Abstract
Substantial barriers exist to the engraftment of hematopoietic cells in mice after in utero transplantation. Although high levels of donor-derived hematopoiesis have been reported in SCID mice, the majority of chimeric recipients exhibit decreasing levels of donor cells over time. To directly test whether the natural killer cell and macrophage activity of the recipients represents a barrier to sustained engraftment, fetal NOD/SCID mice were injected on day 13.5 of gestation with an enriched congenic hematopoietic progenitor cell population. Forty-four percent of pups showed the presence of Ly5.1+ donor cells 4 weeks after transplantation. The mean number of donor-derived nucleated cells in the peripheral blood (PB) was 30%. Although the majority of circulating donor cells were lymphocytes, up to 15% expressed myelomonocytic markers. Serial PB samples from individual mice indicated that the percentage of circulating donor cells increased from 17% to 55% between 4 and 24 weeks. At 6 months posttransplantation, an increased frequency of multilineage, donor-derived cells was also observed in the bone marrow (BM) and the spleen of chimeric recipients. The engraftment of pluripotent hematopoietic stem cells was evaluated by transplanting BM from chimeric mice into irradiated congenic recipients. Irradiated secondary recipients also exhibited multilineage donor-derived hematopoiesis in the PB, BM, and spleen for up to 6 months. These results show that the in utero transplantation of lineage-depleted BM cells into NOD/SCID recipients produces a high frequency of sustained engraftment of allogeneic hematopoietic stem cells.
SEVERAL UNIQUE characteristics of the fetus are thought to make it an ideal recipient for transplantation of hematopoietic stem cells (HSCs). In contrast to steady-state hematopoiesis in the adult, the rapidly developing fetal hematopoietic system provides an expanding hematopoietic microenvironment that should readily support the engraftment of donor HSCs. The early fetal immune system has long been considered to be relatively incompetent, as evidenced by the observation that it does not reject allogeneic tissues.1 Indeed, the in utero transplantation of allogeneic cells is often associated with the development of immunologic tolerance of the recipient to donor antigens but not to third-party antigens.2 3 These studies suggest that the transplantation of HSCs early during ontogeny may provide high levels of donor HSC engraftment without the need for prior bone marrow (BM) ablation. In addition, the in utero transplantation of HSCs may serve as a model system with which to evaluate the contribution of different populations of HSCs to steady-state hematopoiesis.
In utero transplantation holds considerable promise as a therapeutic approach for the treatment of inherited diseases of both the hematopoietic and immune systems.4,5 This strategy may be used to achieve the successful engraftment of donor HSC without the complications associated with the preparative regimens required in postnatal recipients. The ability to transplant HSC into fully disparate HLA recipients would remove the current limitation of identifying a suitable HLA-matched donor. An additional potential benefit of transplantation during early fetal development includes the opportunity to prevent the prenatal toxicity associated with inborn errors of metabolism.6 As well, engraftment of enriched populations of normal HSCs in the setting of inherited immune deficiency syndromes before the exposure of the fetus to environmental pathogens could result in protection against infections in the neonatal period.
Successful in utero transplantation of hematopoietic cells has been reported in a number of species, including nonhuman primates,7-11 sheep,12-16 goats,17 and mice.18-25 In humans, in utero transplantation has provided immune reconstitution in patients with bare lymphocyte syndrome26 and X-linked severe combined immunodeficiency syndrome.27,28 As shown by these studies, the principal limitation of in utero transplantation is the relatively low frequency of engraftment of donor cells. In addition, most chimeric recipients demonstrate low levels of circulating donor cells. Murine SCID recipients have shown higher frequencies of engraftment and higher levels of circulating donor cells than normal mice, suggesting that immune effector cells may provide a barrier to high levels of sustained donor cell engraftment.23 To further evaluate this hypothesis, we have tested the engraftment potential of an enriched population of hematopoietic progenitor cells in NOD/SCID murine recipients. In addition to the absence of functional T cells and B cells associated with the SCID background, NOD/SCID mice also lack natural killer (NK) cell activity and have defects in macrophage function, including antigen presentation.29 This greater degree of immune dysfunction has been associated with high levels of engraftment of human xenografts in these recipients.30 31 We report here that the frequency of chimeric NOD/SCID recipients is at least 44%. Sustained, multilineage engraftment is routinely observed in the peripheral blood (PB), BM, and spleen of these recipients. We also show the long-term multilineage engraftment of donor-derived cells in secondary recipients, thus providing direct evidence of the engraftment of primitive HSCs in this setting.
MATERIALS AND METHODS
Mice. Female C57BL/6-Ly-5.1 donor mice (Jackson Laboratory, Bar Harbor, ME), aged 6 to 8 weeks, were used as a source of BM-derived hematopoietic progenitors in this study. The NOD/Ltz -scid/scid mice used as fetal recipients and the C57BL/6-Ly-5.2 mice used as congenic secondary recipients were bred and maintained in microisolator cages in the animal care facility at Emory University (Atlanta, GA). All experimental procedures were approved by the Institutional Animal Care and Use Committee of the Emory University School of Medicine.
