposed to hypoxia or to a known agonist of PI 3-kinase, platelet-derived growth factor BB (PDGF BB), PI 3-kinase activity was increased twofold (Fig 5C). These data indicate that a small increase of PI 3-kinase activity is sufficient to induce VEGF/HIF-1 promoter activity under hypoxia and is consistent with studies by Klippel et al. showing that low amounts of PI 3-kinase activity were sufficient to activate p70 S6 kinase.51 Taken together these results suggest that PI 3-kinase is a critical downstream effector of Ras in the induction of VEGF under hypoxic conditions.
Modulation of VEGF activity by the p110 and p85 subunits of PI 3-kinase in Ha-ras–transformed cells (NIH3T3R). (A) Stimulation of VEGF expression by cotransfection of increasing concentrations of the wt p110 subunit (0.1, 1, and 10 μg) of PI 3-kinase and 5 μg of wt HIF-1 expression vector. (B) Inhibition of VEGF expression under low-oxygen conditions by a mutant p85 subunit of PI 3-kinase (Δp85). NIH3T3R cells were cotransfected with the mutant p85 (2.5 μg) subunit of PI 3-kinase and a constant amount of reporter plasmid (5 μg; 385 bp; HIF-1 wt [×4]; HIF-1 mut [×5]; Fos-luc) and exposed to hypoxia (except Fos-luciferase) before assaying for luciferase activity. The relative fold induction refers to the ratio of luciferase activity measured in treated cells relative to the activity observed in the untreated controls. Values represent the means of at least three independent transfections. Error bars represent 1 SD of the mean. (C) Thin-layer chromatogram of PI 3-kinase activity from lysates of NIH3T3 and NIH3T3R cells treated with hypoxia and PDGF BB.
Modulation of VEGF activity by the p110 and p85 subunits of PI 3-kinase in Ha-ras–transformed cells (NIH3T3R). (A) Stimulation of VEGF expression by cotransfection of increasing concentrations of the wt p110 subunit (0.1, 1, and 10 μg) of PI 3-kinase and 5 μg of wt HIF-1 expression vector. (B) Inhibition of VEGF expression under low-oxygen conditions by a mutant p85 subunit of PI 3-kinase (Δp85). NIH3T3R cells were cotransfected with the mutant p85 (2.5 μg) subunit of PI 3-kinase and a constant amount of reporter plasmid (5 μg; 385 bp; HIF-1 wt [×4]; HIF-1 mut [×5]; Fos-luc) and exposed to hypoxia (except Fos-luciferase) before assaying for luciferase activity. The relative fold induction refers to the ratio of luciferase activity measured in treated cells relative to the activity observed in the untreated controls. Values represent the means of at least three independent transfections. Error bars represent 1 SD of the mean. (C) Thin-layer chromatogram of PI 3-kinase activity from lysates of NIH3T3 and NIH3T3R cells treated with hypoxia and PDGF BB.
Although many of the downstream targets of PI 3-kinase are still unknown, Akt (protein kinase B) has recently been identified as being a target of PI 3-kinase when cells are stimulated with PDGF BB. We examined the role of Akt in VEGF induction by examining the overexpression of catalytically inactive mutant Akt [HA-Akt(K179M)]52 on VEGF promoter activity. Overexpression of HA-Akt(K179M) inhibited the hypoxic induction of all three VEGF reporter genes transfected in NIH3T3R cells (Fig 6). These results strongly suggest that Akt is used in the hypoxia signaling pathway for VEGF induction in cells possessing an activated Ha-ras oncogene and supports our hypothesis that PI 3-kinase and Akt are downstream effectors of Ras in modulating the transcriptional regulators of VEGF by low-oxygen conditions.
Inhibition of VEGF expression by a catalytically inactive mutant Akt [HA-Akt(K179M)] in Ha-ras–transformed cells (NIH3T3R). NIH3T3R cells were cotransfected with HA-Akt(K179M) (5 μg) plasmid and a constant amount of reporter plasmid (2.5 μg; 1,511 bp; 385 bp; HIF-1 wt), and then exposed to hypoxia before assaying for luciferase activity. Values represent the means of at least three independent transfections. Error bars represent 1 SD of the mean.
Inhibition of VEGF expression by a catalytically inactive mutant Akt [HA-Akt(K179M)] in Ha-ras–transformed cells (NIH3T3R). NIH3T3R cells were cotransfected with HA-Akt(K179M) (5 μg) plasmid and a constant amount of reporter plasmid (2.5 μg; 1,511 bp; 385 bp; HIF-1 wt), and then exposed to hypoxia before assaying for luciferase activity. Values represent the means of at least three independent transfections. Error bars represent 1 SD of the mean.