Donor cell preparations. BM was obtained by flushing the femurs and tibias of donor C57BL/6 mice with Hank's Balanced Salt Solution (HBSS) supplemented with 3% fetal bovine serum and 10 mmol/L HEPES (pH 7.2). To obtain lineage-depleted hematopoietic progenitors, BM cells were incubated with antibodies directed against CD8, Gr-1, Mac-1, Ter119, and B220, as described previously.32 These cells were then washed and incubated with sheep antirat immunomagnetic beads (Dynal, Lake Success, NY). The lineage-positive cells were removed using a magnet, and the nonadherent cells were washed and counted. An aliquot of these cells was stained with a goat antirat second-stage antibody conjugated to phycoerythrin (PE) and with anti-Ly5.1– fluorescein isothiocyanate (FITC) (Pharmingen, San Diego, CA). The degree of the enrichment of lineage-negative/low progenitor cells was then determined by flow cytometry.
In utero transplantation. Cohorts of adult NOD/SCID mice were mated overnight and separated the next morning, a time designated as day 0. On day 13.5 after mating, pregnant dams were anesthetized with methoxyflurane and were maintained using isoflurane. Under aseptic conditions, the uterine horns were exposed, and donor cells were injected through a glass micropipette (∼70 μm) inserted through the uterine wall and into the peritoneal cavity of each fetus under direct visualization. The injection consisted of 0.8 × 106 lineage-depleted BM cells in a maximum volume of 3 μL of HBSS containing 10 mmol/L HEPES, 6 U of heparin, and 3% fetal calf serum. The abdominal incision was closed in two layers using 4-0 silk, and the mice were allowed to complete pregnancy to term.
Analysis of donor-derived hematopoiesis. Four weeks after in utero injection, 200 μL of PB was obtained from the retro-orbital sinus of each recipient under methoxyflurane anesthesia. The red blood cells (RBCs) were sedimented using Dextran T-500 (Pharmacia, Piscataway, NJ) followed by hypotonic lysis. Spleen cell suspensions, free of connective tissue, were obtained by passing spleens through wire mesh. BM cells were obtained by flushing 4 long bones as described above. Nucleated cell pellets were washed and then an aliquot was incubated with a combination of Ly5.1-FITC and Ly5.2-biotin followed by a second incubation with strep-avidin-PE (SA-PE). A second aliquot was stained with Ly5.1-FITC and markers specific for T cells (CD3-PE), B cells (B220-PE), myelomonocytic cells (Mac-1-PE and Gr-1-PE), or NK cells (NK1.1-PE) (antibodies were obtained from Pharmingen, San Diego, CA). The expression of these cell surface antigens was determined by two-color flow cytometry using a FACScan II (Becton Dickinson Immunocytometry Systems [BDIS], San Jose, CA). Data analysis was performed using Paint-A-GatePro software (BDIS).
Long-term reconstitution assay. To determine the pattern of engraftment of in utero-transplanted recipients over time, the PB of a cohort of chimeric recipients was sampled and analyzed for the presence of donor-derived cells for up to 26 weeks posttransplantation. The PB was RBC-depleted as described above and the nucleated cells were stained with Ly5.2-biotin-SA-PE and Ly5.1-FITC and analyzed by flow cytometry. The frequency of donor-derived (Ly5.1) cells is expressed as a percentage of total Ly5+ cells in the PB. At 26 weeks, the PB, BM, and spleen of these recipients were analyzed for the frequency of donor-derived cells and their respective lineage markers expression patterns (see above).
Secondary transplantation. To determine whether the donor-derived hematopoiesis after in utero transplantation was primarily due to transplantable HSCs, BM was harvested from 4-week-old chimeric recipients and transplanted into irradiated secondary recipients (age, 8 to 10 weeks) that were congenic at the Ly5 locus (C57BL/6-Ly-5.2). To ensure that an adequate number of donor-derived stem cells were transplanted, the dose of cells from the primary recipients was adjusted to provide a dose of 2 × 105 Ly5.1 input cells (2 × 106 total BM cells from chimeric recipients containing an average of 8% Ly5.1 cells). Recipient mice received 900 cGy in two equal fractions delivered 3 hours apart using a Gamma Cell 40 irradiator (Nordion, Ottawa, Ontario, Canada). Donor cells were infused intravenously in a total volume of 200 μL of HBSS. Before transplantation, recipient mice were maintained on acidified water (pH 2.2) and after transplantation these animals were treated with aqueous antibiotics for 30 days (106 U/L polymyxin B sulfate and 1.1 g/L neomycin sulfate).
RESULTS
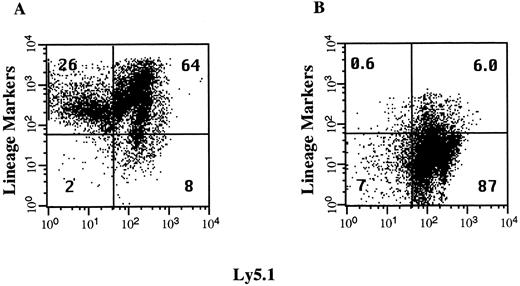
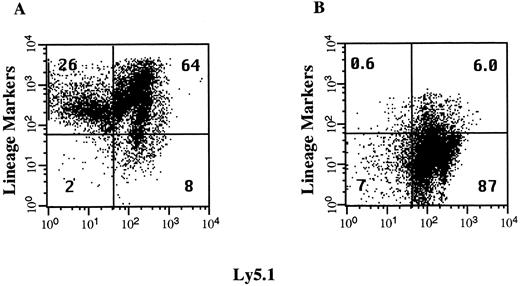
Isolation of lineage-depleted hematopoietic progenitor cells. The volume of cells that can be injected intraperitoneally into day 13.5 fetuses is limited to approximately 3 μL. To increase the dose of HSCs and progenitor cells transplanted, we injected fetal recipients with an enriched population of immature BM cells shown to include all HSCs in the C57BL/6 mouse.33 This population of lineage-depleted cells represents less than 10% of the cells in the BM and the immunomagnetic bead technique we used to deplete the mature lineage-positive cells produced a 10-fold enrichment of these cells (Fig 1). The lineage-positive, Ly5.1− fraction represents up to 26% of the BM cells and consists of erythroid precursors that react with the antibody TER119. This population of cells is reduced by 98% after the lineage-depletion step. The lineage-negative, Ly5.1+ cell fraction increased from 8.2% to 87% in this representative experiment. Each fetal recipient was injected with 0.8 × 106 lineage-depleted cells, which corresponds to the number of progenitor cells found in 8 × 106 BM cells. This dose represents 80-fold more cells than are required to rescue a lethally irradiated adult recipient mouse.
Enrichment of lineage-depleted progenitor cells. Donor C57BL6/Ly5.1 BM cells were incubated with a cocktail of antibodies to lineage-specific markers and with an antibody to Ly5.1. Aliquots of these cells were analyzed by flow cytometry before depletion of lineage-positive cells with immunomagnetic beads (A) and after the enrichment of the lineage-negative hematopoietic progenitors (B). The percentage of total cells in each quadrant is indicated.
Enrichment of lineage-depleted progenitor cells. Donor C57BL6/Ly5.1 BM cells were incubated with a cocktail of antibodies to lineage-specific markers and with an antibody to Ly5.1. Aliquots of these cells were analyzed by flow cytometry before depletion of lineage-positive cells with immunomagnetic beads (A) and after the enrichment of the lineage-negative hematopoietic progenitors (B). The percentage of total cells in each quadrant is indicated.
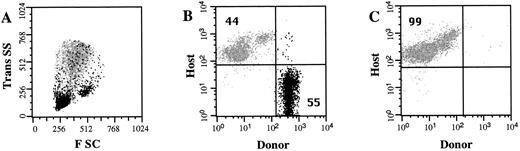
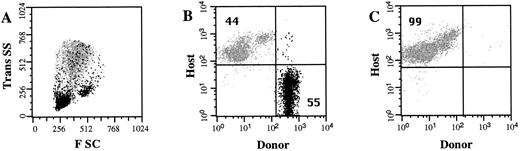
High frequency engraftment of allogeneic hematopoietic progenitor cells. In a series of 9 experiments, a total of 84 embryos were identified (Table 1). To limit the manipulation of the uterus, 31 embryos were not injected due to an unfavorable anatomical orientation. The remaining 53 embryos were injected with lineage-depleted hematopoietic progenitor cells. The number of Ly5.1 donor cells and the number of Ly5.2 host cells in the PB of recipient mice were determined 4 weeks after transplantation. The frequency of Ly5.1 donor cells is expressed as a percentage of total nucleated Ly5+ cells in the PB. Recipients were considered chimeric if a population of ≥1.0% donor cells was detected. Figure 2 shows the presence of Ly5.1 donor cells in the PB of a representative chimeric recipient. In this recipient, 55% of the nucleated PB cells are donor-derived, with the majority of these cells demonstrating a light scatter profile consistent with lymphocytes and monocytes. Of the 34 pups evaluated, 15 (44%) showed the presence of donor cells in the PB 4 weeks after in utero transplantation. This 44% incidence of chimerism represents an underestimation, because a significant number of fetuses were not injected. If the proportion of injected live-born pups is the same as the proportion of noninjected live-born pups, then the frequency of chimeric recipients would be as high as 66% of injected embryos.