DISCUSSION
Ras mutations are the most common oncogenic mutation found in over 40% of all human solid tumors.53 Previous studies have indicated that oncogenic forms of Ras increase VEGF expression8-10 and that it is further increased under a microenvironment low in oxygen.7 The purpose of this study was to identify the immediate downstream effectors of Ras that are used in inducing VEGF under hypoxic conditions and to determine whether they act through the hypoxic-responsive element (HIF-1 binding site) in the VEGF promoter. Although a recent study has implicated a role for PI 3-kinase in VEGF mRNA accumulation based on an inhibitor of PI 3-kinase,54 wortmannin, it is unknown whether this modulation occurs at the level of VEGF mRNA stability or induction, both of which can be modified by hypoxia. Using a genetic approach to modulate VEGF promoter activity, we conclude that PI 3-kinase is a downstream activator of Ras which mediates the rapid induction of VEGF through the HIF-1 regulatory region (Fig 7). The evidence supporting these conclusions is based on three observations. Concentrations of wortmannin as low as 1 nmol/L, which specifically inhibits PI 3-kinase activity, significantly block VEGF induction under hypoxic conditions. Overexpression of a dominant negative mutant of the p85 subunit of PI 3-kinase inhibits VEGF/HIF-1 induction under low-oxygen conditions and overexpression of a wt p110 subunit induces VEGF/HIF-1 induction. Third, Ha-ras–transformed cells exposed to low oxygen exhibit a rapid increase in PI 3-kinase activity.
Based on our results, we propose that the p110 catalytic subunit of PI 3-kinase plays a pivotal role in transmitting the signal to activate VEGF by the combination of oncogenic Ha-ras and hypoxic stress. We have previously shown that Ha-ras–transformed cells possess increased basal levels of VEGF mRNA and a significant increase in VEGF promoter activity under hypoxic conditions.7 The induction of HIF-1 activity after coexpression of the wt of p110 and HIF-1 reporter contruct (Fig 1B) is similar to that obtained under hypoxic conditions, suggesting that the activated p110 subunit is sufficient to mimic hypoxia induced transcriptional activity. According to previous studies, p110 acts as the catalytic subunit of PI 3-kinase.55 We propose that, in NIH3T3R cells, Ha-ras will directly interact with the p110 catalytic subunit of PI 3-kinase28 to stimulate a partial PI 3-kinase activity and increase the basal expression of VEGF. Hypoxia increases PI 3-kinase activity further most probably by altering the interaction of the p85 subunit with other phosphotyrosine-containing proteins. Studies to test this hypothesis are presently under way.
If PI 3-kinase lies downstream of Ras in signaling VEGF induction, what are its downstream effectors? At present, we can exclude p70S6k as one such effector because the macrolide rapamycin that inhibits p70S6k by inducing its dephosphorylation, had little effect on VEGF induction. A second potential downstream effector of PI 3-kinase is the serine/threonine protein kinase Akt that is indirectly activated by PI 3-kinase through its lipid products. Activated Akt, and its effector PI 3-kinase, have been shown to block apoptosis by transducing a survival signal.56 Our studies show that Akt activity is important in the induction of VEGF by hypoxia as overexpression of catalytically inactive mutant Akt inhibits VEGF reporter gene activity in a concentration-dependent manner (Fig 6).
The involvement of PI 3-kinase in the angiogenic and apoptotic programs of Ha-ras–transformed cells has important clinical implications. If PI 3-kinase activity is able to act as transducer of a survival signal to inhibit apoptosis induced by serum withdrawal,57 then increasing the expression of angiogenic mitogens such as VEGF could also act as an additional survival signal to tumor cells exposed to a low-oxygen environment. Thus, PI 3-kinase may not only protect cells from adverse environmental conditions, it may also act to promote tumor evolution by stimulating the angiogenic switch in Ha-ras–transformed cells and allowing the expansion of apoptotically resistant tumor cells. Therefore, PI 3-kinase may represent a selective target for anticancer therapy in cells possessing oncogenic Ras mutations.
Supported by National Institutes of Health (NIH) Grant No. PO1CA67166 to A.J.G. and K.R.L., ACS, Howard Hughes grants, and Naomi Van den Horn Cancer Research Fund to A.J.G. and CA57333 to K.R.L. E.Y.C. was supported by an NIH predoctoral training Grant No. 5T32CA09302.
Address reprint requests to Amato J. Giaccia, PhD, Stanford University, School of Medicine-Radiation Oncology, CBRL, Stanford, CA 94305.

![Fig. 2. Raf-1 is not a downstream effector in the signal transduction pathway used to signal VEGF induction in Ha-ras–transformed cells (NIH3T3R). (A) NIH3T3R cells were stably transfected with a mutant inhibitory allele of Ras [Ras(N17)] or Raf-1 [Raf301] before transient transfection with 5 μg of the 385-bp VEGF reporter plasmid and exposure to hypoxia. Values represent at least five independent transfections. Error bars represent 1 SD of the mean. (B) Transient transfections with Raf301 (0.1, 1, and 10 μg) and 385-bp reporter construct (2.5 μg) and exposure to hypoxia. (C and D) The effect of MAPKs on signaling VEGF induction by hypoxia. Kinase defective alleles of ERK1 and ERK2 were cotransfected with VEGF reporter constructs to examine their effect on inhibiting VEGF induction by low-oxygen conditions. The relative fold induction refers to the ratio of luciferase activity measured in treated cells relative to the activity observed in the untreated controls. Values represent the means of at least three independent transfections. Error bars represent 1 SD of the mean.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/9/10.1182_blood.v90.9.3322/4/m_bl_0055f2.jpeg?Expires=1769216706&Signature=4P50-ubgbsmvGhqwAOwYJbAAUkNVrQ0KoAEI1xgB9lZ-epMDGG4~zYocluJRgPmEM4J3gZ8ar4tPhzJyy~LcUt720e3yp6AOAOp6M4eflVA9WnJzbX5cH7knIKmZRyHaEstsN9hZ~1aYCfCzxATDn03ewE1cPemORSoAv7J~vtC0vduWo4t1tX5Z5gXyRKzsWD4OqxIY0lNVgIgT9Usq1XID86LH545yxF5bipcNddfiwhNkQT173~r2dOGtgAbbsqA1hNKwxH~jIN~8mhp5kNHuQdS2Xb3OcSmOj6O-TGfLf8hphXKB1l692AAKMQ3ZTk4lJ170pn0iXYydxgQhoA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Modulation of VEGF activity by the p110 and p85 subunits of PI 3-kinase in Ha-ras–transformed cells (NIH3T3R). (A) Stimulation of VEGF expression by cotransfection of increasing concentrations of the wt p110 subunit (0.1, 1, and 10 μg) of PI 3-kinase and 5 μg of wt HIF-1 expression vector. (B) Inhibition of VEGF expression under low-oxygen conditions by a mutant p85 subunit of PI 3-kinase (Δp85). NIH3T3R cells were cotransfected with the mutant p85 (2.5 μg) subunit of PI 3-kinase and a constant amount of reporter plasmid (5 μg; 385 bp; HIF-1 wt [×4]; HIF-1 mut [×5]; Fos-luc) and exposed to hypoxia (except Fos-luciferase) before assaying for luciferase activity. The relative fold induction refers to the ratio of luciferase activity measured in treated cells relative to the activity observed in the untreated controls. Values represent the means of at least three independent transfections. Error bars represent 1 SD of the mean. (C) Thin-layer chromatogram of PI 3-kinase activity from lysates of NIH3T3 and NIH3T3R cells treated with hypoxia and PDGF BB.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/9/10.1182_blood.v90.9.3322/4/m_bl_0055f5a.jpeg?Expires=1769216706&Signature=L2Lp4nU6lrfyfyF~IOshzGtF0JftSSwu9XQofoth0rVMqZB5CFKKu7xdW3vbgiBG-hT~~W9ToEk3Rhfy8yuXMQsswvziXZChEjeerN-bfa2fL2fCraiEb4LpgCfM6ABodAWRff-e~cOXfNRsvweiAPs3NgYi-F67CsNgdDrBNgBhL7Y8Tn6xLcE89Fe9YaP9IUfN3Jvxzr3dz5O3r7S~h2QitpR2keGFtxw5UuMR99DmMtPA1YjqFLWjWuEVD3xtYP2lC9LaGAZoaVL~wV50MkDFaTFtxbpb9gZGp3NMYC8pqxyZs-O5hThDNIdf70Sz8yCGACMqc1vI3cQgVL9JcA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Modulation of VEGF activity by the p110 and p85 subunits of PI 3-kinase in Ha-ras–transformed cells (NIH3T3R). (A) Stimulation of VEGF expression by cotransfection of increasing concentrations of the wt p110 subunit (0.1, 1, and 10 μg) of PI 3-kinase and 5 μg of wt HIF-1 expression vector. (B) Inhibition of VEGF expression under low-oxygen conditions by a mutant p85 subunit of PI 3-kinase (Δp85). NIH3T3R cells were cotransfected with the mutant p85 (2.5 μg) subunit of PI 