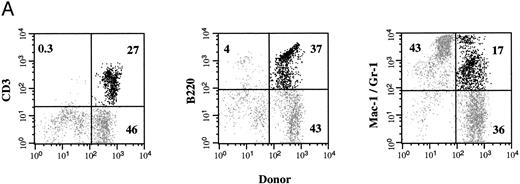
Circulating donor cells in NOD/SCID recipients. Nucleated PB cells were obtained 4 weeks after in utero transplantation and stained with antibodies to Ly5.1 (donor) cells and Ly5.2 (host) cells. The light scatter profile of host cells (gray) and the donor cells (black) is shown in (A). The frequency of Ly5.1 donor cells and Ly5.2 host cells was determined by flow cytometry (B). A control noninjected NOD/SCID mouse is shown in (C). The percentage of total cells that express either the donor or the host cell marker is indicated.
Circulating donor cells in NOD/SCID recipients. Nucleated PB cells were obtained 4 weeks after in utero transplantation and stained with antibodies to Ly5.1 (donor) cells and Ly5.2 (host) cells. The light scatter profile of host cells (gray) and the donor cells (black) is shown in (A). The frequency of Ly5.1 donor cells and Ly5.2 host cells was determined by flow cytometry (B). A control noninjected NOD/SCID mouse is shown in (C). The percentage of total cells that express either the donor or the host cell marker is indicated.
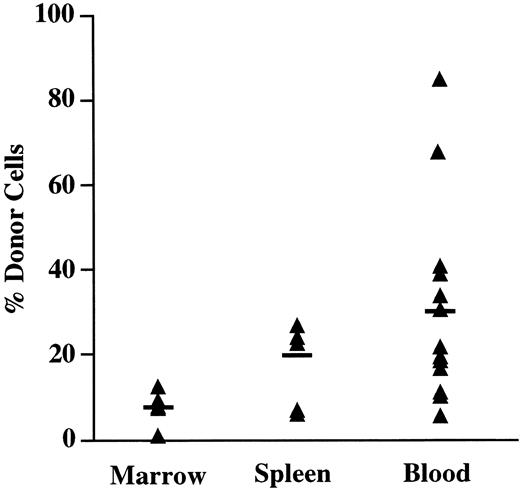
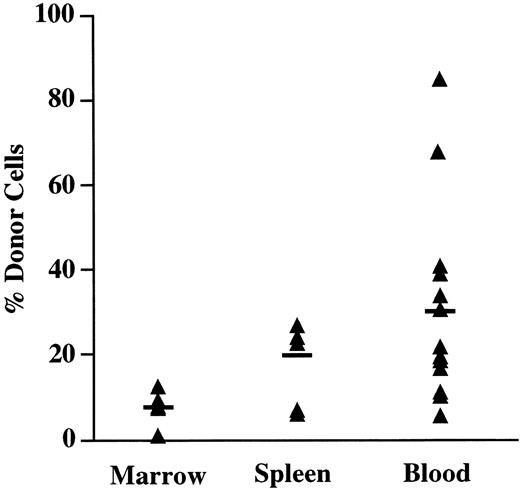
As shown in Fig 3, the mean number of Ly5.1 donor cells in the PB of chimeric recipients at 4 weeks was 30% (range, 5% to 85%). In the spleen, a mean of 20% of the Ly5+ cells were donor-derived, with levels of donor cells ranging from 6% to 34% of nucleated cells. The BM contained the lowest number of donor cells, with a mean of 8% (range, 1% to 12%). However, normalization of the decreased BM cellularity found in NOD/SCID mice was observed with chimeric mice having a mean of 1.64 × 107 cells per femur compared with nonengrafted controls that showed a mean of 0.49 × 107 cells per femur. In all recipients examined, the presence of donor cells in the PB was always predictive of the presence of donor cells in both the BM and in the spleen. In utero recipients without detectable donor cells in the PB failed to demonstrate donor cells in either the BM or the spleen. These findings indicate that engrafted donor cells participate in steady-state hematopoiesis in all the chimeric recipients examined.
Tissue distribution of in utero transplanted cells. The PB, BM, and spleen of fetal recipients of Ly5.1 lineage-depleted progenitors were analyzed for the presence of donor cells 4 weeks after in utero injection. Mice with ≥1% Ly5.1 donor cells were considered chimeric. The percentage of total Ly5+ cells that expressed the Ly5.1 donor marker is shown for individual chimeric mice.The horizontal bar indicates the mean percentage of donor cells in each group.
Tissue distribution of in utero transplanted cells. The PB, BM, and spleen of fetal recipients of Ly5.1 lineage-depleted progenitors were analyzed for the presence of donor cells 4 weeks after in utero injection. Mice with ≥1% Ly5.1 donor cells were considered chimeric. The percentage of total Ly5+ cells that expressed the Ly5.1 donor marker is shown for individual chimeric mice.The horizontal bar indicates the mean percentage of donor cells in each group.