3-kinase and a constant amount of reporter plasmid (5 μg; 385 bp; HIF-1 wt [×4]; HIF-1 mut [×5]; Fos-luc) and exposed to hypoxia (except Fos-luciferase) before assaying for luciferase activity. The relative fold induction refers to the ratio of luciferase activity measured in treated cells relative to the activity observed in the untreated controls. Values represent the means of at least three independent transfections. Error bars represent 1 SD of the mean. (C) Thin-layer chromatogram of PI 3-kinase activity from lysates of NIH3T3 and NIH3T3R cells treated with hypoxia and PDGF BB.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/9/10.1182_blood.v90.9.3322/4/m_bl_0055f5b.jpeg?Expires=1769216706&Signature=vN9Xg9YBisl1D9N2-lfB0wpgImhoR69eZG2MJMu5Z2JEvTlp2Qx-W8GSv2GIBJwQWq1nwNO-vK8cKSDlzyuX3V5e~5uPxvcT7NmFyx01vaxzzIhxML2KxO4ltoEnv8A3zY5xpLCfniy3vilVFa1vI8IINaRUAThXsjvVjHE1basKKgzYVytRVQV7JTJ1EediLAKqcUPL~RavHYkWlsYQVhRu8HAxqL5RoINn8x1ZamH7hkA-X03qTpCQGUyTZIo4vLjDov-UyKbo0qE2Vd~fSQiJ1fHMNIZcAfwAlcLjmpdhxzkFRRf1ujtrftJOLCUir-3~bexNaHEU37bVhn5Jog__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Modulation of VEGF activity by the p110 and p85 subunits of PI 3-kinase in Ha-ras–transformed cells (NIH3T3R). (A) Stimulation of VEGF expression by cotransfection of increasing concentrations of the wt p110 subunit (0.1, 1, and 10 μg) of PI 3-kinase and 5 μg of wt HIF-1 expression vector. (B) Inhibition of VEGF expression under low-oxygen conditions by a mutant p85 subunit of PI 3-kinase (Δp85). NIH3T3R cells were cotransfected with the mutant p85 (2.5 μg) subunit of PI 3-kinase and a constant amount of reporter plasmid (5 μg; 385 bp; HIF-1 wt [×4]; HIF-1 mut [×5]; Fos-luc) and exposed to hypoxia (except Fos-luciferase) before assaying for luciferase activity. The relative fold induction refers to the ratio of luciferase activity measured in treated cells relative to the activity observed in the untreated controls. Values represent the means of at least three independent transfections. Error bars represent 1 SD of the mean. (C) Thin-layer chromatogram of PI 3-kinase activity from lysates of NIH3T3 and NIH3T3R cells treated with hypoxia and PDGF BB.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/9/10.1182_blood.v90.9.3322/4/m_bl_0055f5c.jpeg?Expires=1769216706&Signature=0BoVWR9ORwI9U0dKhsBJgyKmvjAHhfwClCKDQ979qXERe6ODK3knxhvZ0YwA8jgJcjVDjeFG~VWmyTKU82-viFzSE0VrncWsMv35xEMNrt0CnbXUdgTFyGRJ2YMth3WhbFAGRAvNAfLmhS80Vc4-YZN7BWDwR7Qk13rEt0MjA8LYxHek6brK7O6JxXlz-e1j~e2hMxBvmUSq9Ck3eyopObZDBvRXA9T~~UmZ0FI6nNStZUDwKJyb0QFNrSrMIlEhvMDEQBlimpw2-r4rSes56QVZ5gifrhGbhjN05WVQ714dmXvmZnOQXY2NyRDnOG4IPKjh3-AOtrSXrYWJN8T3dg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Inhibition of VEGF expression by a catalytically inactive mutant Akt [HA-Akt(K179M)] in Ha-ras–transformed cells (NIH3T3R). NIH3T3R cells were cotransfected with HA-Akt(K179M) (5 μg) plasmid and a constant amount of reporter plasmid (2.5 μg; 1,511 bp; 385 bp; HIF-1 wt), and then exposed to hypoxia before assaying for luciferase activity. Values represent the means of at least three independent transfections. Error bars represent 1 SD of the mean.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/9/10.1182_blood.v90.9.3322/4/m_bl_0055f6.jpeg?Expires=1769216706&Signature=zWM7ZVyVFSZwkoG3ZqW-90OqeM9j4mAwuuHak9yYjXnf4Ili8rOQpSQWXxhNrlMjRUSPjifAvtCDN7TLjaENmX5il9HIkk7BElPYXhCEhon7-Y5WxmYFtJUixlaUd8HGQWaXMZcQoF9rYTBAdjHqqyN3tsZy3mqzzM-Wz-he1IfdFX-u2bmsoeXb8LgYWGxS-dWtRMr8d5miDsEdA5P-~uzDAAm-W255pebu0MH3jKThDrCYg1lHm2Gt~h-XWPK9uLX4r4QNx3HvAVpkW-a3a-O84bu09o9hQtkuC5xb3IHbRxcW5PwW~2hGdLhDjlRtoZsJkmL0PPJnJTAgeq949w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