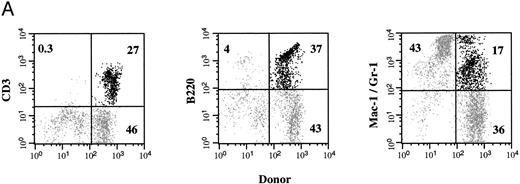
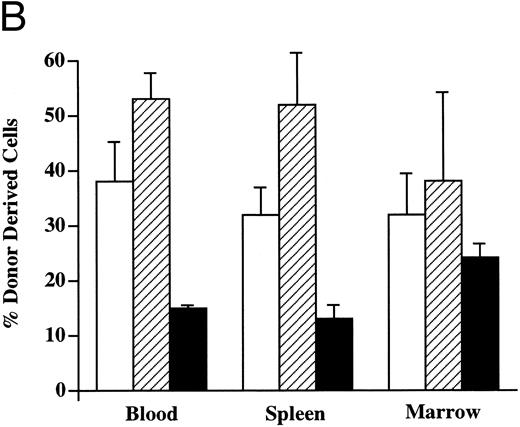
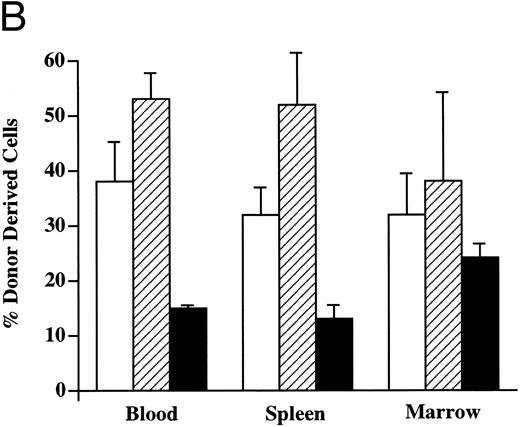
Multilineage hematopoietic reconstitution of chimeric mice. Donor-derived cells in the PB of chimeric recipients predominantly showed the light scatter pattern characteristic of mononuclear cells (Fig 2). When these donor cells were evaluated for the presence of lineage-specific markers at 4 weeks (Fig 4), the majority of these circulating cells expressed the B-cell marker B220 (mean, 53%; range, 45% to 68%). CD3+ T cells were found in the PB with a mean frequency of 37% (range, 18% to 54%), whereas the remaining 15% (range, 14% to 20%) of donor cells expressed the myelomonocytic markers Mac-1/Gr-1. In the spleen, up to 52% of the Ly5.1 cells (range, 10% to 73%) expressed B220, whereas 32% of the donor-derived cells (range, 22% to 58%) expressed CD3. The myelomonocytic markers Mac-1/Gr-1 were detected on 13% of Ly5.1 donor cells (range, 5% to 21%) in the spleen. The BM of chimeric recipients also showed the presence of multilineage donor-derived hematopoiesis. Of the donor-derived cells, 38% (range, 10% to 64%) demonstrated the expression of B220 and 33% (range, 12% to 60%) were CD3+. The fraction of Mac-1/Gr-1–expressing donor cells was highest in the BM, averaging 24% (range, 21% to 31%). Donor-derived multilineage hematopoiesis was present in all three tissues of every chimeric recipient evaluated.
Donor-derived multilineage hematopoiesis. The PB, BM, and spleen of chimeric recipients was evaluated for the presence of Ly5.1+ donor cells 4 weeks after in utero transplantation. (A) The percentage of total cells that express either a lineage-specific marker and/or Ly5.1 in the PB of a representative recipient is indicated. (B) The mean and SEM of the frequency of lineage-specific, donor-derived cells in the PB, BM, and spleen of chimeric recipients (n = 6) is shown. (□) CD3; (▨) B220; (▪) Mac-1/Gr-1.
Donor-derived multilineage hematopoiesis. The PB, BM, and spleen of chimeric recipients was evaluated for the presence of Ly5.1+ donor cells 4 weeks after in utero transplantation. (A) The percentage of total cells that express either a lineage-specific marker and/or Ly5.1 in the PB of a representative recipient is indicated. (B) The mean and SEM of the frequency of lineage-specific, donor-derived cells in the PB, BM, and spleen of chimeric recipients (n = 6) is shown. (□) CD3; (▨) B220; (▪) Mac-1/Gr-1.
In view of the deficiency of NK cells in NOD/SCID mice, we evaluated the potential of donor-derived cells to give rise to NK1.1+ cells. In a cohort of animals examined 3 months after in utero transplantation, a mean of 2.0% (range, 1.0% to 3.2%) of the donor Ly5.1 cells in the spleens of chimeric animals expressed the NK1.1 antigen. This frequency of NK1.1-positive cells is comparable to that observed in normal C57BL/6 spleens (mean, 3.2%). The calculated absolute number of NK1.1-expressing cells was 1.2 × 106 cells per recipient spleen.
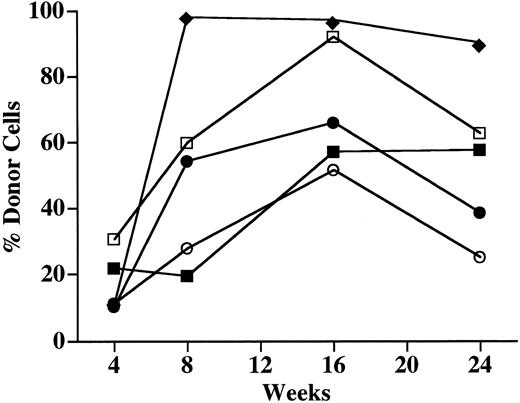
Long-term engraftment of donor-derived cells. To evaluate the patterns of engraftment of donor cells over time, the PB of a group of 5 chimeric mice was examined serially (Fig 5). Similar patterns of donor cell engraftment were observed in all recipient mice. The mean number of Ly5.2 donor cells in the PB increased from 15% at 4 weeks to 52% at 8 weeks and peaked at 72% at 16 weeks. When these chimeric recipients were analyzed again at 26 weeks, the frequency of donor cells had decreased to a mean of 55%.
Engraftment of the PB of NOD/SCID recipients over time. The PB of 5 chimeric recipient mice was sampled at 4, 8, 16, and 24 weeks after in utero transplantation of Ly5.1 lineage-depleted progenitor cells. Total nucleated cells were analyzed for the presence of either the Ly5.1+ (donor) cells or the Ly5.2+ (host) cells. The percentage of Ly5+ cells that were of donor origin in 5 individual animals is shown.
Engraftment of the PB of NOD/SCID recipients over time. The PB of 5 chimeric recipient mice was sampled at 4, 8, 16, and 24 weeks after in utero transplantation of Ly5.1 lineage-depleted progenitor cells. Total nucleated cells were analyzed for the presence of either the Ly5.1+ (donor) cells or the Ly5.2+ (host) cells. The percentage of Ly5+ cells that were of donor origin in 5 individual animals is shown.
To evaluate the long-term multilineage reconstitution of in utero-transplanted cells, the percentage of Ly5.1 donor cells in the PB, BM, and spleen that expressed lineage-specific markers was determined 26 weeks posttransplantation (Table 2). A mean of 43% of the circulating donor cells expressed CD3, 36% expressed B220, and 32% expressed Mac-1/Gr-1. Compared with chimeric recipients at 4 weeks, the frequency of CD3+ cells remained constant, whereas the relative number of B220+ cells decreased by 30%. In contrast, the relative number of Mac-1/Gr-1+ cells more than doubled during this interval (15% to 32%). In the BM, the frequency of total donor cells increased from 8% at 4 weeks to 22% at 26 weeks. Despite this increase in the number of donor cells, the relative frequency of donor cells expressing CD3, B220, and Mac-1/Gr-1 was essentially unchanged. The frequency of donor cells in the spleen increased from 20% at 4 weeks to 68% at 26 weeks. The relative number of CD3+ cells increased from 32% to 56%, whereas B220 B cells declined from a mean of 52% to 40%. The frequency of myelomonocytic (Mac-1/Gr-1+) cells also decreased from 13% to 7% during this interval. These results indicate that the Ly5.1 donor cells contribute to multilineage hematopoiesis for up to 6 months after in utero transplantation.
Secondary transplantation of in utero engrafted Ly5.1 cells. To directly test whether pluripotent HSCs were engrafted in chimeric recipients, we transplanted engrafted donor BM cells into irradiated congenic recipients. The BM of chimeric NOD/SCID recipients typically contained a mean of 8% donor cells (Fig 3) and we injected a dose of total BM cells to provide an equivalent dose of 2 × 105 Ly5.1 donor cells per secondary recipient. All 5 recipient mice survived more than 50 days postirradiation, and when these mice were examined 8 weeks posttransplant, all recipients showed multilineage reconstitution of the PB. To determine the long-term engraftment potential of these Ly5.1 donor-derived cells in irradiated secondary recipients, we analyzed these mice at 26 weeks after secondary transplantation (Table 3). Four of 5 secondary recipients survived for 26 weeks, and the highest levels of donor cells were found in the PB of these animals that demonstrated a mean of 21% cells derived from the original Ly5.1 donor BM. The majority of donor cells were Mac-1/Gr-1–expressing myelomonocytic cells (mean, 54%). B220+ cells represented a mean of 33% of the donor-derived cells, whereas 20% of the donor cells expressed either CD4 or CD8. The BM of these animals demonstrated a mean of 8% donor cells. One third of these cells expressed either CD4 or CD8, B220, or Mac-1/Gr-1. Donor-derived cells in the spleen constituted 15% of nucleated Ly5+ cells. Sixty percent of these donor cells were B220+ and 35% were CD4+ or CD8+, whereas 13% were Mac-1/Gr-1+. The frequency of donor-derived B cells, T cells, and myeloid cells in these secondary recipients is very similar to the distribution found in normal C57BL/6 spleens. These secondary transplantation studies formally show the successful in utero transfer of multipotent Ly5.1 HSCs into NOD/SCID recipients.
DISCUSSION
Lineage-depleted allogeneic hematopoietic progenitor cells produce high levels of hematopoietic engraftment when injected intraperitoneally into day-13/14 fetal NOD/ SCID recipients. The fetal survival in this study was 43% and the frequency of chimeric live-born offspring was 44%. In view of the fact that only 53 of 84 embryos were injected for technical reasons, the true frequency of chimerism could be as high as 66% if the survival of noninjected and injected fetuses was identical. This figure also represents a conservative estimate, because the survival of injected embryos is likely to be less than the noninjected littermates.
The frequency of chimerism in other reported studies of murine in utero transplantation varies significantly and depends on functional characteristics of both the donor and recipient. Most of the earlier studies in murine models have failed to show chimerism in normal fetal recipients.19,34 Using a polymerase chain reaction-based approach to evaluate engraftment, Carrier et al24 have demonstrated the presence of donor cells in up to 50% of nondefective murine in utero recipients. However, the frequency of donor cells was very low, ranging from 0.0001% to a maximum of only 0.6%. In another study using normal C57BL/6 recipients, the engraftment of donor cells was quantitated using Gpi analysis, which has a sensitivity of 1%.22 Allogeneic BM donor cells produced a higher frequency of chimerism (5.2%) than congenic BM cells (0.7%); however, engraftment was transient in both groups. Although it is not clear why these allogeneic cells have a competitive advantage in the early postnatal period, these investigators raise the intriguing possibility that a donor antihost reaction may provide a form of immunologic conditioning. The same study reported that the frequency of chimerism in Wv/Wv recipients approached 40% and that, in contrast to normal recipients, donor cells persisted for greater than 6 weeks and actually increased in number over this time period. This finding is consistent with the hypothesis that stem cell competition is of fundamental importance in the setting of in utero transplantation.
To address the question of whether in utero transplantation of SCID mice would result in engraftment limited to the lymphoid cell compartment, Blazar et al23 transferred unfractionated BM cells into day-13/14 fetal recipients. When congenic BM was used as a source of donor cells, 13% of the recipients were found to be chimeric. In contrast, 28% of recipients of fully allogeneic BM demonstrated the presence of donor cells. Chimeric animals routinely exhibited multilineage donor cell engraftment consistent with the transfer of multipotent hematopoietic progenitor cells. These findings indicate that immunocompromised recipients yield in utero transplantation engraftment rates that are higher for allogeneic than for congenic donors, again suggesting the possibility that a donor antihost effect is also operative in immunodeficient mice.
We chose NOD/SCID mice as in utero recipients in our study to directly test whether the depletion of NK cells and the impaired antigen-presenting activity associated with the NOD background29 would result in a higher frequency of engraftment than that observed in C.B.17 SCID recipients.23 Forty-four percent of the mice analyzed and up to 66% of injected fetal recipients showed long-term engraftment with donor cells, suggesting that the more pronounced immune defect in the NOD/SCID recipients led to a higher incidence of allogeneic donor cell engraftment. Alternatively, this increased rate of engraftment may also in part be due to our use of lineage-depleted hematopoietic progenitor donor cells that represent an 8-fold to 10-fold enrichment in both early progenitor cells and HSCs.33 We injected a mean of 0.8 × 106 cells or the equivalent of 8 × 106 unfractionated BM cells. This cell preparation represents a twofold increase in donor HSC dose compared with the studies of Blazer et al.23 However, it is important to note that, in a previous study from this group, neither doubling the input cell dose nor doubling the number of in utero injections produced a higher frequency of donor cell engraftment.22 To definitively address the effect of cell dose in NOD SCID fetal recipients, a direct comparison of the engraftment potential of unfractionated BM cells and an equivalent number of lineage-depleted cells is required.
When we evaluated the levels of donor-derived cells in NOD/SCID recipients over time, we observed an increase in donor cells in all three hematopoietic tissues. In the PB, the frequency of donor cells in a cohort of 5 animals increased from 15% at 4 weeks to a maximum of 72% at 16 weeks, with a modest decrease to 55% donor cells observed at 24 weeks. These findings are in contrast to the levels of engraftment observed in the PB of normal recipients transplanted with allogeneic donor cells.22 These chimeric recipients had up to 75% donor RBCs by Gpi typing at 5 days of age; however, no donor cells were detected when these chimeric recipients were re-examined at 6 weeks of age. In contrast, in a series of moderately anemic, W41/ W41 in utero recipients transplanted with congenic cells, donor erythroid cells increased from 1% to 5% at 15 days to 50% to 75% at 141 days.21 The frequency of nucleated Ly5.2 donor cells was analyzed during a similar time course and was found to increase from 4.2% to 15.9%. When the levels of allogeneic donor cells in the PB of individual C.B.-17-SCID recipients was determined, three distinct patterns of engraftment were observed.23 Of 15 chimeric mice analyzed between day 39 and day 131 posttransplant, 2 showed an increased frequency of donor cells, 4 showed stable engraftment, and the majority of recipients (n = 9) exhibited a declining number of donor cells. A total of 4 mice in this latter group failed to demonstrate any detectable donor cells in the PB at 131 days posttransplant. Taken together, the data from these studies indicate that the level of donor cell engraftment is highly dependent on both the degree of immunodeficiency and the functional status of the HSC compartment of the in utero recipient.
When we determined the patterns of lineage-specific engraftment in chimeric recipients, we found clear evidence of tissue-specific changes over time. In both the PB and the spleen, the majority of donor-derived cells expressed B220 at 4 weeks. However, by 26 weeks, CD3+ donor cells predominated in both of these tissues. A twofold increase in Mac-1/Gr-1+ cells was found in the PB, whereas a 50% reduction in Mac-1/Gr-1+ cells was observed in the spleen. Although the BM showed almost a threefold increase in donor-derived cells between 4 and 26 weeks, the relative numbers of B cells, T cells, and myelomonocytic cells remained unchanged. A very different pattern of donor cell engraftment was observed in C.B.-17-SCID recipients.23 When tissue-specific levels of multilineage engraftment were evaluated in the SCID recipients that showed either stable or increased numbers of donor cells, a preponderance of donor-derived T cells was found in the PB, BM, and spleen. The frequency of B cells in the PB and spleen was 30% to 50% of that found in control mice, whereas a 90% reduction of B cells was observed in the BM. Less than 1% of donor cells in the BM and 3% of donor cells in the spleen expressed myelomonocytic cell markers. These results show that the in utero transplantation of fetal SCID mice with unfractionated adult BM preferentially gives rise to donor-derived T cells. The functional capacity and specificity of these donor T cells was demonstrated by their tolerance to host antigens, their ability to respond to third-party antigens, and their potential to cause graft-versus-host disease.23 The hematopoietic microenvironment of SCID recipients may favor the development of the T-cell lineage or alternatively an expansion of mature T cells contained in unfractionated donor BM may have produced this result.
The long-term multilineage reconstitution of hematopoiesis in chimeric in utero recipients may be due to the engraftment of self-renewing HSCs or may result from the engraftment of long-lived committed progenitor cells. To formally distinguish between these possibilities, we performed secondary transplantation experiments using irradiated recipients that were congenic to the original BM donor cells at the Ly5 locus. These secondary recipients showed the presence of multilineage donor cells in the PB (21%), BM (8%), and spleen (15%) for up to 6 months, indicating that allogeneic HSCs were engrafted in utero. The majority of the donor cells in the PB were myelomonocytic, whereas donor B cells were the predominant donor cell population in the spleen. The frequencies of the donor cell phenotypes in the tissues of these secondary recipients correspond to the distribution of these cells in irradiated C57BL/6 mice. In contrast, when Blazar et al23 performed secondary transplantation studies using BM derived from chimeric C.B.-17- SCID in utero recipients, they found that the donor cells in the PB were primarily lymphoid, an outcome that was similar to their findings after in utero transplantation. They hypothesized that low levels of HSC engraftment occurred with a preferential central and peripheral expansion of lymphoid cells. We suspect that the higher frequency of myeloid lineage cells in our studies may well reflect a higher level of HSC engraftment in NOD/SCID recipients. Whether higher levels of donor HSC engraftment are the result of our use of an enriched population of donor hematopoietic progenitors or alternatively is the consequence of a decrease in the capacity of NOD/SCID mice to reject allogeneic HSCs has not yet been determined.
In summary, these studies show that high levels of sustained engraftment of donor hematopoietic cells occurs after the in utero transplantation of lineage-depleted hematopoietic progenitor cells into NOD/SCID mice. Secondary transplantation studies indicate that a sufficient number of donor HSCs were transferred into in utero recipients to provide long-term multilineage reconstitution of these secondary hosts. The high frequency of chimeric recipients and patterns of lineage-specific engraftment that are similar to untransplanted recipient mice indicate that this system may serve as a useful model for evaluating the engraftment potential of different sources of HSCs.
ACKNOWLEDGMENT
The authors thank Pamela Lankford-Turner and Laura Hester for their excellent technical assistance and Dr John Wong for reviewing the manuscript. Breeding pairs of NOD/SCID mice were the generous gift of Dr Colin Weber (Emory University).
Supported in part by National Institutes of Health Grants No. HL52965 (W.H.F.) and NS24097 (A.M.Y.), by the Jill Andrews Leukemia Research Fund, and by the Andrew McLemore Memorial Research Fund (D.R.A. and A.M.Y.).
Address reprint requests to William H. Fleming, MD, PhD, BMT Program, Division of Hematology and Oncology, Oregon Health Sciences University, Room 5330, Basic Sciences Building, 3181 SW Sam Jackson Park Rd, Portland, OR 97201-3098.